Abstract
H2SO4-activated carbon generated from the leaves of Magnoliaceae plant-(SACM) is doped in zirconium alginate beads-(SACM@Zr). The active carbon and beads are identified to have affinity for toxic chromate ions and hence studied as adsorbents for chromium remediation of water. The sorbents are characterized using conventional methods including FTIR, FESEM and EDX techniques. The sorption nature is investigated and optimized with respect to initial chromium concentration, adsorbent dosage, contact time, pH and temperature. The adsorption capacities are 28.82 mg/g for active carbon and 37.74 mg/g for beads. Thermodynamic parameters are analyzed. Negative values of ΔG∘ and positive values of ΔH∘ and ΔS° indicate that the chromium adsorption ‘onto’ the adsorbents is spontaneous, endothermic and more disorder prevails at solution/solid interface. Langmuir adsorption isotherms and pseudo second-order kinetics are best models for explaining adsorption process. The adsorbents are successfully applied to treat chromium polluted effluents from Ethiopia Tannery Companies and polluted waters of Leyole and Worka rivers around Kombolcha, located in the north-central part of Ethiopia.

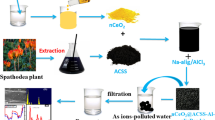
Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Industrialization and urbanization cause the ejection of poisonous heavy metals such as chromium, lead, mercury, cadmium, copper, zinc, nickel and cobalt into the wastewater systems [1,2,3,4]. These metal ions are non-degradable and are accumulated with time in water bodies. So, the toxic pollutants must be removed from the industrial waste waters before discharging them into oceans, rivers etc.
Out of all these heavy metals, chromium is highly toxic. Chromium pollution in waters is mainly from several industrial procedures like leather tanning, steel production, nuclear power plants, electroplating, anodizing of aluminum, textile industries, cutting tools, water-cooling, paints, dyes, paper industries and chromate preparations [5, 6]. Furthermore, natural sources like animals, plants, soils, rocks, volcanic dusts, gases etc. also contribute for chromium pollution.
Chromium exists in trivalent and hexavalent oxidation states in aqueous systems [7]. Chromium (VI) is more toxic than trivalent owing to its carcinogenic, mutagenic and teratogenic characteristics [8,9,10]. Trivalent chromium is an essential element and micronutrient that helps in preserving the normal metabolism of glucose, cholesterol and fat in human bodies, to sustain the good health [11, 12].
Huge volumes of wastewaters with high chromium contents from ˂1 to 10 ppm are produced from many industries. Chromate ions are easily movable in aquatic and soil systems. Furthermore, chromium ions easily enter cell walls and exert poisonous influence in the cell [13, 14]. As per WHO and Bureau of Indian Standards, by considering its toxicity and carcinogenic nature, the maximum levels permitted for hexavalent chromium is 0.05 mg/L [15]. So, it is necessary to remove the chromium from chromium polluted wastewater before releasing it into water bodies.
On expose to high concentration of Cr (VI) beyond the permissible limits, can cause damage to liver, severe epigastria pain, lung cancer, kidney circulation, vomiting, nausea, severe diarrhea, hemorrhage, nerve tissue damage, dermatitis, other health-related complications and even death [16,17,18]. Many traditional procedures based on chemical oxidation and reduction, chemical precipitation, membrane processes, filtration, evaporation techniques, solvent extraction, reverse osmosis, ion exchange and electro-chemical methods are suggested for the removal of chromium from the industrial waste waters [19, 20]. These methods suffer from one or other disadvantages and they are basically non-economical and generates various secondary pollutants. Hence, these methods have limited applications [21].
Adsorption techniques are simple to operate, effective and moreover, they are economical [22, 23]. In this aspect, the bio-materials can be regarded as most appropriate raw materials for the preparation of low cost bio-sorbents. In recent years, many bio-sorbents derived from low-cost natural materials have been studied for the removal of chromium from waste waters.
The surfaces of bio-sorbents are chemically modified with strong oxidizing agents and/or acids to improve their sorption capacities. These include pineapple leaves [24], Hazelnut [25], groundnut husk treated with concentrated sulphuric acid [26], coconut shell treated with nitric acid [27], peanut shell activated with KOH [28], maize corn cob [29] and Casuarina equisetifolia leaves treated with sulphuric acid [30], mango kernel activated with H3PO4 [31], eucalyptus roots activated with H3PO4 [32] and Passiflora foetida plant seeds activated with KOH [33]. Thus bio-sorbents are proving to be simple, effective and moreover they are based on renewable materials. So, developing low-cost adsorbents based on biomaterials possessing high adsorption capacities towards chromium ions is an important aspect of water remediation methods.
In the present investigation, two adsorbents are synthesized. One adsorbent is the active carbon generated from the leaves of Magnoliaceae plant by treating with sulphuric acid (SACM). The other is the said active carbon immobilized in Zirconium alginate beads (SACM@Zr). The chemical composition of beads is so chosen with an aim to enrich the sorption nature due to the presence of functional groups of Zr-alginate besides the easy filtration due to beads structure. The presence of Zr ions is supposed to increase the oxidative functionalities, porosity and thermal stability of beads. These adsorbents have been investigated for their adsorptivities for chromium with respect to different physicochemical characteristics viz., pH of solution, dosage of sorbents, contact-time, initial concentration of chromium and elevated solution temperatures. Extraction parameters have been optimized for high chromium removal. Adsorbents have been characterized with FESEM, EDX and FTIR. Sorption process is analyzed with various isotherm models, kinetic models and thermodynamic studies. The adsorbents developed are applied to real polluted water. The present investigation brings to limelight the chromium adsorptivities of abundantly available bio-materials
2 Materials and methods
2.1 Chemicals and solutions
Solutions and reagents were prepared with A.R grade chemicals and double-distilled water. For investigations, simulated solutions of chromium ions were prepared using A.R. K2Cr2O7. 0.25% of Diphenyl Carbazide in 6 N H2SO4, NaOH, ZrOCl2.8H2O, and Na-alginate were used.
2.2 Preparation of active carbons
2.2.1 Plant description
Of the various plants investigated, the bio-materials of Magnoliaceae plant were found to be effective for the removal of chromium. The plant belongs to the magnolia family and is well grown America, West Indies, South and east- India, SriLanka, Malesia, China, Japan, and Korea. The barks of the plant are used to treat of chronic respirator, lung congestion, abdominal pain, nausea, diarrhea indigestion and sinus infections (Fig. 1).
2.2.2 Preparation of active carbon by treating sulphuric acid
The leaves of Magnoliaceae plant were cut, washed, dried and immersed in conc.H2SO4 for overnight and heated at 1050C in a round bottomed flask under water condenser set-up for 1 h. The bio-material was completely carbonized. The obtained bio-char was filtered, washed with distilled waterfor neutrality and dried at 110°C for 6h in oven. The material was pulverized and sieved to ˂75μm size. This active carbon was named as SACM (sulphuric acid-treated activated carbon of leaves of Magnoliaceaeplant).
2.2.3 SACM embedded in Zr-alginate beads (SACM@Zr)
Sodium alginate (2.0 g) was added to 100 mL distilled-water in a 250-mL beaker. Then the solution was heated to 70°C with thorough stirring to get a gel-like solution. Then, SACM (2.5 g) was added bit-by-bit with thorough stirring of solution. The stirring is continued at the elevated temperature for 2.0 h. Then the resulting solution was allowed to be cooled to room-temperature. This solution was added drop-by-drop into previously cooled (5.0 °C) 3.0% ZrOCl2 solution using a dropper having stem of uniform pore. The drops were solidified to yield beads due to the cross linking of Na-alginate caused by Zr-ions. Then the beads were digested with the mother liquor for overnight and separated, washed and dried at 90°C for 2 h. Thus the Zr-alginate beads doped with the SACM were resulted. These beads were named as SACM @Zr (sulphuric acid-treated activated carbon derived from leaves of Magnoliaceae plant embedded by zirconium alginate beads). The methodology adopted for the synthesis is presented graphically in Fig. 2.
2.3 Surface characterization
FTIR, FESEM and EDX investigations were undertaken to understand nature of adsorption of ‘SACM’ and ‘SACM@Zr’ towards chromate ions. FESEM images of ‘SACM’ and ‘SACM@Zr’ [before and after adsorption of Cr(VI) ions) were taken at 20,000 X magnifications at the acceleration voltage of 15.0 kV. FTIR and EDX spectra of ‘SACM’ and ‘SACM@Zr’ were recorded \before and after\ chromium adsorption. The adsorbents were scanned for the absorption peaks of surface functional groups in the range 4000–500 cm−1 by a Nicolet 6700 FT-IR spectrometer using KBr pellet technique. The results were presented in Figs. 3, 4, to 5.
The Brunauer-Emmett-Teller (BET) surface areas of the adsorbents were determined using nitrogen gas adsorption analyzer at 77 K by Quantachrome NovaWin - Data Acquisition and Reduction for NOVA instruments version 10.01 (Quantachrome Instruments, Boynton Beach, FL, USA). The BET-N2 surface area was obtained by applying the BET equation to the adsorption data.
2.4 Adsorption experiment
Batch adsorption experiments were used as described in the literature [34]. To 100 mL of chromium solutions (50 mg/L) in 250-mL conical flasks, varying quantities of ‘SACM’ and ‘SACM@Zr’ (0.05 to 0.35g) were added. Then, initial pHs of solutions were adjusted from 2 to 12 by using 0.1 M HCl/0.1 M NaOH. The flasks were agitated in orbital shaker for desired times at 300 rpm at room temperature (30±1°C). After a definite time of agitation, the solutions were filtered. The residual chromate in the filtrates was analysed spectrophotometrically by adopting ‘Diphenyl Carbazide’ method [35] using UV-Visible-159 model spectrophotometer (ELICO).
The influence of pH, equilibration-time, dosage of sorbents, initial chromate-concentration and temperature on the sorption of chromium were investigated. For this, the general procedure described above was adopted by varying the targeted parameter while maintaining others at constant (optimum) values. The percentage removal and adsorbed amount of chromium (adsorbent capacity) were calculated by the equations:
and
where m is mass of adsorbent (g), V volume of the solution (L), and C0 and Ci are the initial and final concentrations (mg/L) [36, 37].
The developed procedure was applied to waste-water samples collected from the effluents of Ethiopia Tannery Company and also from the waters of Leyole and Worka rivers located near Kombolcha City, north part of Ethiopia.
3 Results and discussions
3.1 Characterization analysis
3.1.1 BET surface area
The Brunauer-Emmett-Teller (BET) surface areas of the adsorbents, before and after adsorption of chromium were determined and the values were:
-
SACM (before: 395.28; after: 214.51 m2/g)
-
SACM@Zr (before: 478.35; after: 196.43 m2/g)
The decrease in surface areas after Cr (VI) adsorption for both the sorbents, is an indication that Cr(VI) is adsorbed onto the surface of the sorbents.
3.1.2 Fourier transform infrared spectroscopic analysis (FTIR)
FTIR analyses is an important technique to identify the functional groups on the surface of prepared adsorbents [38].The FTIR spectra of ‘SACM’ and ‘SACM@Zr’ before and after chromate sorption were depicted in Fig. 3 A&B. The adsorption band at ~3280 cm-1 for ‘SACM’ and 3328 cm-1 for ‘SACM@Zr’ in the spectrum taken before chromium adsorption is due to the stretching vibrations of ‘–OH’ group. This frequency is shifted to 3334 cm-1 after adsorption for ‘SACM’ and to 3350 cm-1 for ‘SACM@Zr’. The frequency at 2882 cm-1 for ‘SACM’ and 2945.33 cm-1 for ‘SACM@Zr’ in before chromium adsorption spectrum is due to stretching of ‘C-H’ groups. It is shifted to 2915.8 cm-1 and 2973.6 cm-1 of after the adsorption of chromium in ‘SACM’ and ‘SACM@Zr’ spectra respectively. The frequency at 1990.7 cm-1 for ‘SACM’ and 2049 cm-1for ‘SACM@Zr’ is attributed to the allene (C=C=C) group is shifted to 2028 for ‘SACM’ and 2077 cm-1 for ‘SACM@Zr’ in spectra after the adsorption of chromate.
The strong absorption band at 1542 cm-1for ‘SACM’ and 1610 cm-1for ‘SACM@Zr’ in before adsorption spectrum are due to aromatic nature.They are shifted to 1598.4cm-1 for ‘SACM’ and1630 cm-1 for ‘SACM@Zr’ after adsorption of chromate. The sharp bands at 1047 cm-1 for ‘SACM’ and 1125.3 cm-1 for ‘SACM@Zr’ in before adsorption spectrum is due to ‘C-O-C’ stretching vibrations and it is shifted to 1103 cm-1 for ‘SACM’ and 1144 cm-1 for ‘SACM@Zr’ after chromium adsorption.
The peaks around 1050–1000 cm-1 indicated intrinsic vibrations of Cr-O bond. The similar results were observed with the adsorption of chromium on to Fe3O4 [39]. A sharp small peak at 743.7 cm-1 before adsorption pertains to Zr-O (stretching) is shifted to 768 cm-1after adsorption of chromate for ‘SACM@Zr’. This indicates that the functional groups on the surface of adsorbents have a high affinity towards chromium ions [31].The changes in positions and intensities of peaks are the emphatic evidence of chromium adsorption owing to the interactions between chromium ions with the functional groups of adsorbents. The similar results (to higher wavenumbers) were recorded in previous works regarding the adsorption of chromium ions [31, 38, 40].
3.1.3 FESEM analysis
FESEM of ‘SACM’ and ‘SACM@Zr’ taken before and after chromium adsorption was shown in Fig. 4: A to D. Rough, micro pores, spongy, irregular and heterogeneous structure can be noted in the before-adsorption images (Fig. 4 A &C). Similar surface morphology was observed in the adsorbents reported in the literature [31, 40].In the images take after adsorption of chromate, there are marked changes in the images: missing pores or decrease in pore size, and disappearance of some corners and edges (Fig. 4:B & D). After adsorption of chromium ions, the surface morphologies of both the adsorbents slightly changes due to interactions of chromium ions with active sites on the surface of adsorbents [39]. These features reflect that the adsorption of chromate ‘onto’ the surface of the adsorbent. Similar results were observed in previous work where an activated carbon prepared from paper mill sludge was used as an adsorbent to remove chromium from aqueous solution [41].
3.1.4 Energy-dispersive X-ray spectroscopy (EDX)
In Fig. 5 (A) to (D), the EDX spectra of adsorbent ‘SACM’ and ‘SACM@Zr’ taken before and after chromium sorption, were presented. On comparison of the spectra, it is evident that peaks pertaining to chromium are appeared in the spectrum taken after adsorption (Fig. 5 B&D).These chromium peaks are not found in the spectrum of before chromium adsorption (Fig. 5A&C). These features are an emphatic proof for the adsorption of chromate by ‘SACM’ and ‘SACM@Zr’.
3.2 Optimization of extraction parameters
Various experimental conditions were optimized for the maximum extraction of chromate. Investigations were carried out by varying one parameter while maintaining the other experimental conditions constant. Investigations were made by varying the pH from 2 to 12, agitation time: 5 to 70 min, adsorbent dosage from 0.05 to 0.35g/100 mL, at initial chromium concentration: 50 mg/L; rpm: 300 and temp. 30°C for ascertaining the optimum extraction conditions (Fig. 6 A to E). Effect of initial chromium concentration and solution temperature on sorption efficiencies of the two adsorbents were also investigated by keeping all other parameters at optimum levels while varying only the parameter under study.
3.2.1 pH effect
In the removal of metal ions from solution by adsorption technique, pH of the solution is a significant parameter [42]. The metal ion adsorption is depending on the functional groups present on the adsorbent surface and is varying with the pH. By changing the initial pH, the extraction was studied using chromium solution of concentration 50mg/L. The other conditions were kept constant: SACM: 0.25g/100mL, equilibrium time: 50 min; SACM@Zr: 0.2g/100mL; equilibrium time: 40 min; 300 rpm and temp. 303K. The observations were depicted in Fig. 6A. As it is evident from figure that maximum chromium removal occurs at pH:2 for both the sorbents and they were: 95.3 for ‘SACM’ and 98.6% for ‘SACM@Zr’.
pHzpc (point of zero charge) of the ‘SACM’ and ‘SACM@Zr’, were evaluated from the Fig. 7:A&B as 6.3 for ‘SACM’ and 6.6 for ‘SACM@Zr’. The surface of the adsorbent is neutral at these pHzpc values but acquires positive charge below these pHzpc due to protonation and negative charge due to the dissociation of functional groups of adsorbents. Hence, chromate being an anion [43], it shows good adsorption at low pHs [44]. But in basic pHs, as the surface the adsorbent as well as chromate species are negative and hence less adsorption.
3.2.2 Optimization of initial chromium concentration
Adsorption experiments were conducted by changing the initial chromium concentration from 40 to 120 mg/L but keeping constant all other extraction conditions at optimum levels.The experimental results were presented in Fig. 6B.With the raise in chromium concentration from 40 mg/L to 120 mg/L, the adsorption falls from 97.8 to 67.8% for ‘SACM’ and 99.7 to 68.3 % for ‘SACM@Zr’. But the adsorption capacity is increased from 15.65 to 32.54 mg/g for ‘SACM’ and from 19.94 to 40.98 mg/g for ‘SACM@Zr’. As the sorbent dosage is fixed only a fixed number of active sites are available. So, as the concentration of chromate is increased, the number of available ‘active sites per ion’ is decreased and hence, % removal falls. However, the adsorption capacity (qe) is increasing with the increase in concentration of chromate. This may be attributed to the fact that as the concentration of chromate is increased, the concentration gradient between the bulk of the solution and surface of the adsorbent with respect to chromate is also increased (Fig. 6C). This causes the chromate diffuse more towards the surface of adsorbents and thereby resulting more adsorptivities [45].
3.2.3 Optimization adsorbent concentration
The study of effect of adsorbent dosage is very useful to find the optimum amount of adsorbent required for the removal of chromium.The adsorbent doses were varied from 0.05 to 0.35g/100mL while keeping all other extraction conditions at constant levels.The effect of adsorbent dose on the adsorption of chromium was presented in Fig. 6 D. Initially, percentage adsorption of chromate is increased linearly as adsorbent dose is increased and it has reached maximum chromium removal with 0.25g/100 mL for ‘SACM’ and 0.2g/100 mL for ‘SACM@Zr’. After these dosages, steady states were resulted. The maximum removal was found to be 95.3% for ‘SACM’ and 98.6% for ‘SACM@Zr’. With increase in the dosage of the adsorbent, naturally the number of adsorption sites available for adsorption process increase and hence more adsorption is observed initially. But when the adsorbent is further increased, the proportional increase in adsorption is not observed. This may be due to the blocking of pathways for adsorbates to reach to the active sites laid in the matrix of the adsorbent due to aggregation and/or deposition [29, 46].
3.2.4 Optimization of contact time
To assess the optimum time to be allowed between the adsorbents (SACM and SACM@Zr) and the adsorbate, chromate, experiments were conducted by varying the time of contract from 5 to 70 min but maintaining at optimum levels all other conditions of extraction viz.: pH: 2; temperature: 303K; initial chromium concentration: 50 mg/L; agitation speed: 300rpm and adsorbent dose: 0.25 g/100mL for ‘SACM’, 0.2g/100mL for ‘SACM@Zr’. In Fig. 6 E, results were depicted. The maximum extraction, 95.3% was observed after 50 min of equilibration for ‘SACM’ and 98.6% after 40 min for ‘SACM@Zr’. As is viewed from the Fig. 6 E, with increase in time, the adsorption is gradually increased. Steady states were reached at 50 min for ‘SACM’ and 40 min for ‘SACM@Zr’. Initially, the percentage removal of chromium is rapid, but slows down with time and reached a steady state after some time. Initially the availability of active sites is more and so, rate of adsorption is more. With time, the active sites are used-up and hence, the adsorption rate is decreased. As the quantity of the adsorbent is fixed (0.25 g/100 mL for ‘SACM’ and 0.20 g/100 mL for ‘SACM@Zr’, the active sites are fixed. When all the sites are engaged with the adsorption of chromate, steady states are resulted [47, 48].
3.2.5 Temperature effect
To assess the influence of solution temperature on the chromium adsoption, experiments were conducted at different temperature viz., 303, 313, 323 and 333 K. by equilibrating 100 mg/L of chromate solution at pH: 2.0; adsorbent dosage: 0.25 g/100mL for SACM, 0.2 g/100mL for SACM@Zr ; agitation time: 50 min for SACM and 40 min for SACM@Zr. The results were depicted in Fig. 8A. As temperature increases from 303 to 333 K, percentage removal of chromate was increased from 74.8 to 85.5% for SACM and 77.9 to 87.8% for SACM@Zr. The increase in temperature increases the % removal. This indicates that the adsorption process is favourable at high temperatures. Increase in solution temperature, enhances the vibrational kinetic energy of the functional groups present on the surface of the adsorbents and thereby decrease the surface-layer thickness. This results in opening of the hidden active sites in the adsorbents. Further, the adsorbate ions acquire more kinetic energy. These factors help in the penetration of adsorbate (chromate) deeper into the adsorbent and hence, more adsorptivities with increase in temperature [49,50,51].
3.2.6 Thermodynamic studies
Various thermodynamic parameters, namely ∆H, ∆S, and ∆G, were evaluated according to procedures in the literature [52, 53].The concern equations are:
∆G = − RTlnKd, \( \mathrm{Kd}=\frac{\mathrm{qe}}{\mathrm{Ce}} \) and \( lnKd=\frac{\Delta \mathrm{S}}{\mathrm{R}}-\frac{\Delta \mathrm{H}}{\mathrm{R}\mathrm{T}} \), where, qe = amount of chromium adsorbed at equilibrium, Kd = distribution coefficient, Ce = equilibrium concentration of chromium, R = gas constant and T =Temperature ( Kelvin). ∆H and ∆S values were calculated from lnKd Vs. (1/T) plots, Fig. 8B. The values were noted in Table 1.
The negative ΔG° values indicate the spontaneity of adsoption process. As the magnitude of –ve values increase; it reflects that increase in temperature is beneficial to chromium adsorption (Fig. 8B).
Positive ΔH° values indicate endothermic nature of adsorption process [54, 55]. ΔH values were: 20.11kJ/mol for ‘SACM’ and 19.84 kJ/mol for ‘SACM@Zr’. The magnitude of these values indicates that the adsorption of chromium ions ‘onto’ the surface of ‘SACM’ and ‘SACM@Zr’ is chemical in nature. The adsorption is physical if the values are: 2.1–20.9 kJ/mol and chemical: 20.9-418.4 kJ/mol [56].
Positive ΔS° values reflect the randomness or disorder at the interface of solution and solid [57]. As ‘SACM@Zr’ has high value than ‘SACM’, disorderliness is more for the former than the latter. If the disorder is more, the randomness in the movement of chromate is more. This allows the chromate ions to penetrate deeply into the adsorbent layer. Therefore, in the case of ‘SACM@Zr’, the adsorption capacity is expected to be greater than ‘SACM’.
3.3 Nature of adsorption
Data was modeled by four isotherm concepts: Freundlich, Langmuir, Temkin, and Dubinin–Raduskovich. Needed calculations were done to compare the theoretical and experimental adsorption isotherms. These procedures give us a better understanding of the behavior of chromium in solution. Numerous parameters were evaluated and also the correlation coefficient (R2), which reveals a good fit between the experimental data and the theoretical adsorption isotherm equations, were considered to compare the models.The linear plots of the above said four isotherms for the adsorbents, ‘SACM’ and ‘SACM@Zr’ were presented in Fig. 9 and the constants together with the correlation coefficient were presented in Table 2.
The correlation coefficient, R2, values decrease in the order: Langmuir (0.9974) > Freundlich (0.0.8137) > Temkin (0.8133) > Dubinin-Radushkevich (0.7539) for ‘SACM’; Langmuir (0.9979) > Freundlich (0.9258) >Temkin (0.9153) > Dubinin-Radushkevich (0.691) for ‘SACM@Zr’.The correlation coefficient values were found to be higher for the two adsorbents in Langmuir adsorption isotherm model. The dimensionless separation factor values (RL=0.0099 for ‘SACM’, RL=0.0037 for ‘SACM@Zr’) were found to be in the range between zero to one. These two facts indicate the favorability of the Langmuir-isotherm and confirm the monolayer adsorption on the surface of adsorbents [57] and also homogenous presence of active sites on the surface of adsorbent. From the Freundlich isotherms, the 1/n values are: 0.1537 for ‘SACM’ and 0.1176 for ‘SACM@Zr’. As the slope, 1/n, values range between 0 and 1, the adsorption is favorable [58].
To assess the nature of adsorption, the magnitude of D-R mean free energy (E) and Temkin heat of sorption (B) values are used. The Dubinin-Radushkevich mean free energy, E, was found to be 1.581 and 3.536 kJ/mol for ‘SACM’ and ‘SACM@Zr’ respectively. Temkin heat of sorption (B) values was found to be as 3.4601 and 3.4176 J/mol for ‘SACM’ and ‘SACM@Zr’ respectively. Physisorption is dominated if, E, and B values are less than 20kJ/mol [55, 59]. Hence, the adsorption of chromate is more oriented towards physiosorption.
3.4 Modeling of kinetic data
Four kinetic models: pseudo first and second order, Bangham's pore diffusion and Elovich, were employed (Fig. 10). In these models, correlation coefficient (R2) value is used to find the best fitting model. If the value of the correlation coefficient (R2) is close to 1, greater linearity can be achieved. Linear plots of these models were shown in Fig. 10 and the slopes and intercepts of the corresponding linear plots along with correlation coefficient (R2) values were presented in the Table 3.
Higher correlation coefficient (R2) values are obtained from pseudo second-order kinetics for ‘SACM’ as well as for ‘SACM@Zr’. This reflects that pseudo second-order model is better fit to explain kinetics of adsorption in the two adsorbents, SACM and SACM@Zr.
3.5 Interference of co-ions
Interference of co-ions (two-fold excess) that naturally exist in water was investigated under the same optimum extraction conditions using ‘SACM’ and ‘SACM@Zr’ as adsorbents. The optimum conditions of maximum extraction are: pH 2; initial chromium concentration: 50mg/L; co-ions concentration: 100mg/L;SACM: 0.25g/100mL, equilibrium time: 50 min; SACM@Zr: 0.2g/100mL; equilibrium time: 40 min; 300 rpm and temp. 303K.
In the absence of co-ions, the maximum extraction was found to be 95.3% and 98.6% for ‘SACM’ and ‘SACM@Zr’ respectively. But, in presence of co-ions, the percent extraction of adsorbents was decreased. For example, in presence of Sulphate ions, the percent extraction was decreased from 95.3 to 81.0% with SACM and from 98.6 to 83.0% with SACM@Zr. With phosphate as interfering ion, the extraction was reduced from 98.6 to 83.0 % for SACM and 85.0 % for SACM@Zr. This is due to the interference of co-ions with the chromium ions. The extent of interference of co-ions on chromium ions depends on several factors such as charge, size, polarizability, electro negativity difference etc [60].The observations were summarized in Fig. 11 (A) & (B). It may be noted that many of the cations as well as anions marginally affected the % of chromium extraction except sulphates and phosphates.
3.6 Recycling of adsorbents
Investigations were made to regenerate spent sorbents with various solutions of acids, bases, or salts or their blends. This study is essential to assess the reversibility of sorption process and to have cost effective procedure. It was observed that 0.01N NaOH solution was effective in removing the adsorbed chromium ions from the matrix of adsorbent. The spent adsorbent was digested overnight in the said solution, filtered, washed with distilled water and dried at 90°C.
Thus, regenerated adsorbents were employed for the treatment of chromium polluted simulated water. In Fig. 12, the results were noted. It may be inferred that up to the eight cycles of regeneration, the loss of adsorption capacities is marginal. So, by repetitively adopting the same regenerated adsorbent, complete chromium removal may be effected.
3.7 Applications
Samples from discharges of Ethiopia Tannery Companies and chromium polluted waters of Leyole and Worka rivers around Kombolcha, located in the north-central part of Ethiopia were collected. These samples were subjected to treatment with the present developed ‘SACM’ and ‘SACM@Zr’ for the removal of Cr (VI). In Table 4, results were noted. It may be inferred that the adsorbents are effective in the removal of chromate.
3.8 Comparative study
The adsorbents developed were compared with various previously reported adsorbents for removing chromium from water. The adsorption capacity values of different adsorbents reported in the literature were compared to each other by considering of their experimental conditions, such as higher chromium concentration, lower pH etc., which favours higher adsorption capacity. The data was summarized in Table 5. It can be inferred from the table that the adsorbents developed in this study, namely ‘SACM’ and ‘SACM@Zr’, have higher adsorption capacities than reported in the literature. There are some other adsorbents in the literature, bio-graphene foam [4], KOH-AC [67], etc., which have high adsorption capacity than the adsorbents in the present work.
But, the preparation of adsorbents in the present work is simple and low adsorbent dose, 0.25g/L and 0.2g/L is sufficient for the maximum removal, 95.3 and 98.6% with initial chromium concentration 50mg/L at pH 2 for SACM and SACM@Zr respectively.
In addition, when these adsorbents were used to treat chromium polluted waters of different areas, 100% extraction was observed. All these confirmed the suitability of these adsorbents in large scale applications.
4 Conclusions
An activate carbons derived from the leaves of Magnoliaceae plant (SACM) and Zirconium-alginate beads embedded with active-carbon, SACM@Zr, are successfully investigated for chromate removal from wastewater. The adsorbents are synthesized and characterized by using FTIR, FESEM and EDX techniques. The adsorption efficiencies of the two adsorbents towards chromate removal from waste water are investigated with respect to various parameters such as initial concentration, adsorbent dosage, contact-time, pH and elevated temperatures. The optimized conditions for the maximum extraction are: pH: 2, initial concentration: 50 mg/L; sorbent concentration: 2g/L for SACM@Zr and 2.5g/L for SACM; contact time: 40 min SACM@Zr and 50 min for SACM; temperature:303 K.
The thermodynamic parameters ∆H°, ∆S° and ∆G°, are evaluated. The negative value of ∆G∘ and the positive value of ∆H∘ indicate that the adsorption of chromate ‘onto’ the surface of adsorbent is spontaneous and endothermic. The positive ∆S° values indicate randomness at the solid/liquid interface and hence, increase in adsorptivities. Langmuir isotherm with R2 values 0.9979 for ‘SACM@Zr’ and 0.9974 for ‘SACM’, describe the experimental data of the two adsorbents well and show that the adsorption of chromate ‘onto’ the adsorbents is mono-layer and homogeneous. The Langmuir monolayer adsorption capacities for chromate are: 37.74 mg/g for ‘SACM@Zr’ and 28.82mg/g for ‘SACM’. The data are in good agreement with the pseudo second-order kinetics model with R2 values: 0.9986 for ‘SACM@Zr’ and 0.9988 for ‘SACM’. The two adsorbents, ‘SACM’ and ‘SACM@Zr’, have been successfully applied to treat Cr-polluted effluents from Ethiopia Tannery Companies and waters of Leyole and Worka rivers around Kombolcha, located in the north-central part of Ethiopia. Thus, the present developed adsorbents are proving to be promising adsorbents for removing chromium from polluted water.
References
Khamis M, Jumean F, Abdo N (2009) Speciation and removal of chromium from aqueous solution by white, yellow and red UAE sand. J Hazard Mater 169:948–952
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: A review. J Environ Manag 92:407–418
Babu AN, Reddy DS, Kumar GS, Ravindhranath K, Mohan GK (2018) Removal of lead and fluoride from contaminated water using exhausted coffee grounds based bio-sorbent. J Environ Manag 218:602–612
Krebsz M, Pasinszki T, Tung TT, Nine MJ, Losic D (2021) Multiple applications of bio-graphene foam for efficient chromate ion removal and oil-water separation. Chemosphere 263:127790. https://doi.org/10.1016/j.chemosphere.2020.127790
Gholami F, Mahvi AH, Omrani GA, Nazmara S (2006) Removal of chromium (VI) from aqueous solution by Ulmus leaves. Iran J Environ Healt 3:97–102
Babu AN, Mohan GK, Ravindhranath K (2016) Removal of Chromium (VI) from polluted waters using adsorbents derived from Chenopodium album and Eclipta prostrate plant materials. Int J Chem Tech Research 9(03):506–516
Goyal N, Jain SC, Banerjee UC (2003) Comparative studies on the microbial adsorption of heavy metals. Adv Environ Res 7:311–319
US, (2009) Department of Health and Human Services, Toxicological profile for chromium, Public health services agency for toxic substances and diseases registry, Washington, DC.
Praveen P, Loh KC (2016) Thermodynamic analysis of Cr(VI) extraction using TOPO impregnated membranes. J Hazard Mater 314:204–210
Ravulapalli S, Kunta R (2018) Enhanced removal of chromium (VI) from wastewater using active carbon derived from Lantana camara plant as adsorbent. Water Sci Technol 78(6):1377–1389
Alloway BJ (1995) Heavy Metals in Soils, Blackie Academic and Professional, London, edn. 2, p. 368.
Kimbrough DE, Cohen Y, Winer AM, Creelman L, MabuniCA (1999) Critical assessment of chromium in the environment critical reviews in environmental science and technology. Crit Rev Environ Sci Technol 29(1):1–46
Singh IB, Singh DR (2002) Cr (VI) removal in acidic aqueous solution using iron-bearing industrial solid wastes and their stabilisation with cement. Environ Technol 23(1):85–95
Gil RA, Cerutti S, G’asquez JA, Olsina RA, Martinez LD (2006) Pre-concentration and speciation of chromium in drinking water samples by coupling of online sorption on activated carbon to ETAAS determination. Talanta 1065(68):1065–1070
World Health Organization (WHO) (2004) Guidelines for drinking-water quality (third ed.). Recommendations. WHO, Geneva 1:334–335
Katz F, Slem H (1994) The biological and environmental chemistry of chromium (pp. 51–58). New York: VCH.
Kotas J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107(3):263–283
Mohan GK, Babu AN, Kalpana K, Ravindhranath K (2019) Removal of chromium (VI) from water using adsorbent derived from spent coffee grounds. Int J Environ Sci Technol 16(1):101–112
Cushnie GC Jr (1985) Electroplating wastewater pollution control technology. Noyes Publications Park Ridge:30–39
Kobya M (2004) Removal of Cr(VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: kinetic and equilibrium studies. Bioresour Technol 91:317–321
Deveci H, Kar Y (2013) Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis. J IndEng Chem 19:190–196
Cetin G, Kocaoba S, Akcin G (2013) Removal and recovery of chromium from solutions simulating tannery wastewater by strong acid cation exchanger. J Chem 7
Ravulapalli S, Kunta R (2017) Defluoridation studies using active carbon derived from the barks of Ficusracemosa plant. J Fluor Chem 193:58–66
Ponou J, Kim J, Wang LP, Dodbiba G, Fujita T (2011) Sorption of Cr (VI) anions in aqueous solution using carbonized or dried pineapple leaves. Chem Eng J 172(2-3):906–913
Cimino G, Passerini A, Toscano G (2000) Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res 34(11):2955–2962
Dubey SP, Gopal K (2007) Adsorption of chromium (VI) on low cost adsorbents derived from agricultural waste material: a comparative study. J Haza Materials 145(3):465–470
Babel S, Kurniawan TA (2004) Cr (VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54(7):951–967
Al-Othman ZA, Ali R, Naushad M (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247
Garg UK, Kaur MP, GargVK SD (2007) Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J Hazard Mater 140(1-2):60–68
Ranganathan K (2000) Chromium removal by activated carbons prepared from Casurinaequisetifolia leaves. Bioresour Technol 73(2):99–103
Rai MK, Shahi G, Meena V, Meena R, Chakraborty S, Singh RS, Rai BN (2016) Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resource-Efficient Technol 2:S63–S70
Aparna B, Gupta SK (2016) Removal of Cr(VI) from waste water using root of Eucalyptus tree. Int J Eng Technol Sci Res 3(11):37–42
Velumani K, Kumar PE, Sivakumar V (2016) Adsorption of Chromium (VI) using a nonconventional adsorbent. Rasayan J Chem 9(2):149–159
Metcalf and Eddy (2003) Wastewater Engineering: Treatment of Reuse, McGraw Hill Co., New York, edn 4.
Vogel AI (1961) A Textbook of Quantitative Inorganic Analysis, Including Elementary Instrumental Analysis, 3rd edn. John Wiley and Sons, Inc., New York, NY, USA.
Dehghani MH, Tajik S, Panahi A, Khezri M, Zarei A, Heidarinejad Z, Yousefi M (2018) Adsorptive removal of noxious cadmium from aqueous solutions using poly urea-formaldehyde: A novel polymer adsorbent. MethodsX 5:1148–1155
Babu AN, Krishna Mohan GV, Kalpana K, Ravindhranath K (2018a) Removal of fluoride from water using H2O2-treated fine red mud doped in Zn-alginate beads as adsorbent. J Environ Chem Eng 6(1):906–916. https://doi.org/10.1016/j.jece.2018.01.014
Khalil U, Shakoor MB, Ali S, Ahmad SR, Rizwan M, Alsahli AA, Alyemeni MN (2021) Selective Removal of hexavalent chromium from wastewater by Rice Husk: Kinetic, isotherm and spectroscopic Iivestigation. Water 13:263. https://doi.org/10.3390/w13030263
Amir M, Anwar ul HAS, Salma B (2020) Effective Adsorption of hexavalent chromium and divalent nickel ions from water through Polyaniline, Iron Oxide, and their composites. Appl Sci 10:2882. https://doi.org/10.3390/app10082882
Samson OO, Adedibu CT (2016) Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-suef Univ J Basic Appl Sci 5:377–338
Gorzin F, Abadi MMBR (2018) Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorpt Sci Technol 36(1–2):149–169
Windholz M (1976) The Merck Index, vol. 802, Merck & Company, Whitehouse Station, NJ, USA, 9th edition.
Sanchez-Polo M, Rivera-Utrilla J (2002) Adsorbent− adsorbate interactions in the adsorption of Cd (II) and Hg (II) on ozonized activated carbons. Environ Sci Technol 36(17):3850–3854
Argun ME, Dursun S, Ozdemir C, Karatas M (2007) Heavy metal adsorption by modified oak sawdust: thermodynamics and kinetics. J Hazard Mater 141(1):77–85
Simha P, Yadav A, Pinjari D, PanditAB (2016) On the behaviour, mechanistic modelling and interaction of biochar and crop fertilizers in aqueous solutions. Resource-Efficient Technol 2(3):133–142
Jung C, Heo J, Han J, Her N, Lee SJ, Oh J, Yoon Y (2013) Hexavalent chromium removal by various adsorbents: powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Sep Purif Technol 106:63–71
Yu B, ZhangY SA, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J Hazard Mater 80(1-3):33–42
Krishna Mohan GV, Naga Babu A, Kalpana K, Ravindhranath K (2017) Removal of chromium (VI) from water using adsorbent derived from spent coffee grounds. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-017-1593-7
Inglezakis VJ, Loizidou MM, Grigoropoulou HP (2004) Ion exchange studies on natural and modified zeolites and the concept of exchange site accessibility. J Colloid Interface Sci 275(2):570–576
Mansooreh D, Simin N, Mojtaba K (2014) Removal of 2,4-Dichlorophenolyxacetic acid (2,4-D) herbicide in the aqueous phase using modified granular activated carbon. J Environ Health Sci Eng 12:28
Sujitha R, Ravindhranath K (2017) Extraction of phosphate from polluted waters using calcium alginate beads doped with active carbon derived from A. aspera plant as adsorbent. J Anal Method Chem 2017:1–13. https://doi.org/10.1155/2017/3610878
Fan C, Zhang Y (2018) Adsorption isotherms, kinetics and thermodynamics of nitrate and phosphate in binary systems on a novel adsorbent derived from corn stalks. J Geochem Explor 188:95–100
Biftu WK, Ravulapalli S, Kunta R (2020) Effective de-fluoridation of water using Leucaenaluecocephala active carbon as adsorbent. Int J Environ Res 14:415–426. https://doi.org/10.1007/s41742-020-00268-z
Ngah WW, Hanafiah MAKM (2008) Adsorption of copper on rubber (Heveabrasiliensis) leaf powder: Kinetic, equilibrium and thermodynamic studies. Biochem Eng J 39(3):521–530
RavulapalliS RK (2019) Novel adsorbents possessing cumulative sorption nature evoked from Al2O3 nanoflakes, C. urens seeds active carbon and calcium alginate beads for defluoridation studies. J Taiwan Ins Chemical Eng 101:50–63
Sun C, Li C, Wang C, Qu R, Niu Y, Geng H (2012) Comparison studies of adsorption properties for Hg(II) and Au(III) on polystyrene-supported bis-8-oxyquinoline-terminated open-chain crown ether. Chem Eng J 200(202):291–299
Altundogan HS, Altundogan S, Tümen F, Bildik M (2000) Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag 20(8):761–767
Biftu WK, Ravindhranath K (2020a) Synthesis of nanoZrO2 via simple new green routes and its effective application as adsorbent in phosphate remediation of water with or without immobilization in Al-alginate beads. Water Sci Technol 81:2617–2633. https://doi.org/10.2166/wst.2020.318
Atkins P (1999) Physical chemistry, 6th edn. Oxford University press, London, pp 857–864
Onyango MS, Kojima Y, Aoyi O, Bernardo EC, Matsuda H (2004) Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent cation-exchanged zeolite F-9. J Colloid Interface Sci 279:341–350
Baral SS, Das SN, Rath P (2006) Cr(VI) removal from aqueous solution by adsorption on treated sawdust. Biochem Eng J 31(3):216–222
Anandkumar J, Mandal B (2009) Removal of Cr (VI) from aqueous solution using Bael fruit (Aeglemarmeloscorrea) shell as an adsorbent. J Hazardous Mat 168(2-3):633–640
Dakiky M, Khamis M, Manassra AMerEb M (2002) Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv Environ Res 6(4):533–540
Aditya GVV, Pujitha BP, Babu NC, Venkateswarlu P (2012) Biosorption of chromium onto Erythrina Variegata Orientalis leaf powder. Korean J Chem Eng 29(1):64–71
Alaerts GJ, Jitjaturant V, Kelderman P (1989) Use of coconut shell-based activated carbon for chromium (VI) removal. Water Sci Technol 21(12):1701–1704
Selvi K, Pattabhi S, Kadirvelu K (2001) Removal of Cr (VI) from aqueous solution by adsorption onto activated carbon. Bioresour Technol 80(1):87–89
Feng B, Shen W, Shi L, Qu S (2018) Adsorption of hexavalent chromium by polyacrylonitrile-based porous carbon from aqueous solution. R Soc Open Sci 5:171662
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biftu, W.K., Suneetha, M. & Ravindhranath, K. Zirconium-alginate beads doped with H2SO4-activated carbon derived from leaves of Magnoliaceae plant as an effective adsorbent for the removal of chromate. Biomass Conv. Bioref. 13, 5991–6006 (2023). https://doi.org/10.1007/s13399-021-01568-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01568-w