Abstract
Biochar application to soil has been projected as an approach to improve soil quality, which can also influence soil microbial activities. In this experiment, we have utilized four dissimilar feedstocks derived biochar amendments. The highest microbial biomass carbon was found in Lantana camara (LC) biochar followed by pine needle (PN), maize stalk (MS) and lowest in black gram (BG) biochar. The dehydrogenase activity in different biochar treatment increased significantly along with control with increase in incubation days except pine biochar where dehydrogenase decreased. Biochar application in soil increased acid phosphatase compared to control. The highest alkaline phosphatase was found in MS and it was 20.56, 31.27, 42.52, 57.62 and 69.56 at 1, 7, 30, 60 and 90 days of incubation, respectively. The highest urease was found in LC followed by BG, MS and lowest in PN among the biochar at both the biochar application rate. The biochar application augmented the protease enzyme activity in soil, which might be due to augmenting the accessibility of inorganic nitrogen. The highest fluorescein diacetate was found in LC and it was 10.12, 17.62, 24.62, 32.86 and 37.56 at 1, 7, 30, 60 and 90 days of incubation, respectively. The increased biological indicator was more at 2.5 t/ha biochar application rate than 5.0 t/ha i.e. lower concentration of biochar enhanced more than higher concentration. This laboratory study demonstrated that biochar application can profit incubated acid soils by improving microbial biomass carbon up-lift while increasing potential soil enzyme activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biochar is broadly utilized as a soil amendment. Biochar addition to soil has the prospective to progress soil quality and helps the resilience of agro-ecosystems [1]. Biochar has dissimilar characteristics, stability and constancy depending on the biomass type, pyrolysis temperature and production procedures [2]. Soil enzymes are associated in different biochemical processes in soil system, like mineralization of soil organic matter and further biogeochemical nutrient cycling [3]. Due to the high sensitivity to changes in microbial biomass carbon, enzyme activities can also be used as indicators of environmental and soil quality [4]. Biochar addition to acidic soils is particularly advantageous because it has pH more than 7.0 [5]. Therefore, tracking biological indicators like soil enzymes in response to biochar addition over time may facilitate us to understand the nutrient cycles as well as mechanisms for regulating soil quality and functions [6]. Researches with biochar are desirable due to the exclusive interactions connected to type of biochar, charring environment used, and application rate [7]. Application of biochar into soil system could have numerous direct or indirect influences on soil biota due to the transform of some abiotic factors like soil pH or altered substrate quality as a source of energy [8]. Biochar application has been demonstrated to influence the biomass carbon and enzymatic dynamics with organic carbon availability as biochar has noteworthy quantity of dissolved organic carbon [9]. The residence time of biochar in soil is normally hundreds to thousands of years and thus, microbial community structure and functions changes could continue for a longer period [10]. Research connected with the influence of biochar on soil microbes are still inadequately understood, and mostly due to the inconsistency of the biomass that produces the biochar and the quantity of organic carbon present [11]. Biochar is normally efficient for supporting microbial enlargement, improving nutrient accessibility, thus influencing crop production [12]. Largely, the net augment in CO2 discharge following biochar addition to soil comes into view to be a short-lived result, whereas for incubations over a longer time period, the normal emission of CO2 is typically not or even negatively affected for huge application rates [13]. Soils system has complex population of thousands of divergent microbes, and it was revealed that biochar shows a great influence on their function and composition [14]. Biochar produced at low temperature can increase enzyme activity more than high temperature pyrolyzed biochars [15]. The reason is that lesser amount of aromatic structures and higher amount of quickly degradable compounds [16]. Microbial populations are principally dependable for decay of the organic matter vis-a-vis a number of enzymes and therefore, biochar addition is a technique of replenishing besmirched soil quality through development of soil biota status, which generally implies an augment in enzyme activity [13]. Nevertheless, the response of microbial biomass carbon and soil enzymes dynamics to biochar application and the major issues that force their resultant performance have rarely been studied. Hence, keeping the significance of feedstocks derived biochar amendment, the investigation was carried out to examine the influence of biochar technology on soil microbial biomass carbon and enzyme dynamics under laboratory incubation condition in mid hill ecosystem of Sikkim organic state of India. The intention of such research work was to determine the influence of dissimilar feedstocks derived biochar on microbial biomass carbon (MBC), dehydrogenase (DHA), acid phosphatase, alkaline phosphatase, urease, protease and fluorescein diacetate activity at 2.5 and 5.0 t ha−1 biochar application rate at different incubation period. Investigation was also carried out to examine the influence of different biochar type (dissimilar feedstock) on the MBC and soil enzyme activity.
2 Materials and methods
2.1 Incubation study location
A study was conducted under laboratory condition (incubation study) at ICAR Research Complex for NEH Region, Sikkim Centre, Gangtok, Sikkim, India to evaluate the effect of four different feedstocks derived four biochar namely MS (maize stalk) biochar, LC (Lantana camara) biochar, PN (pine needle) biochar and BG (black gram) biochar on different soil biological properties. The soil sampling area was located at 1350 m above the mean sea level (msl) and represents sub-tropical mid hill location of Sikkim and lies at 27°20′N latitude and 88°37′E longitude. The sampling area received an annual rainfall of more than 3000 mm.
2.2 Soil sampling and analysis
Soil samples were collected in polythene bags from the surface layer (0–15 cm). The soil sample was sandy loam to clay loam in texture, acidic in pH and high in organic matter content (also organic carbon). After removing all the debris, roots and stones the moist soil samples were sieved through 2 mm. Half of the soil samples were air-dried, stored (room temperature) followed by chemical analysis. The rest of half of soil samples were used for microbiological analysis. To stabilize the disturbed microbial activity during the period of soil sampling, the samples were kept at 4 °C in plastic bags for a few days, followed by analysis within 2 weeks.
2.3 Chemicals and glassware
The reagents used for microbial biomass carbon and enzyme dynamics study were of analytical grade (AR) and purchased from different repute company in India. The reagents were procured from M/S Qualigen India. Different solvents like chloroform, methanol etc. were glass distilled before use and were purchased from M/S Merck India Ltd.
2.4 Biochar amendment preparation
Four different biochar viz. MS, LC, PN and BG biochar were prepared from maize stalk, Lantana camara, pine needle and black gram biomass. The biomass was inserted into kiln, combusted (heating rate 10 °C min−1 and holding temperature 4.0 h) and the temperature was maintained by electrically operated manual switch connected to the kiln. After preparing, the biochar were collected with shovel/scoop, dried at 100 °C (24 h), pulverized to fine powder, sieved through 0.2 mm and used for further study.
2.5 Experimental design
A short-term incubation study was conducted under laboratory utilizing four different biochar to assess their impact on different soil biological properties. The biochar application dose was 2.5 t/ha and 5.0 t/ha. The calculated amount of biochar was applied to 10 g soil in each 100 mL beaker for incubation study. The microbial biomass carbon study was conducted at 365 days of incubation period. Whereas, the soil enzyme activity (dehydrogenase, phosphatase, urease, protease, fluorescein diacetate) studies were conducted at 90 days of incubation period. Twelve sets of each biochar treated soil, along with control were maintained for microbial biomass study. But, eight sets of each biochar treated soil, along with control were maintained for soil enzyme activity. Experiments were carried out in triplicate. All the beakers were placed in incubator at 27 ± 1 °C temperature (60% of maximum water-holding capacity) and about 90% relative humidity was maintained. Constant weight of the beakers was maintained throughout the experiment by replenishing the lost water every alternate day. Samples were withdrawn at 0, 1, 3, 5, 7, 10, 15, 30, 45, 60, 90, 150, 225, 300 and 365 days interval depending upon analysis element.
2.6 Analysis of microbial biomass carbon
Chloroform fumigation extraction method as per Jenkinson and Powlson [15] and modified by Vance et al. [17] was followed for estimation of microbial biomass carbon in soil. Field moist soils (10 g on oven dry basis) were fumigated with ethanol-free-chloroform (25 ml) for 24 h in a vacuum desiccator. The soil samples were allowed for evacuation and fumigation removal. Then, samples were extracted with 0.5 (M) K2SO4 (1:2.5 soil: solution ratio) by an oscillating shaker for 30 min at 500 rpm and filtered (Whatman No. 42). Non-fumigated (without ethanol-free-chloroform) soil samples were also extracted with 0.5 (M) K2SO4. The extracts were subjected to oxidation using wet oxidation diffusion method as per Snyder and Trofymow [18]. For this, 5 ml of extract was transferred in diffusion tube. It was then acidified with 0.025 (M) H2SO4 followed by digestion for 2 h at 120 °C. Then, the diffusion tube was allowed to remain undisturbed for 12 h. In a shell vial (~ 6 ml capacity) 4 ml of 0.1 (N) NaOH was kept. The shell vial was kept over the indentation inside diffusion tube. The amount of CO2-C evolved was trapped into the shell vial. After 12 h, the shell vial was taken out from the diffusion tube. In the presence of an excess of 1 (M) BaCl2, the unspent alkali in the shell vial was titrated against 0.02 (N) HCl to stabilize the trapped CO2-C. Calculation of microbial biomass carbon was done using an efficiency factor (Kc) 0.25 [19].
2.7 Analysis of soil enzyme activity
2.7.1 Dehydrogenase
Soil dehydrogenase activity was measured as per the methodology of Casida et al. [13] by reducing 2,3,5-triphenyltetrazolium chloride (TTC). For this 5 g soil sample mixed with 50 mg of CaCO3 and 1 ml of 3% (w/v) TTC. Then, it was incubated at 37 ± 1 °C for 24 h where dehydrogenase enzyme converted TTC to 2,3,5-triphenylformazan (TPF) and the produced TPF was extracted with acetone solvent (3 × 15 ml). Then, the extracts were filtered followed by spectrophotometric analysis at 485 nm.
2.7.2 Phosphomonoestaerase (Acid and alkaline phosphatase) activity
As per the methodology of Tabatabai and Bremner [20], acid and alkaline phosphatase activity in soil was measured. For estimation of acid phosphatase p-nitrophenhyl phosphate tetrahydrate (pH 6.5) solution was used and for alkaline phosphatase the same solution of pH 11.0 was used. For these assay, soil samples were incubated at 37 ± 1 °C for 1 h. Then, yellow colour filtrate obtained using Whatman No. 42 filter paper was estimated using UV–VIS double beam spectrophotometer at 440 nm. Then, calibration graph was plotted using standards containing 0 to 50 µg of p-nitrophenol solution and from that amount of p-nitrophenol released was calculated. Acid and alkaline phosphatase activities were expressed as µg p-nitrophenol g−1 soil h−1.
2.7.3 Urease
Urease activity was estimated as per the methodology of Tabatabai and Bremner [21]. For this, incubation study was carried by taking 5 g of soil with 5 ml of 0.05 M tris-(hydroxymethyl)-aminomethane (THAM) buffer (pH 9.0) and 1 ml of 0.02% urea solution for 2 h at 37 ± 1 °C. After incubation, the excess urea was extracted with KCl-PMA solution, the extractant measured colorimetrically at 527 nm. Urease activity was expressed as (µg urea hydrolysed or µg tyrosine produced g−1 h−1).
2.7.4 Protease
Protease activity was measured as per the methodology of Ladd and Butler [22]. For this, 1 g of soil (oven-dry equivalent of field-moist soil) was mixed with 5 ml of 50 mM trisaminomethane (Tris) buffer (pH 8.1) and 5 ml of 2% Na-caseinate and incubated at 50 ± 1 °C for 2 h. During incubation, aromatic amino acids were released and extracted. The remaining substrate was precipitated with 0.92 M trichloroacetic acid. Using folin-Ciocalteu reagent the released tyrosine was measured colorimetrically at 700 nm. Protease activity was expressed as mg tyrosine produced g− soil h−1.
2.7.5 Fluorescein diacetate hydrolyzing activity
Fluorescein diacetate hydrolytic activity (FDHA) was measured as per the methodology of Green et al. [23]. By the action of hydrolytic enzymes in soil, the released fluorescein from fluorescein diacetate was measured. Fluorescein diacetate hydrolyzing activity was expressed as mg of fluorescein kg−1 oven dry soil hr−1.
2.8 Statistical analysis
All the data generated during the entire period of investigation was statistically analysed using the ‘F’ using the procedure of Gomez and Gomez [16]. LSD values at P = 0.05 were used to determine the significance of difference between the treatment means. This analysis was done using the statistical package ‘STATISTICS’.
3 Results and discussion
For mineral nutrient preservation and conversion related to soil quality and health, the soil MBC and enzyme activity had been acknowledged as a potential indicator. The initial soil biological properties of collected sample under laboratory condition have been presented in Table 1. Results revealed that the microbial biomass carbon was 295 mg kg−1 soil, dehydrogenase 27.46 µg TPF produced g−1 h−1, acid and alkaline phosphatase 96.24 and 27.64 µg p-nitrophenol g−1 h−1, urease 95.46 µg urea hydrolysed, protease 95.46 µg urea hydrolysed, protease 14.15 µg urea hydrolysed, fluorescein diacetate hydrolyzing 16.38 mg of fluorescein kg−1 oven dry soil hr−1, invertase activity 55.78 mg glucose eq. g−1 h−1. The influences of biochar application are also in conformity with different researcher who mentioned that the potential activities of dehydrogenase, cellulase, protease and invertase augmented in soils amended with dissimilar types and doses of biochars produced at unlike pyrolysis condition. Maximum number of biochar is alkaline in pH. The application of biochar therefore may perhaps augment the soil pH through its liming potential, creating the soil environment additional favorable for soil microbes as well as enzymes.
3.1 Microbial biomass carbon
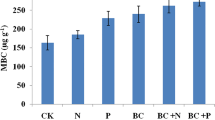
The addition of organic manures to soils could excite microbe’s biomass via supplying degradable carbon substances and nutrients [24]. This explained that the more amount of soil microbial biomass carbon was found in the biochar amended soil than biochar unamended soil and the soil getting organic sources of manures as well as fertilizers [25]. Biochar may also manipulate the abundance of microbes via different mechanism like soil physicochemical characters changes by creating micro-habitats, which significantly supply huge niches for microbial enlargement. This gives details the augmented soil microbial biomass carbon found in biochar amended soil than without biochar amended soil. Effect of biochar type at 5.0 t/ha on microbial biomass carbon at different days of incubation period has been presented in Fig. 1. The microbial biomass carbon for control was 270.3, 273.1, 278.1, 288.1 and 298.6 at 0, 30, 90, 225 and 365 days of incubation, respectively. The microbial biomass carbon for MS biochar was 272.6, 279.1, 296.5, 331.3 and 366.4 at 0, 30, 90, 225 and 365 days of incubation, respectively. The microbial biomass carbon for LC was 270.4, 280.1, 301.1, 343.1 and 385.5 at 0, 30, 90, 225 and 365 days of incubation, respectively. The microbial biomass carbon for PN biochar was 271.5, 279.6, 298.4, 336.7 and 374.5 at 0, 30, 90, 225 and 365 days of incubation, respectively. The microbial biomass carbon for BG biochar was 270.2, 277.4, 293.6, 326.5 and 358.5 at 0, 30, 90, 225 and 365 days of incubation, respectively. Results revealed that the microbial biomass carbon in different biochar treatment increased significantly along with control with increase in incubation days. The highest microbial biomass carbon was found in LC followed by PN, MS and lowest in BG among the biochar. Thus, biochar application in soil increased microbial biomass carbon compared to control. Effect of biochar type at 2.5 t/ha on microbial biomass carbon at different days of incubation period has been presented in Fig. 1. The results followed the similar trend as observed in case of 5.0 t/ha application rate. But the increased in microbial biomass carbon in all the biochar treatment was more at 2.5 t/ha application rate than 5.0 t/ha i.e. lower concentration of biochar enhanced more in microbial biomass carbon than higher concentration. Besides, microbes present in soil are sensitive to the soil environment changes [14]. Rather than pyrolysis temperature and type of feedstock, the organic carbon present in biochar significantly influenced different soil biological properties like microbial biomass carbon [26]. The maximum constructive combined influences of biochar on soil microbial biomass carbon were found while biochar was jointly used with decomposed waste N-fertilizer [27]. A raise in soil microbial biomass carbon was found while biochar application rate was augmented, in spite of quantity of nitrogen applied [28]. After biochar application, the improved microbial biomass carbon can be attributed to encouraging priming effect of biochar [29]. Nevertheless, published results of biochar applications to soil microbial biomass carbon are quite contradictory [30]. Due to the presence of water soluble low molecular organic substances (trace amount) in biochar, it might trigger priming effects [31]. Peanut biochar addition can also augment the microbial biomass carbon in a short-term period due to rise in soil organic carbon [32].
3.2 Dehydrogenase activity
As an intracellular enzyme, the dehydrogenase (DHA) serves as an index for soil microbiological action due to its function in soil organic matter oxidation (biological). The DHA action has been utilized fruitfully as a factor for assessment of degree of restoration of degraded acid soils [33]. Dehydrogenase activity under different type and 2.5 t/ha rate of biochar application at various incubation intervals has been presented in Fig. 2. The dehydrogenase for control was 18.57, 20.01, 19.97, 20.97 and 21.61 at 1, 7, 30, 60 and 90 days of incubation, respectively. The dehydrogenase for MS biochar was 19.61, 25.61, 24.61, 34.69 and 38.08 at 1, 7, 30, 60 and 90 days of incubation, respectively. The dehydrogenase for LC biochar was 20.61, 24.59, 28.61, 38.56 and 35.88 at 1, 7, 30, 60 and 90 days of incubation, respectively. The dehydrogenase for PN biochar was 14.2, 23.62, 22.26, 17.62 and 18.08 at 1, 7, 30, 60 and 90 days of incubation, respectively. The dehydrogenase for BG biochar was 20.61, 21.32, 29.58, 37.91 and 40.28 at 1, 7, 30, 60 and 90 days of incubation, respectively. Results revealed that the dehydrogenase in different biochar treatment increased significantly along with control with increase in incubation days except pine biochar where dehydrogenase decreased. The highest dehydrogenase was found in BG followed by MS, LC and lowest in PN among the biochar. Thus, biochar application in soil increased dehydrogenase compared to control except LC biochar. Dehydrogenase activity under different type and 5.0 t/ha rate of biochar application at various incubation intervals has also been presented in Fig. 2. The results followed the similar trend as observed in case of 2.5 t/ha application rate. But the increase in dehydrogenase in all the biochar treatment was more at 5.0 t/ha application rate than 2.5 t/ha except LC biochar where higher application rate decreases more in dehydrogenase activity than lower application rate. The probable mechanisms through which dissimilar biochar addition in soil changes enzyme activities are (a) biochar’s more specific surface area, pore space and ability to sorb various substrates on its exterior or (b) biochar addition modify the features of soil to manipulate soil enzyme activity [34, 35]. The interaction of dehydrogenase, which only works within the cell, is typically linked to microbiological activity and respiration and furthermore reflects the greatness of soil microbial procedure [36]. Soil dehydrogenase activity augmented only with application of manure biochar produced at 300 °C while no effect with the same biochar derived at 500 °C [37]. Biochar produced at low temperature pyrolysis augmented the enzyme activity more than high temperature pyrolyzed biochars [38]. The reason may be the lower concentration of aromatic structures and more quantity of easily degradable substances [39].
3.3 Acid phosphatase activity
The acid phosphatase enzyme activity is unswervingly connected to available phosphorus and soil organic carbon [40]. Acid phosphatase activity under different type and 2.5 t/ha rate of biochar application at various incubation intervals has been presented in Fig. 3. The acid phosphatase for control was 45.27, 46.28, 52.61, 54.62 and 55.67 at 1, 7, 30, 60 and 90 days of incubation, respectively. The acid phosphatase for MS biochar was 47.56, 55.61, 67.56, 75.61 and 81.07 at 1, 7, 30, 60 and 90 days of incubation, respectively. The acid phosphatase for LC biochar was 46.91, 55.61, 67.56, 88.61 and 90.07 at 1, 7, 30, 60 and 90 days of incubation, respectively. The acid phosphatase for PN biochar was 44.86, 48.86, 45.86, 49.56 and 51.46 at 1, 7, 30, 60 and 90 days of incubation, respectively. The acid phosphatase for BG biochar was 46.62, 54.82, 65.91, 78.16 and 85.57 at 1, 7, 30, 60 and 90 days of incubation, respectively. Results revealed that the acid phosphatase in different biochar treatment increased significantly along with control with increase in incubation days. The highest acid phosphatase was found in LC followed by BG, MS and lowest in PN among the biochar. Thus, biochar application in soil increased acid phosphatase compared to control. Acid phosphatase activity under different type and 5.0 t/ha rate of biochar application at various incubation intervals has been also presented in Fig. 3. The results followed the similar trend as observed in case of 2.5 t/ha application rate. But the increase in acid phosphatase in all the biochar treatment was more at 2.5 t/ha application rate than 5.0 t/ha. Augmented soil enzyme activity as a result of biochar application could be taking place directly during co-location of enzyme-biochar followed by interaction with surface of biochar or indirectly during augmented microbial activity, biomass, soil organic matter and finally changes in microbial community organization [41]. Due to the presence of plentiful micro-pores, more surface area and surface functional groups or augmented soil aggregation in biochar, it can physico-chemically immobilize soil enzyme activity [42,43,44,45,46]. Biochars may sorb more substrate due to its more specific surface area and porosity and, consequently, decrease extremely soluble substrate availability for soil enzymes, which may hinder the reaction rate of enzyme [46, 47]. Moreover, biochar is able to immobilize native soil organic carbon due to its large surface area and porosity [48]. The immobilization process may be due to direct sorption of dissolved microbial enzymes and organic matter onto surfaces of biochar and within pore spaces, which ultimately results in hindering soil enzyme activity [49, 50].
3.4 Alkaline phosphatase
Alkaline phosphatase activity under different type and 2.5 t/ha rate of biochar application at various incubation intervals has been presented in Fig. 4. The alkaline phosphatase for control was 19.56, 20.56, 22.23, 22.97 and 23.62 at 1, 7, 30, 60 and 90 days of incubation, respectively. The alkaline phosphatase for MS biochar was 20.56, 31.27, 42.52, 57.62 and 69.56 at 1, 7, 30, 60 and 90 days of incubation, respectively. The alkaline phosphatase for LC biochar was 21.52, 32.64, 45.62, 61.27 and 65.85 at 1, 7, 30, 60 and 90 days of incubation, respectively. The alkaline phosphatase for PN biochar was 19.02, 25.75, 45.29, 57.56 and 61.61 at 1, 7, 30, 60 and 90 days of incubation, respectively. The alkaline phosphatase for BG biochar was 18.89, 24.86, 33.86, 42.46, 61.62 and 68.46 at 1, 7, 30, 60 and 90 days of incubation, respectively. Results revealed that the alkaline phosphatase in different biochar treatment increased significantly with increase in incubation days along with control. The raise in alkali phosphatase through biochar addition may be due to a chemical improvement of enzyme activity which occurred by interaction with biochar. It augmented gradually over the incubation period which recommended that biochar offers a proper habitation for the phytase-producing microorganisms. As soil holds huge quantity of organic-P, the microorganisms living in biochar particles pore space can liberate the biochar-phosphorus and also from the organic matter which increased the enzyme activity. The highest alkaline phosphatase was found in MS followed by BG, LC and lowest in PN among the biochar. Thus, biochar application in soil increased alkaline phosphatase compared to control. Alkaline phosphatase activity under different type and 5.0 t/ha rate of biochar application at various incubation intervals has been also presented in Fig. 4. The results followed the similar trend as observed in case of 2.5 t/ha application rate. But the increase in acid phosphatase in all the biochar treatment was more at 5.0 t/ha application rate than 2.5 t/ha. The higher rate of rice straw biochar addition at 50 mg/kg can inhibit the alkaline phosphatase activity whereas alkaline phosphatase activity indicated an augment with biochar addition at 10 mg/kg [51, 52]. In unfertile tropical soils, the enzymes involved in carbon, nitrogen and phosphorus cycles were considerably augmented by biochar application, however for fertile soil, biochar addition resulted in lower phosphomonoesterase activity [53,54,55,56,57]. The unpredictable role of different biochar on soil enzyme activities may be due to its more dependence on soil type [58].
3.5 Urease activity
The urease enzyme is recognized to hydrolyse the nitrogenous compound (protein). The action of urease was acknowledged as an extremely successful soil enzymatic indicator for nitrogen mineralization of applied organic manure and soil organic matter [59,60,61]. In our research findings, extensively enhanced soil urease activities with four dissimilar biochar possibly will be attributed to exogenous addition of enzymes along with enhancement of microbial enlargement. Urease activity under different type and 2.5 t/ha rate of biochar application at various incubation intervals has been presented in Fig. 5. The urease for control was 60.59, 66.45, 65.46, 66.49 and 67.59 at 1, 7, 30, 60 and 90 days of incubation, respectively. The urease for MS biochar was 61.58, 68.56, 71.37, 75.56 and 79.64 at 1, 7, 30, 60 and 90 days of incubation, respectively. The urease for LC biochar was 62.65, 69.57, 78.56, 82.56 and 85.69 at 1, 7, 30, 60 and 90 days of incubation, respectively. The urease for PN biochar was 59.56, 57.52, 58.17, 63.17 and 65.16 at 1, 7, 30, 60 and 90 days of incubation, respectively. The urease for BG biochar was 61.62, 62.59, 72.46, 79.56 and 83.64 at 1, 7, 30, 60 and 90 days of incubation, respectively. Results revealed that the urease in different biochar treatment increased significantly with increase in incubation days along with control. The highest urease was found in LC followed by BG, MS and lowest in PN among the biochar. Thus, biochar application in soil increased urease compared to control. Urease activity under different type and 5.0 t/ha rate of biochar application at various incubation intervals has been also presented in Fig. 5. The results followed the similar trend as observed in case of 2.5 t/ha application rate. But, the increase in urease in all the biochar treatment was more at 5.0 t/ha application rate than 2.5 t/ha except LC biochar. A noteworthy expand in soil urease activity (31.1–37.6%) having optimistic correlation of soil enzyme activities with soil organic carbon and pH was observed after biochar application in a two year paddy field in China [62, 63].
3.6 Protease activity
The protease enzyme is recognized to hydrolyse nitrogen compound in soil [64]. Protease activity under different type and 2.5 t/ha rate of biochar application at various incubation intervals has been presented in Fig. 6. The protease for control was 10.12, 12.62, 11.26, 13.01 and 13.62 at 1, 7, 30, 60 and 90 days of incubation, respectively. The protease for MS biochar was 11.62, 17.62, 22.61, 28.46 and 30.26 at 1, 7, 30, 60 and 90 days of incubation, respectively. The protease for LC biochar was 10.56, 12.62, 22.62, 21.56 and 23.73 at 1, 7, 30, 60 and 90 days of incubation, respectively. The protease for PN biochar was 10.01, 9.45, 9.02, 8.15, 8.98, 9.11 and 9.98 at 1, 7, 30, 60 and 90 days of incubation, respectively. The protease for BG biochar was 12.91, 17.62, 22.62, 24.89 and 25.46 at 1, 7, 30, 60 and 90 days of incubation, respectively. Results revealed that the protease in different biochar treatment increased significantly along with control with increase in incubation days except pine biochar where protease activity decreased. The highest protease was found in MS followed by BG, LC and lowest in PN among the biochar. Thus, biochar application in soil increased protease compared to control except LC biochar which decreased protease activity. In this experiment, the biochar application augmented the protease enzyme activity in soil, which might be due to augmenting the accessibility of inorganic nitrogen. Protease activity under different type and 5.0 t/ha rate of biochar application at various incubation intervals has been also presented in Fig. 6. The results followed the similar trend as observed in case of 2.5 t/ha application rate. But the increase in protease in all the biochar treatment was more at 5.0 t/ha application rate than 2.5 t/ha except LC biochar where higher application rate decrease more in protease activity than lower application rate. The protease enzyme acts as significant function in soil organic N-mineralization through hydrolysis of protein to polypeptide and oligo-peptide to amino acid [65,66,67]. The synergistic role of fresh biomass and its resulted biochar on soil enzymes are stalwartly linked to enhanced soil physico-chemical characters, and the application of various labile-C substances in the biochar for enzyme reactions [68]. The role of biochar addition on soil enzyme activity was mostly because of its influence on soil microbial biomass as because enzyme activities are connected with live microbe’s cell and abiotic elements as extracellular enzyme [69, 70].
3.7 Fluorescein diacetate activity
Fluorescein diacetate activity (FDA) under different type and 2.5 t/ha rate of biochar application at various incubation intervals has been presented in Table 2. The fluorescein diacetate for control was 9.12, 11.62, 13.92, 12.68 and 14.62 at 1, 7, 30, 60 and 90 days of incubation, respectively. The fluorescein diacetate for MS biochar was 10.91, 14.26, 22.36, 28.56 and 31.28 at 1, 7, 30, 60 and 90 days of incubation, respectively. The fluorescein diacetate for LC biochar was 10.12, 17.62, 24.62, 32.86 and 37.56 at 1, 7, 30, 60 and 90 days of incubation, respectively. The fluorescein diacetate for PN biochar was 9.08, 7.85, 5.45, 9.46 and 11.27 at 1, 7, 30, 60 and 90 days of incubation, respectively. The fluorescein diacetate for BG biochar was 9.26, 17.56, 23.52, 31.62 and 35.42 at 1, 7, 30, 60 and 90 days of incubation, respectively. Results revealed that the fluorescein diacetate in different biochar treatment increased significantly with increase in incubation days along with control. The highest fluorescein diacetate was found in LC followed by BG, MS and lowest in PN among the biochar. Thus, biochar application in soil increased fluorescein diacetate compared to control significantly except LC biochar which increase non-significantly (slight increase). Fluorescein diacetate activity under different type and 5.0 t/ha rate of biochar application at various incubation intervals has been also presented in Table 2. The results followed the similar trend as observed in case of 2.5 t/ha application rate. But, the increase in fluorescein diacetate in all the biochar treatment was more at 5.0 t/ha application rate than 2.5 t/ha. In our findings, augmenting the biochar application rates enhanced the soil enzyme activity (except PN biochar) and such surveillance was mentioned by various recognized researchers utilizing other types of biochar. The PN biochar may have an antagonistic effect on soil enzyme activity because pine needle contains resin (oleoresin). The augmented action of FDA might be either due to encouragement of a particular type of microbes by different biochar or enlargement of biomass in reply to primarily labile carbon. The FDA exists in the major decomposers like soil bacteria and fungi and is mediated by various enzymes, for example, esterase, proteases and lipases [71,72,73]. Therefore, the FDA could be precious to determine various responses happening in soil [74]. Even though investigations have shown uplifted in FDA hydrolysis in soils amended with biochar, the response (decrease/no change/increase) has varied due to biochar type, application rates, and the complex mechanisms of action of type of soil with biochar [75,76,77].
4 Conclusion
We concluded that the effect of four dissimilar biochar applications on microbial biomass carbon and soil enzyme activity was extremely variable. The feedstock type has a great significant influence on biochar and obviously, significantly enhancement on MBC and enzyme activity with four different biochar was observed. Besides, rate of biochar application also played a crucial role to influence the MBC and soil enzyme. Some properties of soil biological indicator were more influenced by higher biochar application, while some were by lower biochar application rate. Thus, there is an urgent call for further estimation of positive and negative long term results of biochar on the soil biology. The existing work suggests that the employ of MS, LC and BG biochar (but not PN biochar) as a soil amendment might be adopted as an efficient measure to improve MBC and enzyme activity. Finally, more research under actual field conditions is necessary to verify the advantages of biochar application.
References
Collard F-X, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sustain Energy Rev 38:594–608
Fidel RB, Laird D, Thompson AML (2013) Evaluation of modified Boehm titration methods for use with biochars. J Environ Qual 42:1771–1778
Singh M, Gupta B, Das SK (2015) Assessment of economic viability of different agroforestry systems in Giri catchment, Himachal Pradesh. Econ Aff 60(3):557–561
Yuan H, Lu T, Huang H, Zhao D, Kobayashi N, Chen Y (2015) Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J Anal Appl Pyrol 112(3):284–289
Barman H, Roy A, Das SK (2015) Evaluation of plant products and antagonistic microbes against leaf blight (Alternaria alternata), A devastating pathogen of Tomato. Trends Biosci 8(13):3374–3377
Zhang A, Cheng G, Hussain Q, Zhang M, Feng H, Dyck M, Wang X (2017) Contrasting effects of straw and straw–derived biochar application on net global warming potential in the Loess Plateau of China. Field Crop Res 205:45–54
Das SK (2013) Integrated nutrient management using only through organic sources. Pop Kheti 1(4):126
Zhao L, Cao X, Masek O, Zimmerman A (2013) Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J Hazard Mater 256–257:1–9
Barman H, Roy A, Das SK, Singh NU, Dangi DK, Tripathi AK (2016) Antifungal properties of some selected plant extracts against leaf blight (Alternaria alternata) in tomato. Res Crop 17(1):151–156
Das SK, Ghosh GK, Avasthe RK (2021) Conversion of crop, weed and tree biomass into biochar for heavy metal removal and wastewater treatment. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01334-y
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Biores Technol 102:3488–3497
Das SK, Ghosh GK, Avasthe RK, Kundu MC, Choudhury BU, Baruah K, Lama A (2021) Innovative biochar and organic manure co-composting technology for yield maximization in maize-black gram cropping system. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01519-5
Barman H, Roy A, Das SK (2015) Evaluation of plant products and antagonistic microbes against grey blight (Pestalotiopsis theae), a devastating pathogen of tea. Afr J Microbiol Res 9(18):1263–1267
Barman H, Das SK, Roy A (2018) Zinc in soil environment for plant health and management strategy. Univers J Agric Res 6:149–154
Das SK, Ghosh GK, Avasthe RK, Choudhury BU, Mishra VK, Kundu MC, Roy A, Mondal T, Lama A and Dhakre DS (2021) Organic nutrient sources and biochar technology on microbial biomass carbon and soil enzyme activity in maize-black gram cropping system. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01625-4
Mukherjee I, Das SK, Kumar A (2016) Degradation of flubendiamide as affected by elevated CO2, temperature, and carbon mineralization rate in soil. Environ Sci Pollut Res 23(19):19931–19939
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nature Commun 1(5):1–9
Roy A, Das SK, Tripathi AK, Singh NU (2015) Biodiversity in North East India and their conservation. Progress Agric 15(2):182–189
Wisniewski D, Golaszewski J, Bialowiec A (2015) The pyrolysis and gasification of digestate from agricultural biogas plant. Arc Environ Prot 41:70–75
Das SK, Avasthe RK (2015) Carbon farming and credit for mitigating greenhouse gases. Curr Sci 109(7):1223
Mukherjee I, Das SK, Kumar A (2012) A Fast Method for determination of flubendiamide in vegetables by liquid chromatography. Pestic Res J 24(2):159–162
Kavitha B, Reddy PVL, Kim B, Lee SS, Pandey SK, Kim KH (2018) Benefits and limitations of biochar amendment in agricultural soils: a review. J Environ Manag 227:146–154
Das SK, Avasthe RK, Singh R, Babu S (2014) Biochar as carbon negative in carbon credit under changing climate. Curr Sci 107(7):1090–1091
Mate CJ, Mukherjee I, Das SK (2015) Persistence of spiromesifen in soil: influence of moisture, light, pH and organic amendment. Environ Monit Assess 187(2):7. https://doi.org/10.1007/s10661-014-4207-6
Mukherjee I, Das SK, Kumar A (2018) Atmospheric CO2 level and temperature affect degradation of pretilachlor and butachlor in Indian soil. Bull Environ Contam Toxicol 100(6):856–861
Das SK, Avasthe RK, Yadav A (2017) Secondary and micronutrients: deficiency symptoms and management in organic farming. Innov Farm 2(4):209–211
Brewer CE, Schmidt-Rohr K, Satrio JA, Brown RC (2009) Characterization of biochar from fast pyrolysis and gasification systems. Environ Prog Sustain Energy 28(3):386–396
Das SK, Ghosh GK (2017) Soil hydro-physical environment as influenced by different biochar amendments. Int J Bio-resour Stress Manag 8(5):668–673
Al-Wabel MI, Hussain Q, Usman ARA, Ahmad M, Abduljabbar A, Sallam AS, Ok YS (2017) Impact of biochar properties on soil conditions and agricultural sustainability: a review. Land Degrad Dev 29(7):2124–2161
Das SK (2019) Soil carbon sequestration strategies under organic production system: a policy decision. Agrica 8(1):1–6
Roy A, Das A, Das SK, Datta M, Datta J, Tripathi AK, Singh NU (2018) Impact analysis of National Agricultural Innovation Project (NAIP): a paradigm shift in income and consumption in Tripura. Green Farm 9(3):559–564
Das SK, Avasthe RK, Singh M, Roy A (2018) Managing soil fertility under organic production system through integrated approach. Green Farm 9(3):449–454
Hung CY, Tsai WT, Chen JW, Lin YQ, Chang YM (2017) Characterization of biochar prepared from biogas digestate. Waste Manag 66:53–60
Das SK, Avasthe RK (2018) Plant nutrition management strategy: a policy for optimum yield. Acta Sci Agric 2(5):65–70
Singh NS, Mukherjee I, Das SK, Varghese E (2018) Leaching of clothianidin in two different Indian soils: effect of organic amendment. Bull Environ Contam Toxicol 100(4):553–559
Das SK, Avasthe RK, Singh M, Yadav A (2018) Soil health improvement using biochar application in Sikkim: a success story. Innov Farm 3(1):48–50
Karim AA, Kumar M, Mohapatra S, Singh SK (2019) Nutrient rich biomass and effluent sludge wastes co-utilization for production of biochar fertilizer through different thermal treatments. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.04.330
Das SK, Avasthe RK (2018) Development of innovative low cost biochar production technology. J Krishi Vigyan 7(1):223–225
Roy A, Singh NU, Tripathi AK, Yumnam A, Sinha PK, Kumar B, Das SK (2017) Dynamics of pulse production in north-east region of India — a state-wise analysis. Econ Aff 62(4):655–662
Das SK, Avasthe RK (2017) Livelihood improvement of rural tribal farmers through soil health management, input support system and training — a success story. Innov Farm 2(3):171–173
Das SK, Avasthe RK, Sharma K, Singh M, Sharma P (2017) Soil fertility assessment in different villages of east Sikkim district. Indian J Hill Farm 30(1):14–16
Li S, Chen G (2018) Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag 78:198–207
Das SK, Ghosh GK, Avasthe RK (2017) Biochar amendments on physico-chemical and biological properties of soils. Agrica 6(2):79–87
Sharma M, Rana M, Sharma P, Das SK (2016) Effect of different organic substrates and plant botanicals on growth and flowering of chincherinchee (Ornithogalum thyrosides Jacq). Indian J Hill Farm 29(2):72–74
Gopi R, Avasthe RK, Kalita H, Kapoor C, Yadav A, Babu S, Das SK (2016) Traditional pest and disease management practices in Sikkim himalayan region. Int J Bio-resour Stress Manag 7(3):471–476
Sharma P, Sharma K, Das SK (2016) Ethno medicinal plants uses in health care by the Himalayan tribal people in India. Pop Kheti 4(3):41–45
Liu CH, Chu W, Li H, Boyd SA, Teppen BJ, Mao J, Zhang W (2019) Quantification and characterization of dissolved organic carbon from biochars. Geoderma 335:161–169
Gopi R, Kapoor C, Kalita H, Das SK, Avasthe RK (2015) A new report of downy mildew on buckwheat (Fagopyrum esculentum) caused by Perenospora sp Sikkim. J Mycopathol Res 53(2):95–297
Roy A, Dkhar DS, Tripathi AK, Singh NU, Kumar D, Das SK, Debnath A (2014) Growth performance of agriculture and allied sectors in the North East India. Econ Aff 59(Special):783–795
Singh M, Das SK, Avasthe RK (2018) Effect of multipurpose trees on production and soil fertility on large cardamom based agro forestry system in Sikkim Himalaya. Indian J Agrofor 20(2):25–29
Mukherjee I, Das SK, Kumar A, Shukla L (2020) Sludge amendment affects the persistence, carbon mineralization and enzyme activity of atrazine and bifenthrin. Bull Environ Contam Toxicol 105(2):291–298
Das SK, Ghosh GK, Avasthe RK (2020) Ecotoxicological responses of weed biochar on seed germination and seedling growth in acidic soil. Environ Technol Innov 20(4):101074. https://doi.org/10.1016/j.eti.2020.101074
Mukome FND, Zhang X, Silva LCR, Six J, Parikh SJ (2013) Use of chemical and physical characteristics to investigate trends in biochar feedstocks. J Agric Food Chem 61:2196–2204
Das SK, Ghosh GK, Avasthe RK (2020) Evaluating biomass-derived biochar on seed germination and early seedling growth of maize and black gram. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00887-8
Das SK, Ghosh GK, Avasthe RK (2020) Valorizing biomass to engineered biochar and its impact on soil, plant, water, and microbial dynamics: a review. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00836-5
Das SK (2020) Influence of phosphorus and organic matter on microbial transformation of arsenic. Environ Technol Innov 19(3):100930. https://doi.org/10.1016/j.eti.2020.100930
Das SK, Avasthe RK (2020) Packages of organic nutrient management as soil policy for upgrading cropping system to restore soil productivity, organic agriculture, Shaon Kumar Das, IntechOpen, https://doi.org/10.5772/intechopen.91928. Available from: https://www.intechopen.com/books/organic-agriculture/packages-of-organic-nutrient-management-as-soil-policy-for-upgrading-cropping-system-to-restore-soil
Wang M, Zhu Y, Cheng L, Andserson B, Zhao X, Wang D, Ding A (2018) Review on utilization of biochar for metal-contaminated soil and sediment remediation. J Environ Sci 63(1):156–173
Das SK, Ghosh GK (2020) Soil Health Management Through Low Cost Biochar Technology. In: Singh J, Singh C (eds) Biochar Applications in Agriculture and Environment Management. Springer. https://doi.org/10.1007/978-3-030-40997-5_9
Gopi R, Avasthe RK, Kalita H, Yadav A, Das SK, Rai D (2020) Eco-friendly management of tomato late blight using botanicals, bio-control agents, compost tea and copper fungicides. Indian J Agric Sci 90(1):35–39
Das SK, Avasthe RK (2018) Soil organic nutrients management through integrated approach: a policy for environment & ecology. Environ Anal Ecol Stud 4(1):1–8
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214
Das SK, Ghosh GK, Avasthe RK (2020) Applications of biomass derived biochar in modern science and technology. Environ Technol Innov 21:101306. https://doi.org/10.1016/j.eti.2020.101306
Crane-Droesch A, Abiven S, Jeffery S, Torn MS (2013) Heterogeneous global crop yield response to biochar: a meta-regression analysis. Environ Res Lett 8(4):044–049
Das SK, Ghosh GK, Avasthe RK, Sinha K (2021) Compositional heterogeneity of different biochar: effect of pyrolysis temperature and feedstocks. J Environ Manag 278(2):111501. https://doi.org/10.1016/j.jenvman.2020.111501
Das SK, Ghosh GK, Avasthe RK, Sinha K (2020) Morpho-mineralogical exploration of crop, weed and tree derived biochar. J Hazard Mater 407(5):124370. https://doi.org/10.1016/j.jhazmat.2020.124370
Kamara A, Kamara A, Mansaray M, Sawyerr PE (2014) Effects of biochar derived from maize stover and rice straw on the germination of their seeds. Am J Agric 2:246–249
Das SK, Ghosh GK, Avasthe RK (2020) Biochar application for environmental management and toxic pollutant remediation. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01078-1
Das SK, Ghosh GK, Avasthe RK (2020) Application of biochar in agriculture and environment, and its safety issues. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01013-4
Jaiswal AK, Elad Y, Graber ER, Frenkel O (2014) Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol Biochem 69:110–118
Das SK, Avasthe RK, Kalita H, Yadav A and Gopi R (2020) Organic soil nutrient practices in Sikkim and impact at field level for tribal farmers’: a success story. Biotica Res Today. 2, 2 (Feb. 2020), 24–26
Singh M, Gupta B, Das SK (2018) Soil organic carbon density under different agroforestry systems along an elevation gradient in north-western Himalaya. Range Manag Agrofor 39(1):8–13
Singh M, Gupta B, Babu S, Avasthe RK, Das SK (2017) Fodder, fuelwood consumption pattern and energy dynamics along elevation gradient in Giri Catchment, Himachal Pradesh India. Indian J Agric Sci 87(2):261–265
Das SK, Avasthe RK, Singh M (2016) Carbon-negative biochar from weed biomass for agricultural research in India. Curr Sci 110(11):2045–2046
Robertson SJ, Rutherford PM, Lo’ pez-Gutie´rrez JC, Massicotte HB (2012) Biochar enhances seedling growth and alters root symbioses and properties of sub-boreal forest soils. Can J Soil Sci 92:329–340
Pluchon N, Gundale MJ, Nilsson MC, Kardol P, Wardle DA (2014) Stimulation of boreal tree seedling growth by wood-derived charcoal: effects of charcoal properties, seedling species and soil fertility. Funct Ecol 28:766–775
Li Y, Yang Y, Shen F, Yang G, Zhang Y, Deng S, Zhang J, Zeng Y, Hu Y (2017) Mitigating biochar phytotoxicity via lanthanum (La) participation in pyrolysis. Environ Sci Pollut Res 24:10267–10278
Acknowledgements
The author Shaon Kumar Das is thankful to the Director, ICAR Research Complex for NEH Region, Umiam, Meghalaya, India and Department of Soil Science and Agricultural Chemistry, Palli Siksha Bhavana, Visva Bharati, Shantiniketan, India for providing necessary facility during the entire period of the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, S.K., Ghosh, G.K., Mishra, V.K. et al. Utilizing dissimilar feedstocks derived biochar amendments to alter soil biological indicators in acidic soil of Northeast India. Biomass Conv. Bioref. 13, 10203–10214 (2023). https://doi.org/10.1007/s13399-021-01670-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01670-z