Abstract
Formaldehyde and BTEX have been used for different industries and other activities. They release many ways that negatively affect the environment and human health due to inadequate ventilation and rapidly increasing oscillation conditions. The widely used technology, which activated carbon, is emerging new studies on air pollutants removal. This study was produced activated carbon for formaldehyde and BTEX removal from Aesculus hippocastanum L. biomass. It was used as organic waste that is abundant in nature. The carbonization was taken place at 600 °C and chemical activation by mixing with ZnCl2. The produced activated carbon has a surface area is 1858.42 m2/g. The adsorption capacity acquired from experimental data of VOCs were range from 638 to 1114 µg/g for AC-KN, respectively. The removal capacity of produced activated carbon was investigated in a batch reactor. Also, Freundlich and Langmuir’s isotherms were applied in the study. Accordingly, the experimental data were found compatible with both and a hybrid structure that the formaldehyde and BTEX adsorption by AC-KN was better fitted into the Freundlich model. Overall, the study showed that the produced AC-KN from the Aesculus hippocastanum L. biomass has a perfect potential in the removal of the formaldehyde and BTEX from indoor air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The emission of toxic gaseous, volatile organic compounds (VOCs), and odorous is increased with the development process, which has increased raw materials and processes in industrialization management including modern technologies [65]. Air pollution is increasing in urban and industrial areas [27]. The WHO reported that more than 8 million people died every year due to exceeding limits of air pollutants [44]. They cause air pollution which occurs in different forms such as small or bigger particles, which can create danger according to the concentration levels in the indoor or outdoor environment for humans and all livings [21, 24, 26, 49, 51]. Especially the effects of a high amount of different pollutants in indoor air cause negativities to human beings due to people spending most of their time indoors such as house, office, and schools [40, 57]. Various oscillations take place in harmful air pollutants like benzene, toluene, ethylbenzene, xylene, and formaldehyde (HCHO). They are the most common pollutant that is flammable, pungent odor, colorless, and easily evaporate under room conditions in the indoor air [7, 33]. They easily come from insufficient ventilation, cigarette smoke, medical wastes, wood-based products, paint polishing products, cosmetics, and solvent-based chemicals in the buildings (especially in unventilated indoor air) [38, 58]. Indoor air quality (IAQ) is up to 10 times more dangerous than outdoor air quality in terms of human health. The worried people then try reduced indoor air quality in living spaces [30]. Also, they are contributing to ozone formation, global warming, and greenhouse gases from all these sources that lead to react with other radicals [10, 14, 19, 20, 53].
The high concentration of HCHO and BTEX creates some disorders that rise above 100 µg/m3 for 30 min it can because of itching and reddening of the eye [37]. Additionally, it gives rise to respiratory system disorders, premature birth, disorders gene distribution, Alzheimer’s disease, and even cancer [3, 18, 31]. Arrangements need to keep formaldehyde levels in the atmosphere between 61.4 and 492 µg/m3 in some countries [47]. The formaldehyde usually present ranges from 61.4 to 2333.2 µg/m3 depending on the temperature and age of the house in the indoor environment [17, 39]. The WHO recommends a limit of formaldehyde for 30-min indoor environment of 122.8 µg/m3 [62]. Some studies show that it can be cancer for a high value of 2000 µg/m3 [41]. Similarly, the BTEX are used for different industrial activities as several concentrations in the treatment of some gases includes benzene, ethylbenzene, xylene, and toluene [6, 56]. They are caused to nervous and respiratory diseases and several cancer types although limit values are given for benzene as 5 µg/m3 [59]. The American Conference of Governmental Industrial Hygienists (ACGIH) reported that the benzene limit value is 0.5 ppm for an 8-h time-weighted average due to working exposure [1, 13, 15, 52].
The many studies on the removal of HCHO and BTEX on industrial scale or indoor air have applied various removal methods such as condensation, adsorption, absorption, combustion, and biological oxidation [67]. Among the many removal methods like them, adsorption is frequently the preferred method due to practicality, lack of energy, low cost, and low environmental impact [66]. Activated carbon is widely chosen for the removal of undesirable substances such as bad odors and environmental pollutants from silica gel, zeolite, and metal–organic lattice [5, 32, 35, 42]. They have used waste raw materials that are easy to access, cheap, and readily available as agricultural waste or lignin-based such as peach stones, olive stone, rice bran, acorn shell, coffee residue, hazelnut bagasse, and tobacco stem [3, 36, 48, 50, 54]. Especially, gas purification is processed with activated carbon that is used as gas-phase applications such as chemical solvent [9]. Activated carbon application areas are increasing rapidly, and it is frequently used in filter production [23]. The surface area of activated carbon used in gas treatment should be relatively higher than the surface area of activated carbon used in wastewater treatment. In addition, granular or cylindrical form is preferred rather than powder form [22]. After the gas purification and removal methods, the recovery of the released gases has been the subject of research. Capturing and recovering the elements lost by being released from the flue gas has started to be applied in the recycling industries [46, 63]. By determining the gases to be recovered, activated carbons with higher micropore volume and higher desorption rate are preferred (> 3000 m2/g). However, if gas removal rather than recovery is desired, it is sufficient to have an average surface area (800–3000 m2/g) [8]. Its performance and efficiency may hinge on certain circumstances (adsorbent type, residence time, and usage area) [2, 16].
In this study, AC was generated by classical chemical activation and pyrolysis method from Aesculus hippocastanum L. which is organic waste and collected as solid waste from nature. In addition, commercial activated carbon was purchased and compared each other in the experimental study. The adsorption efficiency of produced activated carbon (AC-KN) and commercial activated carbon (AC-COM) were investigated at different concentrations of formaldehyde and BTEX in a batch reactor. In other words, there are very limited studies on the capture of them with ACs in batch reactors. Therefore, this study is important in terms of the removal of formaldehyde and BTEX for the evaluation of agricultural wastes and the removal of indoor air pollutants.
2 Materials and methods
2.1 Preparation of AC-KN
The Aesculus hippocastanum L. as lignocellulosic biomass was collected from Kastamonu City, Türkiye. All samples were washed with distilled water and allowed to dry at 110 °C for 24 h in a controlled oven. After the samples were dried at 105 °C. They were ball milling (Retsch PM100) and then sieved (Retsch AS200) at 0–250 mesh. Zinc chloride (ZnCl2) from Merck was used for chemical activation as powder. The raw material was mixed ZnCl2 as 1–5 M and then was heated at 200 °C for 25 min in a controlled oven. It brought consistency by stirring without stopping until a paste. Then it was allowed to stand at room temperature for 1 day. After the remaining water was evaporated in the oven at 105 °C for 24 h. Pyrolysis was carried out in a stainless steel reactor with high temperatures (1200 °C) and N2 (120 mL/min). The N2 flow under a constant increasing the temperature until 600 °C (10 °C/min) and stayed for 1 h in a muffle furnace. Then they were washed with hot deionized water to remove the excess of ZnCl2 until pH between 6 and 7. It was put into the controlled oven for 24 h to dry, and the AC-KN was obtained. Also, commercial activated carbon (AC-COM) was purchased from Merck for comparison of adsorption capacity performance in this study.
2.2 Characterization of AC-KN
The AC-KN were analyzed with SEM–EDX (FEI Quanta FEG 250) and BET (Quantachrome Nova 2200e) that were degassed at 250 °C for 24 h before BET surface area measurements and pore size. Surface area measurements and pore size distributions of degassed samples were carried out using Quantachrome Nova 2200e series device by using pure nitrogen gas as an adsorbent in a liquid nitrogen environment (77 K). In addition, SEM–EDX images of the samples were taken using Zeiss SUPRA 50 VP microscope.
2.3 Adsorption experiment
2.3.1 Formaldehyde adsorption
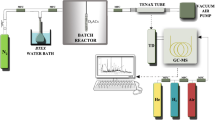
The batch reactor specially designed from quartz glass with two outlets from upper side. C0 values were obtained after injection of different concentrations of formaldehyde into the reactor (volume of 2 L). The carrier gas N2 and formaldehyde concentration are withdrawn from the gas-washing bottle by the pump (SKC The AirChek XR5000). The analysis was carried out under three different conditions: (i) non-AC, (ii) AC-COM, and (iii) AC-KN. Each experiment consisted of three replicates and the mean values of the results in the analysis. The amount of adsorbed formaldehyde was determined by a spectrophotometer at 412 nm wavelength. The EPA 323 method is based on the reaction of formaldehyde with the acetylacetone and its read by a spectrophotometer. Firstly, the spectrophotometer was calibrated for the quantitative determination of formaldehyde. Then calibration curve was drawn through eight concentration levels (0, 50, 100, 150, 250, 350, 500, and 750 µg/L) [60]. The N2 gas (120 mL/min) was used during experiments in Fig. 1.
While 0.1 g of ACs were placed at the same time, the HCHO was injected into the reactor. Then it was evaporated at 25 °C via water bath. A tube made of silica gel (Sigma-Aldrich) was connected to the outlet of the pump to remove moisture from the atmosphere. Forty milliliter of ultra-pure water was added to two gas washing bottles at each measurement. The flow rate of the pump was regulated with 200 mL/min. Each experiment occurs a total of 1 h (10-min intervals). Then vials were filled with 2 mL samples and 2 mL acetylacetone reagent. They were mixed for 1 min in each repetition. It was placed in the water bath at 60 °C for 10 min. After cooling samples were read by spectrophotometer.
2.3.2 BTEX adsorption
The BTEX removal was achieved by modifying the formaldehyde experimental setup with a sorbent tube (Tenax TA). The experiments were analyzed by TD-GC/MS (Markes Unity-2 TD and Agilent 6890 N Network Gas Chromatograph and Agilent 5975 Series). Many studies have used high separation performed with US-EPA TO-17 Method [28]. The standards of the BTEX used standard commercial mixtures at a concentration of 200 μg/mL in methanol (Ultra Scientific brand Aromatic Hydrocarbons Mixture DWM-550–1) from Supelco, USA. The column was an Agilent J&W DB-5 ms Ultra Inert column 30 m × 0.25 mm × 0.25 μm film thickness. The carrier gas was He with the constant flow as 1.0 mL/min, 50 kPa. The injector was used in splitless mode. The programmed temperature ramp for the oven was set as follows: (i) isothermal at 38 °C for 10 min, (ii) increase of 10 °C/min up to 300 °C, (iii) actual duration of the run was approximately 36 min, while the post-run cleaning took 2 min at 300 °C.
3 Results and discussion
3.1 Surface properties of AC-KN
The BET surface area was measured to identify the structural characteristics of the AC-KN between 400 and 900 °C with 1–5 M in Fig. 2.
When the surface areas were examined, the highest values were found at 1 M (550 °C and 1062 m2/g), while it was found at 3 M (700 °C and 1858.42 m2/g) and 5 M (600 °C and 1600 m2/g). The micropore volumes (Vmicro) for 1 M, 3 M, and 5 M were calculated as 0.25 cm3/g, 0.49 cm3/g, and 0.34 cm3/g, respectively.
3.2 SEM–EDX analysis
The SEM micrographs were identified as homogeneity that an increased porosity of the raw material is visible. The AC-COM overtly reveals regular porosity although AC-KN demonstrates an irregular and rough surface in Fig. 3.
The oxygen contents of AC-KN were decreased which is significant changes in the structure of raw material with activation process from the removal of hydroxyl functional groups in the cellulose structure in EDX analysis. The FTIR-ATR analysis of pore size distribution results showed similar data of surface areas in Fig. 4.
The samples were investigated by the nitrogen adsorption isotherm DFT method. Their porosity usually consists of the micro- (0–20 Ẳ) and mesopores (20–500 Ẳ) that figure supported by SEM micrographs due to changes in surface morphology. The carbon and oxygen contents of ACs were compared that AC-KN has higher carbon and lower oxygen content except for AC-COM in Table 1.
3.3 Quality assurance and quality control
The calibration was prepared as 10 ng/μL, 20 ng/μL, 40 ng/μL, 60 ng/μL, 80 ng/μL, 100 ng/μL, and 200 ng/μL. It was loaded by 1 μL of each concentration (R2 > 0.986 is a correlation for 3 replicates). The Tenax tubes were cleaned and stored at − 18 °C until taken to the sampling time without any contamination. Before starting experiments, all tubes were checked in TD-GC–MS (cleaned according to USEPA TO-17 protocol). Tubes were changed every 15 min, with a total sampling time of 1 h by GC–MS in SIM mode.
3.4 Investigation of blank values
The GC–MS calibration parameters were performed with BTEX standard and also were found retention time, correlation coefficient, average, and accuracy of each gas in Table 2.
All cleaned tubes were checked whether any contamination of the environment and blind samples were given in Fig. 5.
3.5 Efficiency of BTEX removal
The removal efficiency (RE) was calculated with Eq. (1) for the performance of ACs in Fig. 6. Initial concentration (C0) and with adsorbent use (Ce) in equilibrium (µg/m3) was defined in the process. The initial concentration of HCHO is in the range of 5–500 µg/m3.
The efficiency of BTEX gases, which are required to be removed with AC-COM, is seen to be lower than AC-KN (57.69–78.25%). While these values are low especially in benzene at 63.29%, these values are high in o-xylene at 85.56%. The adsorption capacity of the COM-AC and COM-KN in benzene were 441 and 844 mg/g; in toluene were 423 and 876 µg/g; in ethylbenzene were 488 and 812 µg/g; in m-p xylene were 587 and 1070 µg/g; and in o-xylene were 603 and 1114 µg/g, respectively.
3.6 Sorption equilibrium modeling of BTEX
The adsorption data of BTEX on the AC-COM and AC-KN were examined with Langmuir and Freundlich isotherms that were used at different initial concentrations. The Langmuir isotherm explains the physical or chemical adsorption of adsorption on the surface of the adsorbent better than other isotherms [12]. Adsorption conditions were plotted with equilibrium values at different concentration values according to Langmuir isotherm in Fig. 7.
Freundlich gives information about surface heterogeneity and an exponential distribution of active sites and their energies [43]. Adsorption conditions were plotted with equilibrium values at different concentration values according to Freundlich isotherm in Fig. 8.
3.7 Efficiency of formaldehyde removal
The experiments were performed with 12 different initial concentrations ranging from 170 to 110,000 µg/m3. The highest yield obtained was 170 ± 14.92 µg/m3, and the lowest yield was 110,000 ± 497.21 µg/m3 in all concentrations. It was to have 47.83% for AC-COM and 73.37% for AC-KN at the lowest concentration of 170 µg/m3 and 26.39% for AC-COM and 32.89% for AC-KN at the highest concentration of 110,000 ± 497.21 µg/m3. The experiments show that firstly the removal efficiency rapidly increases depending on the time and then the efficiency decreases with filling the solid surface of the adsorbent in Fig. 6. However, adsorbent pores clogged earlier period due to the higher concentration of formaldehyde, so yield was significantly an earlier time in Fig. 9.
The high concentration of formaldehyde led to the rapid establishment of the adsorbent balance. Adsorption efficiency decreased after equilibrium. The AC-COM removal efficiency was constantly remained and then was not seem to change much after 30 min at the end of the study. The adsorption capacity of the COM-AC and COM-KN were 321 and 638 µg/g, respectively.
3.8 Sorption equilibrium modeling of formaldehyde
When the calculated \(n\) value is greater than one which indicates the intensity of the adsorption process. Logarithmic states that the log qe to log Ce graph of AC-COM (A) and AC-KN (B) and plotted with equilibrium values at different concentration values and constants drawn by equilibrium data in Freundlich Isotherm. The graph was drawn linearly for values of 170 and 7650 μg/m³ (low concentration) although high concentration range from 30000 to 110000 μg/m³ was not drawn linearly for AC-COM and AC-KN in Fig. 10.
3.9 Assessment and comparison
The assessment of the holding capacity and performance on AC-COM and AC-KN was helpful to ensure comprehension for capturing indoor air pollutants. The comparison was based on the adsorption of prevalent indoor air pollutants as HCHO and BTEX from several initial concentrations on AC-KN derived from lignocellulosic biomass. Many studies of various indoor air pollutants such as sulfur dioxide, hydrogen sulfide, toluene, and ammonia were conducted with ACs from lignocellulosic biomass varied by type of reactor [11, 25, 45]. Mirzaie et al. [34] reported that benzene vapor adsorption was studied in a fixed bed reactor with different operating conditions (VOC concentration from 700 to 1000 ppm). Scope of study AC was produced with nitric acid (HNO3), and adsorption capacity was found as 640 mg/g. Zhou et al. [66] used removal and recovery with commercial AC for removal of methanol, acetone, and toluene in pressure swing adsorption. These molecules can recover at low and high vacuum due to having smaller kinetic diameters and greater polarity. Awad et al. [4] produced activated carbon nanofibers from electrospun polyacrylonitrile/cellulose nanocrystal fibers for VOC adsorption. The pore size distribution indicated large volumes of micropores and mesopores as smooth morphology that was exhibited a high surface area of 3497 m2/g. The performance of produced adsorbent was higher pore and mesoporosity than commercial adsorbent. Zhang et al. [66] were generated activated carbon from biomass-derived with H3PO4 chemical activation. They studied toluene adsorption from air, and adsorption capacity was reached 417 mg/g. Chen et al. [7] prepared activated carbon via Ni-KOH (a porous graphitized carbon) for VOC removal (under humid conditions). Humidity conditions had a negative effect on the adsorption process. Li et al. [30] were evaluated toluene adsorption (321.9 mg/g) and ethyl acetate adsorption (240.4 mg/g) on activated carbon from straw agricultural biowastes via ZnCl2/PM, w/w of 1.6. Lara-Ibeas et al. [29] studied HCHO adsorption with carbon, MOF, silica, and zeolite at a formaldehyde gaseous flow of 164 ppb. The adsorption capacity, which varies according to the adsorbent they use, was found to be between 4.5 ± 0.6 and 34.9 ± 8 μg/g. This study also showed how strongly the humidity rate affects the adsorption capacity. Similarly, their compatibility with adsorption isotherms for multilayer adsorption was investigated for gas removal that was researched; the most suitable isotherm is Freundlich than others [55, 61, 64]. The common idea of these studies, produced activated carbon has a high surface area under optimum conditions from lignocellulosic and agricultural residues. In addition, it is an indication that various pollutants in the indoor air can be easily removed with this activated carbon.
4 Conclusion
In this research, the adsorption of HCHO and BTEX on lignocellulosic biomass-based (lignin is a large fraction of biomass) was studied and compared the performance between AC-COM and AC-KN with several concentrations (activating agent, temperature, pH, humidity, and raw material). The removal of VOCs is closely related to AC-KN’s high surface area, and polarity as the main pore size has a better adsorption effect on small molecules than on large molecules in a batch reactor. In particular, o-xylene was adsorbed quite successfully compared to others. The experimental data are studied and fit to adsorption isotherms. The excessive presence of VOCs in indoor air threatens human health. The continuous active removal of these pollutants is quite laborious and expensive. Therefore, passive removal with adsorbent is a much smarter method. However, some problems arise such as easy accessibility, low cost, desorption, and long service life arise. For this reason, it is very reasonable to use activated carbons produced from materials with high surface area and waste in nature for passive removal of VOCs.
Data availability
Not applicable.
References
ACGIH (2011) Threshold limit values for chemical substances and physical agents and biological exposure indices. American Conference of Governmental Industrial Hygienists, Cincinnati
Aivalioti M, Papoulias P, Kousaiti A et al (2012) Adsorption of BTEX, MTBE and TAME on natural and modified diatomite. J Hazard Mater 207:117–127. https://doi.org/10.1016/j.jhazmat.2010.01.053
Amiri MJ, Roohi R, Arshadi M et al (2020) 2, 4-D adsorption from agricultural subsurface drainage by canola stalk-derived activated carbon: insight into the adsorption kinetics models under batch and column conditions. Environ Sci Pollut Res 1–15.https://doi.org/10.1007/s11356-020-08211-7
Awad R, Mamaghani AH, Boluk Y et al (2021) Synthesis and characterization of electrospun PAN-based activated carbon nanofibers reinforced with cellulose nanocrystals for adsorption of VOCs. Chem Eng J 410:128412. https://doi.org/10.1016/j.cej.2021.128412
Bellat JP, Bezverkhyy I, Weber G, Royer S, Averlant R, Giraudon JM, Lamonier JF (2015) Capture of formaldehyde by adsorption on nanoporous materials. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2015.07.078
Ceron-Breton JG, Bretón RMC, Kahl JD, Rico GS, Lozada SEC et al (2021) Concentrations, sources, and health risk associated with exposure to BTEX at ten sites located in an urban-industrial area in the Bajio Region, Mexico. Air Qual Atmos Health 14(5):741–761. https://doi.org/10.1007/s11869-021-00976-1
Chen PW, Kuo TC, Liu ZS et al (2021) Assessment of the mutagenicity of two common indoor air pollutants, formaldehyde and toluene. Indoor Air. https://doi.org/10.1111/ina.12832
Clinger JC, O’Shaughnessy PT (2019) Breakthrough analysis for filtering facepiece respirators impregnated with activated carbon. J Occup Environ Hyg 16(7):423–431. https://doi.org/10.1080/15459624.2019.1594838
Destro F, Hur I, Wang V, Abdi M, Feng X, Wood E et al (2021) Mathematical modeling and digital design of an intensified filtration-washing-drying unit for pharmaceutical continuous manufacturing. Chem Eng Sci 244:116803. https://doi.org/10.1016/j.ces.2021.116803
Dinh TV, Kim SY, Son YS, Choi IY, Park SR, Sunwoo Y et al (2015) Emission characteristics of VOCs emitted from consumer and commercial products and their ozone formation potential. Environ Sci Pollut Res 22(12):9345–9355. https://doi.org/10.1007/s11356-015-4092-8
El-Hashemy MAES, Alotaibi NF (2021) Purification of benzene-laden air by static adsorption of benzene onto activated carbon prepared from Diplotaxisacris biomass. Biomass Convers Biorefinery 1–15.https://doi.org/10.1007/s13399-021-01462-5
Fu C, Zhang L, Zhang K, Xiao B, Liu J, Luan Q, Liu J (2021) Effects of air-prepared atmosphere on the Pb2+ adsorption of sludge-based adsorbent. Biomass Convers Biorefinery 1–13.https://doi.org/10.1007/s13399-021-01706-4
Gałko G, Rejdak M, Tercki D, Bogacka M et al (2021) Evaluation of the applicability of polymeric materials to BTEX and fine product transformation by catalytic and non-catalytic pyrolysis as a part of the closed loop material economy. J Anal Appl Pyrol 154:105017. https://doi.org/10.1016/j.jaap.2021.105017
Giovanis E (2018) The relationship between teleworking, traffic and air pollution. Atmos Pollut Res. https://doi.org/10.1016/j.apr.2017.06.004
Goldstein AH, Nazaroff WW, Weschler CJ et al (2020) How do indoor environments affect air pollution exposure? Environ Sci Technol 55(1):100–108. https://doi.org/10.1021/acs.est.0c05727
González-García P (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications.
Guo M, Yu W, Zhang S, Wang H et al (2020) A numerical model predicting indoor volatile organic compound volatile organic compounds emissions from multiple building materials. Environ Sci Pollut Res 27(1):587–596. https://doi.org/10.1007/s11356-019-06890-5
Haffner MJ, Oakes P, Demerdash A, Yammine KC, Watanabe K, Loukas M, Tubbs RS (2015) Formaldehyde exposure and its effects during pregnancy: recommendations for laboratory attendance based on available data. Clin Anat. https://doi.org/10.1002/ca.22623
Hajizadeh Y, Mokhtari M, Faraji M, Mohammadi A, Nemati S, Ghanbari R, Abdolahnejad A, Fard RF, Nikoonahad A, Jafari N, Miri M (2018) Trends of BTEX in the central urban area of Iran: a preliminary study of photochemical ozone pollution and health risk assessment. Atmos Pollut Res. https://doi.org/10.1016/j.apr.2017.09.005
Huang S, Wei W, Weschler LB, Salthammer T, Kan H, Bu Z, Zhang Y (2017) Indoor formaldehyde concentrations in urban China: preliminary study of some important influencing factors. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.02.187
Isinkaralar O, Isinkaralar K, Ekizler A, Ilkdogan C (2017) Changes in the amounts of CO2 and particulate matter in Kastamonu Province depending on weather conditions and locations. J Chem Biol Phys Sci 7(3):643–650. https://doi.org/10.24214/jcbps.D.7.3.64350
Isinkaralar K (2020) Removal of formaldehyde and BTEX in indoor air using activated carbon produced from horse chestnut (Aesculus hippocastanum L.) Shell. Ph.D. Thesis Hacettepe University, Institute of Science and Engineering, Department of Environmental Engineering. Ankara, Turkey
Jaiswal KK, Dutta S, Banerjee I, Pohrmen CB et al (2021) Photosynthetic microalgae–based carbon sequestration and generation of biomass in biorefinery approach for renewable biofuels for a cleaner environment. Biomass Convers Biorefinery 1–19.https://doi.org/10.1007/s13399-021-01504-y
Janhäll S (2015) Review on urban vegetation and particle air pollution–deposition and dispersion. Atmos Environ 105:130–137. https://doi.org/10.1016/j.atmosenv.2015.01.052
Javed M, Zahoor M, Mazari SA, Qureshi SS, Sabzoi N, Jatoi AS, Mubarak NM (2021) An overview of effect of process parameters for removal of CO 2 using biomass-derived adsorbents. Biomass Convers Biorefinery 1–19.https://doi.org/10.1007/s13399-021-01548-0
Karacocuk T, Sevik H, Isinkaralar K, Turkyilmaz A, Cetin M (2021) The change of Cr and Mn concentrations in selected plants in Samsun city center depending on traffic density. Landscape Ecol Eng. https://doi.org/10.1007/s11355-021-00483-6
Karmakar D, Deb K et al (2021) Ecophysiological responses of tree species due to air pollution for biomonitoring of environmental health in urban area. Urban Climate 35:100741. https://doi.org/10.1016/j.uclim.2020.100741
Kim MJ, Seo YK, Kim JH et al (2020) Impact of industrial activities on atmospheric volatile organic compounds in Sihwa-Banwol, the largest industrial area in South Korea. Environ Sci Pollut Res 27:28912–28930. https://doi.org/10.1007/s11356-020-09217-x
Lara-Ibeas I, Megias-Sayago C, Louis B, Le Calvé S (2020) Adsorptive removal of gaseous formaldehyde at realistic concentrations. J Environ Chem Eng 8(4):103986. https://doi.org/10.1016/j.jece.2020.103986
Li Z, Li Y, Zhu J (2021) Straw-based activated carbon: optimization of the preparation procedure and performance of volatile organic compounds adsorption. Materials 14(12):3284. https://doi.org/10.3390/ma14123284
Liu X, Zhang Y, Wu R, Ye M, Zhao Y, Kang J, Ma P, Li J, Yang X (2018) Acute formaldehyde exposure induced early Alzheimer-like changes in mouse brain. Toxicol Mech Methods. https://doi.org/10.1080/15376516.2017.1368053
Liu Y, Jia H, Li C et al (2019) Efficient removal of gaseous formaldehyde by amine-modified diatomite: a combined experimental and density functional theory study. Environ Sci Pollut Res 26:25130–25141. https://doi.org/10.1007/s11356-019-05758-y
Mirzaei A, Leonardi SG, Neri G (2016) Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: a review. Ceram Int 42(14):15119–15141. https://doi.org/10.1016/j.ceramint.2016.06.145
Mirzaie M, Talebizadeh AR, Hashemipour H (2021) Mathematical modeling and experimental study of VOC adsorption by Pistachio shell–based activated carbon. Environ Sci Pollut Res 28(3):3737–3747. https://doi.org/10.1007/s11356-020-10634-1
Na CJ, Yoo MJ, Tsang DCW, Kim HW, Kim KH (2019) High-performance materials for effective sorptive removal of formaldehyde in air. J Hazard Mater 366:452–465. https://doi.org/10.1016/j.jhazmat.2018.12.011
Nanda S, Mohammad J, Reddy SN, Kozinski JA, Dalai AK (2014) Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers Biorefinery 4(2):157–191. https://doi.org/10.1007/s13399-013-0097-z
Nielsen GD, Larsen ST, Wolkoff P (2013) Recent trend in risk assessment of formaldehyde exposures from indoor air.
Niu Z, Kong S, Zheng H, Yan Q, Liu J, Feng Y et al (2021) Temperature dependence of source profiles for volatile organic compounds from typical volatile emission sources. Sci Total Environ 751:141741. https://doi.org/10.1016/j.scitotenv.2020.141741
Park SH, Lim HB, Hong HJ, Kim HS, Yoon DK, Lee HW et al (2021) Health risk assessment for multimedia exposure of formaldehyde emitted by chemical accident. Environ Sci Pollut Res 28(8):9712–9722. https://doi.org/10.1007/s11356-020-11403-w
Paterson CA, Sharpe RA, Taylor T, Morrissey K (2021) Indoor PM2. 5, VOCs and asthma outcomes: a systematic review in adults and their home environments. Environ Res 111631. https://doi.org/10.1016/j.envres.2021.111631
Penney D, Benignus V, Kephalopoulos S, Kotzias D, Kleinman M, Verrier A (2010) Guidelines for indoor air quality. WHO Guidelines 9:454. https://doi.org/10.1186/2041-1480-2-S2-I1
Pivokonsky M, Kopecka I, Cermakova L, Fialova K, Novotna K, Cajthaml T et al (2021) Current knowledge in the field of algal organic matter adsorption onto activated carbon in drinking water treatment. Sci Total Environ 149455.https://doi.org/10.1016/j.scitotenv.2021.149455
Ramadoss R, Subramaniam D (2019) Removal of divalent nickel from aqueous solution using blue-green marine algae: adsorption modeling and applicability of various isotherm models. Sep Sci Technol 54(6):943–961. https://doi.org/10.1080/01496395.2018.1526194
Rehfuess E, World Health Organization (2006) Fuel for life: household energy and health. World Health Organization
Restrepo SYG, Rocha MH, Lora EES, Venturini OJ, Cobas VRM, Maya DMY (2021) Design and operation of a gas cleaning system for biomass gasification in a two-stage air-blown downdraft gasifier to meet quality requirements of solid oxide fuel cells. Biomass Convers Biorefinery 1–27.https://doi.org/10.1007/s13399-021-01796-0
Robert B, Nallathambi G (2021) Indoor formaldehyde removal by catalytic oxidation, adsorption and nanofibrous membranes: a review. Environ Chem Lett 1–29.https://doi.org/10.1007/s10311-020-01168-6
Ryu DY, Shimohara T, Nakabayashi K, Miyawaki J, Park JI, Yoon SH (2019) Urea/nitric acid co-impregnated pitch-based activated carbon fiber for the effective removal of formaldehyde. J Ind Eng Chem 80:98–105. https://doi.org/10.1016/j.jiec.2019.07.036
Saleem J, Shahid UB, Hijab M, Mackey H, McKay G (2019) Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers Biorefinery 9(4):775–802. https://doi.org/10.1007/s13399-019-00473-7
Savas DS, Sevik H, Isinkaralar K, Turkyilmaz A et al (2021) The potential of using Cedrus atlantica as a biomonitor in the concentrations of Cr and Mn. Environ Sci Pollut Res 1–8.https://doi.org/10.1007/s11356-021-14826-1
Scherzinger M, Kaltschmitt M (2021) Thermal pre-treatment options to enhance anaerobic digestibility–a review. Renew Sustain Energy Rev 137:110627. https://doi.org/10.1016/j.rser.2020.110627
Sevik H, Isinkaralar K, Isinkaralar O (2018) Indoor air quality in hospitals: the case of Kastamonu Turkey. J Chem Biol Phys Sci Sect D 9(1):067–073. https://doi.org/10.24214/jcbps.D.9.1.06773
Sovacool BK, Griffiths S, Kim J, Bazilian M (2021) Climate change and industrial F-gases: a critical and systematic review of developments, sociotechnical systems and policy options for reducing synthetic greenhouse gas emissions. Renew Sustain Energy Rev 141:110759. https://doi.org/10.1016/j.rser.2021.110759
Sun J, Shen Z, Zhang Y, Zhang Z, Zhang Q, Zhang T et al (2019) Urban VOC profiles, possible sources, and its role in ozone formation for a summer campaign over Xi’an, China. Environ Sci Pollut Res 26(27):27769–27782. https://doi.org/10.1007/s11356-019-05950-0
Teimouri Z, Salem A, Salem S (2019) Regeneration of wastewater contaminated by cationic dye by nanoporous activated carbon produced from agriculture waste shells. Environ Sci Pollut Res 26(8):7718–7729. https://doi.org/10.1007/s11356-018-04094-x
Tham YJ, Latif PA, Abdullah AM, Shamala-Devi A, Taufiq-Yap YH (2011) Performances of toluene removal by activated carbon derived from durian shell. Biores Technol 102(2):724–728. https://doi.org/10.1016/j.biortech.2010.08.068
Turkyilmaz A, Cetin M, Sevik H, Isinkaralar K, Saleh EAA (2020) Variation of heavy metal accumulation in certain landscaping plants due to traffic density. Environ Dev Sustain 22(3):2385–2398. https://doi.org/10.1007/s10668-018-0296-7
Turkyilmaz A, Sevik H, Isinkaralar K, Cetin M (2018) Using Acer platanoides annual rings to monitor the amount 520 of heavy metals accumulated in air. Environ Monit Assess 190:578. https://doi.org/10.1007/s10661-018-5216956-0
Turkyilmaz A, Sevik H, Isinkaralar K, Cetin M (2019) Use of tree rings as a bioindicator to observe atmospheric heavy metal deposition. Environ Sci Pollut Res 26(5):5122–5130. https://doi.org/10.1007/s11356-018-3962-2
UNION P (2008) Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Official Journal of the European Union
USEPA (2020) —Measurement of Formaldehyde Emissions From Natural Gas-Fired Stationary Sources—Acetyl Acetone Derivitization Method Environmental Protection Agency. https://www.epa.gov/sites/default/files/2020-12/documents/method_323.pdf. Accessed 9/7/2020
Wang H, Wang B, Li J, Zhu T (2019) Adsorption equilibrium and thermodynamics of acetaldehyde/acetone on activated carbon. Sep Purif Technol 209:535–541. https://doi.org/10.1016/j.seppur.2018.07.076
WHO (2000) Air Quality Guidelines for Europe, 2nd edn, European Series, No. 91. WHO Regional Publications, Copenhagen
Wu J, Ahn J, Lee J (2021) Characterization of gold deportment and thiosulfate extraction for a copper‑gold concentrate treated by pressure oxidation. Hydrometallurgy 105771.https://doi.org/10.1016/j.hydromet.2021.105771
Xu X, Guo Y, Shi R, Chen H, Du Y, Liu B et al (2021) Natural honeycomb-like structure cork carbon with hierarchical micro-mesopores and N-containing functional groups for VOCs adsorption. Appl Surf Sci 565:150550. https://doi.org/10.1016/j.apsusc.2021.150550
Yusoff MNAM, Zulkifli NWM, Sukiman NL, Chyuan OH, Hassan MH, Hasnul MH et al (2021) Sustainability of palm biodiesel in transportation: a review on biofuel standard, policy and international collaboration between Malaysia and Colombia. Bioenergy Res 14(1):43–60. https://doi.org/10.1007/s12155-020-10165-0
Zhang G, Lei B, Chen S, Xie H, Zhou G (2021) Activated carbon adsorbents with micro-mesoporous structure derived from waste biomass by stepwise activation for toluene removal from air. J Environ Chem Eng 9(4):105387. https://doi.org/10.1016/j.jece.2021.105387
Zhu L, Shen D, Luo KH (2020) A critical review on VOCs adsorption by different porous materials: species, mechanisms and modification methods. J Hazard Mater 389:122102. https://doi.org/10.1016/j.jhazmat.2020.122102
Acknowledgements
This study is produced from the Ph.D. thesis titled “Removal of Formaldehyde and BTEX in Indoor Air Using Activated Carbon Produced from Horse Chestnut (Aesculus hippocastanum L.) Shell” conducted at Hacettepe University, Ankara, Türkiye, Graduate School of Science and Engineering, Department of Environmental Engineering.
Author information
Authors and Affiliations
Contributions
KI, raw material collection and/or processing analysis and/or interpretation; AT, materials and processing analysis; GG, conceptualization, methodology, and literature search.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Isinkaralar, K., Gullu, G. & Turkyilmaz, A. Experimental study of formaldehyde and BTEX adsorption onto activated carbon from lignocellulosic biomass. Biomass Conv. Bioref. 13, 4279–4289 (2023). https://doi.org/10.1007/s13399-021-02287-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02287-y