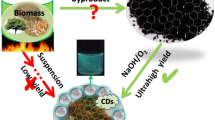
Abstract
We introduce a simple, low-cost, and environment friendly method to obtain high-fluorescence carbon dots and activated carbon via a one-step hydrothermal process using banana peels. The dispersion of carbon dots generated strong, bright-blue photoluminescence (average diameter 3–6 nm), which could be further used in biosensing, electronics, and catalysis applications research. Moreover, the precipitation accumulated at the bottom of the hydrothermal process contained activated carbon with a highly porous structure and large specific surface area (294.6 m2 g−1), which could be used as a supercapacitor electrode. The three-electrode cell exhibited excellent capability and stability of the activated carbon as the working electrode in various aqueous electrolytes, with a high specific capacitance (199 F g−1) in an aqueous electrolyte (1-M KOH) and a high energy density of 54.15 Wh kg−1 at a current density of 0.5 A g−1. Thus, based on the excellent characteristics of the carbon dots and the strong electrochemical properties of the activated carbon as an electrode material, the banana peels, as an abundant bioresource, can provide two excellent produces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon materials from biomass sources have a long history of synthesis and application in all aspects of human life [1]. Currently, biomass-derived carbon materials are widely used in various applications, such as energy storage (batteries and supercapacitors), biosensing, electronics, and photocatalysis. Biomass-derived carbon materials have become popular owing to their low-cost, high specific surface area, and high conductivity, stability, and versatility [2]. Popular biomass sources include rice husk [3], sugarcane bagasse [4], coconut shell [5], and soybean root [6]. To create environmentally friendly biomass-derived carbon materials of superb quality; the hydrothermal process is strongly preferred mainly due to its short preparation time and yield of high-quality carbon materials [7, 8]. The carbon materials can be obtained using the hydrothermal process in a cleaning solvent under self-generated pressures and controllable temperatures (100–800 °C) [9].
Currently, the hydrothermal process is employed to obtain two separate outputs from biomass sources: porous carbon materials and carbon dots. Porous carbon materials are commonly found in batteries and supercapacitors; these materials can be obtained from many sources, such as rice husk, sugarcane bagasse, coconut shell, and soybean root [3,4,5,6]. Carbon dots are used in various applications, such as biomedicine (nanomedicine), sensors (chemical sensors, biosensors, and bioimaging), catalysis (photocatalysis), optoelectronics (light-emitting diode devices), and energy conservation/storage (organic solar cells and supercapacitors) [10,11,12]. Carbon dots are derived from various biomass sources, such as orange juice [13], sugarcane bagasse pulp [14], flour [15], and ascorbic acid aqueous [16].
Among the various applications used in biomass-derived carbon materials, the supercapacitor is one of the best energy storage devices with high energy density, long lifetime, and fast charge–discharge capability [17]. However, the supercapacitor contains costly electrode materials, such as carbon nanotube, graphene, and rare metal oxide [17]. Therefore, it is important to identify new, reasonably priced electrode materials. Biomass-derived carbon materials for supercapacitors originate from many groups owing to their high conductivity, large specific surface area, adjustable porosity, and excellent stability [9]. Moreover, in light of climate change and diminishing natural resources, choosing a biomass source as input for the synthesis of porous carbon materials for the electrode supercapacitors has considerable economic and environmental benefits.
In our experiment, unlike previous ones, we relied on the one-step hydrothermal process for introducing a promising method for the synthesis of porous carbon materials and carbon dots. The precursor in our experiment is the banana peel because it is abundant and safe, has low-cost, and is easy to harvest. Banana trees are grown widely in tropical countries as one of the largest fruit crops, especially in South Asia (Vietnam, Indonesia, Malaysia, Thailand) [18]. Up to now, there are various kinds of research on banana tree such as banana peel-derived porous carbon materials for batteries [19], banana stem-derived porous carbon-derived carbon foam for the electrochemical determination [20], banana peels for gas adsorption [21], porous carbon from activated banana peels for electrochemical cell [22], and synthesis of carbon dots from banana peels for biological application [23]. Different from many previous reports, in this work we would like to introduce one fast and safety step to synthesize banana peel for two products: activated carbon and carbon dots with high quality.
The synthesis of the porous carbon materials and carbon dots employing the hydrothermal process containing banana peels in deionized (DI) water and ethanol (0.5/0.5 ratio) involves the bottom-up approaches [10, 24, 25], which yield high-quality porous carbon materials and nontoxic carbon dots. Compared with previous reports on banana peels, our experiment introduces a one-step preparation method for porous carbon materials and carbon dots using only banana peels. More importantly, this experiment offers new insights into the application of one biomass precursor to various outputs.
2 Experiment
2.1 Materials
Banana peels were obtained from a supermarket. Ethanol (C2H5OH, 99.9%), carbon nanotube multiwalls (CNT MWs), and poly(vinylidene fluoride) (PVDF, MW 534,000) were purchased from Sigma-Aldrich, and 1-methyl-2-pyrrolidinone (C5H9NO) was purchased from Alfa Aesar.
2.2 Preparation and characterization of carbon dots and porous carbon powder
In the experiment, 0.04 g of dried banana peels was added to 80 mL of a mixed solution of DI water and ethanol (0.5/0.5 ratio). This mixture was placed in a stainless steel clave for the hydrothermal process and kept at 200 °C for 24 h. After that, a yellow solution was obtained with precipitation at its bottom. The dark yellow precipitation was collected carefully, and the suspension was separated. Then, using DI water and ethanol, the dark yellow precipitation was washed several times and dried at 100 °C for 1 h to obtain the biomass-derived porous carbon material. The yellow suspension solution was filtrated several times using a 0.2-μm syringe filter to obtain the carbon dot solution.
Ultraviolet–visible spectroscopy (UV–VIS, PerkinElmer LAMBDA 1050), photoluminescence (PL) spectroscopy (Hitachi, F-7000 FL Spectrophotometer), transmission electron microscopy (TEM, JEM-2100F), and X-ray photoelectron spectroscopy (XPS), combined with Auger electron spectroscopy (AES) (Microlab 350), were applied to study the carbon quantum dots. The porous carbon material was examined by X-ray powder diffraction (XRD) using a CuKα tube (Bruker D2), Raman spectroscopy using a He/Ne laser (excitation wavelength, 632.8 nm) (HORIBA Jobin Yvon), scanning electron microscopy (SEM, Hitachi SU8000), transmission electron microscope (TEM, JEM-2100F, Joel), and Brunauer–Emmett–Teller (BET, Micromeritics, ASAP 2020). The SEM measurement of activated carbon was prepared from solution of 100 mg carbon powder in 10 ml ethanol with ultrasonic in 1 h and dropped onto silicon substrate and dried at 80 °C for 6 h. The TEM measurement of carbon dots was prepared by the following steps: the solution of carbon dots were dropped onto Cu grid and dried at 80 °C in vacuum oven in 48 h before measurement. For XPS of carbon dots measurement, the carbon dots were dropped many times onto silicon substrate and dried 80 °C for overnight.
2.3 Preparation of the activated carbon electrode and three-electrode cell characterization
The solution coating method was applied to prepare the biomass-derived porous carbon electrode and an active slurry. Firstly, the porous carbon (8 mg, 80 wt%), carbon nanotube single wall (1 mg, 10 wt%), and PVDF (1 mg, 10 wt%) were mixed in 0.4 mL of N-methyl-2-pyrrolidone (NMP) to obtain the slurry. Then, the slurry was coated on a carbon paper substrate (1 × 2 cm) and dried at 70 °C for 48 h. The weight of each electrode was 2 mg.
The three-electrode system, including platinum foil as the counter electrode, Ag/AgCl reference electrode, and porous carbon electrode as the working electrode, was used to conduct electrochemical characterization. The aqueous electrolyte potassium hydroxide (KOH) solution 1 M was used in the experiment. Electrochemical workstation Zarneh Zenium (Z 2.23, Germany) was used to examine the electrochemical properties, such as cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge–discharge (GCD).
The specific capacitance (C, F g−1) of the three-electrode system for the activated carbon electrode was calculated via charge–discharge curves using the following equation [26]:
Furthermore, energy density (E, W h kg−1) and power density (P, W kg−1) were calculated using the following equations [26]:
where I (A) denotes the discharge current; ∆t (s), the discharge time; ∆V, the potential voltage; and m (g), the mass of the activated carbon material for the working electrode.
3 Results and discussion
3.1 Banana peel-derived carbon dots
As presented in Fig. 1, the carbon dots were prepared via the one-step hydrothermal process in a mixture of DI water and ethanol. This method yielded green carbon dots and activated carbon without contamination.
Figure 2 a presents the UV–VIS spectra of carbon dots with strong blue luminescence under UV light, indicating excellent optical properties [14]. The UV–VIS absorption exhibited a peak at around ~ 280 nm, which is attributed to the π–π* transition of the C=C band and the n–π* transition of the C=O band [14,15,16], formed after the hydrothermal process during the preparation of the carbon dots. Moreover, XPS was applied to analyze the surface functional group of the carbon dots. As Fig. 2 a shows, the carbon dot solution is bright yellow and transparent in daylight, but changes to intense blue under UV light (see insets), indicating the excellent absorption of carbon particles in the solvent. The PL spectra of the carbon dots under various excitation wavelengths from 300 to 460 nm are presented in Fig. 2 b. The wide excitation range of PL measurements indicates that the maximum fluorescence emission is around 450 nm, with an excitation wavelength of 380 nm. The carbon dots in the experiment exhibited excellent fluorescence intensity, which can be employed for other applications using fluorescence.
TEM and the high-resolution TEM of carbon dots are presented in Fig. 3. The TEM image in Fig. 3 a indicates that the distribution of the carbon dots is very uniform in the copper grid, albeit with different sizes. The high-resolution TEM of carbon dots in Fig. 3 b indicates that the diameter size of the carbon dots ranges from 3 to 6 nm. Although carbon dots have varying diameters, no deterioration of the PL properties is noticeable. In addition, the carbon dots in Fig. 3 b indicate the lattice finger (diameter, approximately 0.128 nm) of the high-resolution TEM images, illustrating the crystalline phase of the carbon dots.
To determine the chemical compositions of the carbon dots, AES was employed to investigate the XPS measurements (see Fig. 4). The XPS survey spectrum in Fig. 4 a suggests the existence of carbon and oxygen elements. The two noticeable peaks at 285 and 532 eV are attributed to carbon and oxygen, respectively. As presented in Fig. 4 b, the deconvolution of the C 1s spectra can be observed in three carbon states of C–C at 284.6 eV, C–O at 286 eV, and C=O at 288.2 eV [16, 27,28,29]. The O 1s spectra can be divided into two oxygen states of C–O at 531.6 eV and C=O at 532.8 eV [28,29,30]. The XPS results suggest that the carbon dots with the hydroxyl and carbonyl groups originate from carbohydrate banana peels.
3.2 Banana peel-derived activated carbon
The activated carbon from the banana peels was obtained successfully through the hydrothermal process. Moreover, its structure and morphology were investigated by XRD, Raman spectroscopy, BET measurement, and SEM. The XRD pattern of the banana peel-derived activated carbon in Fig. 5 a exhibits a strong peak at 2θ = 22.6° and a broad peak at 43.5°, corresponding to 002 and 001 lattice planes, respectively [31]. The weak 002 peak indicates the low graphitic structure after the hydrothermal process, suggesting an amorphous phase in the activated carbon powder [26, 32].
Furthermore, the activated carbon quality was evaluated by Raman spectroscopy. The Raman spectrum in Fig. 5 b exhibits two peaks at around 1360 cm−1 (D band) and 1590 cm−1 (G band). The D band indicates the disordered graphite structure, and the G band corresponds to the crystalline graphitic carbon [33, 34]. The intensity ratio between the D band and the G band (ID/IG) denotes the defect sites and the graphitization degrees in the activated carbon sample [28, 29, 35]. According to the Raman spectrum, the ID/IG ~ 0.84 ratio indicates the lower graphitic phase in the sample. This finding agrees with the XRD pattern, which suggests a high amorphous carbon phase in the banana peel-derived activated carbon sample.
To determine the properties of the porous structure via the specific surface area of the banana peel-derived activated carbon material, BET measurement was performed, as presented in Fig. 5 c and Fig. 5 d. Moreover, the nitrogen adsorption/desorption isotherm was investigated to calculate the specific surface area of the activated carbon, as presented in Fig. 5 c. The activated carbon exhibits a high specific surface area of 294.6 m2 g−1, which is a high value for the hydrothermal process. This result indicates the high value of the activated carbon without any chemical activation, suggesting a green synthesizing process of the activated carbon from the banana peels. The uptake of the adsorption of N2 in Fig. 5 c exhibits micropores. The gradual increase of N2 isotherm at the high relative pressure of P/P0 of 1.0 indicates the existence of macropores in the sample. Moreover, the large, open hysteresis loop from 0.9 to 0.2 of P/P0 indicates the existence of abundant mesopores. In general, the banana peel-derived activated carbon has a porous structure with a few micropores and many mesopores and macropores [36,37,38,39,40]. As presented in Fig. 5 d, the pore-size distribution in the activated carbon, via the Barrett–Joyner–Halenda (BJH) model, exhibits an adsorption average pore width of 21.2 Å. The BJH adsorption average pore width is 55.7 Å, whereas the BJH desorption average pore width is 57.1 Å. These results confirm the suitability of the porous structure of the banana peel-derived activated carbon for the electrode material in energy storage applications.
Moreover, SEM was employed to investigate the surface morphology and porous structure of the activated carbon material. Fig. 6 presents the SEM images and energy-dispersive spectroscopy (EDS) of the activated carbon material. The visible porous structure of the activated carbon with a rough surface indicates the excellent quality of the material after the hydrothermal process in the cleaning solvent without chemical activation. The porous structure of the activated carbon material creates a perfect surface environment for the diffusion of the ions in the charge–discharge process for supercapacitor electrodes [32].
The supercapacitor electrodes using this porous structure can enhance their capability via the excellent porous electrode layer and the good electrode/electrolyte interface. Furthermore, EDS was used to examine the elements of the banana peel-derived activated carbon. The EDS spectra of the activated carbon in Fig. 5 c show that the soot consists of 80.46% of carbon weight, 15.67% of oxygen weight, and 3.86% of silicon weight (i.e., the silicon substrate for SEM analysis).
To investigate the electrochemical behavior of the banana peel-derived activated carbon for the electrode supercapacitor application, the three-electrode system was used for the banana peel-derived activated carbon as the working electrode. Figure 7 a presents the CV curves at various scan rates from 10 to 180 mV s−1. The CV curves have large and identical shapes, even at the high rate of 180 mV s−1, indicating excellent charge–discharge behavior and electrochemical stability [26, 32]. Moreover, in the experiment, the scan rate increased with an increase in the current output of the CV curves, implying an acceptable ability rate [39].
EIS was applied to investigate the equivalent series resistance during the charge–discharge process, as presented in the Nyquist plot of Fig. 7 b. The Nyquist plot consists of three regions as follows: a visible high-frequency semicircle indicating the charge transfer resistance, a 45° sloop line illustrating diffusion resistance, and a low-frequency vertical line showing diffusion resistance [39,40,41]. According to Fig. 7 b, the equivalent series resistance of the activated carbon electrode has a low value of 1.6 Ω, indicating the excellent conductivity of the activated carbon electrode during the electrochemical analysis. This equivalent series resistance contains a combination of the ionic resistance of the electrolyte, the resistance of the electrode–electrolyte interface, and the intrinsic resistance of the activated carbon material [39, 41]. The charge storage properties of the activated carbon electrode were investigated using the galvanostatic charge–discharge (GCD) measurement, as presented in Fig. 7 c. The GCD curves at current densities ranging from 0.5 to 5 A g−1 have triangular shapes, indicating the electrical double-layer behavior. The GCD curves preserve their triangular shape even at the high current density of 5 A g−1, suggesting the higher stability of the electrode. Based on Eq. (1), the highest specific capacitance has a value of 199 F g−1 at the current density of 0.5 A g−1, and the lowest specific capacitance has a value of 57.14 F g−1 at the current density of 5 A g−1. The specific capacitance decrease and the current density increase are presented in Fig. 7 d. The Ragone plot in Fig. 7 e illustrates the relationship between energy density and current density. The highest energy density is 54.14 Wh kg−1 at the power density of 350 W kg−1 at the minimum current density of 0.5 A g−1. When the current density reached 5 A g−1, the energy density was 15.55 Wh kg−1 at the power density of 3500 W kg−1.
In summary, the high electrochemical performance of the banana peel-derived activated carbon indicates the excellent potential of the electrode supercapacitor application. In comparison with other reports of biomass-derived carbon materials with the chemical activating agent (Table 1), one salient feature of this experiment is its use of the hydrothermal process to obtain porous carbon materials without any activating agent, such as KOH, ZnCl2, FeCl3, Na2CO3, and K2CO3 [3,4,5,6, 31, 34]. Although the synthesis did not involve the use of any activating agent, the porous carbon materials from the banana peels have large surface areas and high specific capacitance. The SEM and BET results reveal that the porous carbon materials in the experiment have excellent porosity in the presence of micropores, mesopores, and macropores. Moreover, the porous electrodes not only provide an excellent layer for the diffusion of ions but also create a perfect electrode–electrolyte interface to enhance the specific capacitance of the supercapacitors.
4 Conclusions
In the face of fossil fuel and global warming crises, agricultural products are promising sources for the synthesis of materials for various applications, ranging from biotechnology to energy storage and conservation and environmental treatment. This study used biomass sources to obtain useful products, such as carbon dots and porous carbon materials, using the green and straightforward hydrothermal process. More importantly, banana peels proved to be an excellent choice because they are low-cost, they are abundant in the tropical countries, and they are easy to harvest.
The banana peels emanating from the hydrothermal process in this experiment provide not only high-quality carbon dots but also porous carbon materials. Carbon dots in this experiment have an average diameter of around 3 to 6 nm with strong blue fluorescence. Furthermore, the activated carbon from the hydrothermal process has a porous structure with a high specific surface area of 294.6 m2 g−1. To investigate the compatibility of the activated carbon with the energy storage applications, the electrochemical of the three-electrode system was investigated. The activated carbon exhibited an excellent electrochemical performance, with the highest specific capacitance of 199 F g−1 at the current density of 0.5 A g−1. The energy density of the activated carbon exhibited an acceptable value of 54.14 W h kg−1 at the power density of 350 W kg−1. In short, this report introduced a green method for obtaining carbon dots and activated carbon from banana peels for fluorescence and energy storage applications.
References
Hu B, Wang K, Wu L, Yu SH, Antonietti M, Titirici MM (2010) Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Mater 22(7):813–828
Herou S, Schlee P, Jorge AB, Titirici M (2018) Biomass-derived electrodes for flexible supercapacitors. Curr Op Green Sust Chem 9:18–24
Liang Y, Yang C, Dong H, Li W, Hu H, Xiao Y, Zheng M, Liu Y (2017) Facile synthesis of highly porous carbon from rice husk. ACS Sustain Chem Eng 5(8):7111–7117
Rufford TE, Hulicova-Jurcakova D, Khosla K, Zhu Z, Lu GQ (2010) Microstructure and electrochemical double-layer capacitance of carbon electrodes prepared by zinc chloride activation of sugar cane bagasse. J Power Sources 195(3):912–918
Sun L, Tian CG, Li MT, Meng X, Wang L, Wang R, Yin J, Fu H (2013) From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J Mater Chem A 1(21):6462–6470
Guo N, Li M, Wang Y, Sun X, Wang F, Yang R (2016) Soybean root-derived hierarchical porous carbon as electrode material for high-performance supercapacitors in ionic liquids. ACS Appl Mater Interfaces 8(49):33626–33634
Gao Z, Zhang Y, Song N, Li X (2017) Biomass-derived renewable carbon materials for electrochemical energy storage. Mater Res Lett 5(2):69–88
Wang J, Nie P, Ding B, Dong S, Hao X, Dou H, Zhang X (2017) Biomass derived carbon for energy storage devices. J Mater Chem A 5(6):2411–2428
Deng J, Li M, Wang Y (2016) Biomass-derived carbon: synthesis and applications in energy storage and conversion. Green Chem 18(18):4824–4854
Wang Y, Hu A (2014) Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2(34):6921–6939
Zhang J, Yu SH (2016) Carbon dots: large-scale synthesis, sensing and bioimaging. Mater Today 19(7):382–393
Wu ZL, Liu ZX, Yuan YH (2017) Carbon dots: materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J Mater Chem B 5(21):3794–3809
Sahu S, Behera B, Maiti TK, Mohapatra S (2012) Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun 48(70):8835–8837
Thambiraj S, Shankaran DR (2016) Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl Surf Sci 390:435–443
Qin X, Lu W, Asiri AM, Al-Youbi AO, Sun X (2013) Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury(II) ions. Sensors Actuators B 184:156–162
Jia X, Li J, Wang E (2012) One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale. 4(18):5572–5575
Wang F, Wu X, Yuan X, Liu Z, Zhang Y, Fu L, Zhu Y, Zhou Q, Wu Y, Huang W (2017) Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem Soc Rev 46(22):6816–6854
Padam BS, Ti HS, Chye FY, Abdullah MI (2014) Banana by-products: an under-utilized renewable food biomass with great potential. J Food Sci Technol 51(12):3527–3545
Xue M, Lu W, Chen C, Tan Y, Li B, Zhang C (2019) Optimized synthesis of banana peel derived porous carbon and its application in lithium sulfur batteries. Mater Res Bull 11:269–280
Rajesh M, Veeramani V, Chen S-M (2014) Heteroatom-enriched and renewable banana-stem-derived porous carbon for the electrochemical determination of nitrite in various water samples. Sci Rep 4:4679
Niya AA, Rufford TE, Zhu Z (2016) Nitrogen-doped carbon foams synthesized from banana peel and zinc complex template for adsorption of CO2, CH4, and N2. Energy Fuel 30:7298–7309
Fasakin O, Dangbegnon JK, Momodu DY, Madito MJ, Oyedotun KO (2018) Synthesis and characterization of porous carbon derived from activated banana peels with hierarchical porosity for improved electrochemical performance. Electrochim Acta 262:187–196
Atchudan R, Edison TNJI, Perumal S, Muthuchamy N, Lee YR (2020) Hydrophilic nitrogen-doped carbon dots from biowaste using dwarf banana peel for environmental and biological applications. Fuel. 275:117821
Du Y, Guo S (2016) Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale. 8(5):2532–2543
Roy P, Chen PC, Periasamy AP, Chen YN, Chang HT (2015) Photoluminescent carbon nanodots: synthesis, physicochemical properties and analytical applications. Mater Today 18(8):447–458
Zequine C, Ranaweera CK, Wang Z, Singh S, Tripathi P, Srivastava ON, Gupta BK, Ramasamy K, Kahol PK, Dvornic PR, Gupta RK (2016) High per formance and flexible supercapacitors based on carbonized bamboo fibers for wide temperature applications. Sci Rep 6:31704
Liu M, Xu Y, Niu F, Gooding JJ, Liu J (2016) Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst. 141(9):2657–2664
Chen W, Hu C, Yang Y, Cui J, Liu Y (2016) Rapid synthesis of carbon dots by hydrothermal treatment of lignin. Materials. 9(3):184
Lu W, Qin X, Asiri AM, Al-Youbi AO, Sun X (2013) Green synthesis of carbon nanodots as an effective fluorescent probe for sensitive and selective detection of mercury(II) ions. J Nanopart Res 15(1):1344
Feng J, Wang WJ, Hai X, Yu YL, Wang JH (2016) Green preparation of nitrogen-doped carbon dots derived from silkworm chrysalis for cell imaging. J Mater Chem B 4(3):387–393
Sudhan N, Subramani K, Karnan M, Ilayaraja N, Sathish M (2017) Biomass-derived activated porous carbon from rice straw for a high-energy symmetric supercapacitor in aqueous and non-aqueous electrolytes. Energy Fuel 31(1):977–985
Li Y, Zhang Q, Zhang J, Jin L, Zhao X, Xu T (2015) A top-down approach for fabricating free-standing bio-carbon supercapacitor electrodes with a hierarchical structure. Sci Rep 5:14155
Liu X, Zhou Y, Zhou W, Li L, Huang S, Chen S (2015) Biomass-derived nitrogen self-doped porous carbon as effective metal-free catalysts for oxygen reduction reaction. Nanoscale. 7(14):6136–6142
Lu B, Hu L, Yin H, Xiao W, Wang D (2016) One-step molten salt carbonization (MSC) of firewood biomass for capacitive carbon. RSC Adv 6(108):106485–106490
Ma F, Wan J, Wu G, Zhao H (2015) Highly porous carbon micro flakes derived from catkins for high-performance supercapacitors. RSC Adv 5(55):44416–44422
Xie L, Sun G, Su F, Guo X, Kong Q, Li X, Huang X, Wan L, song W, Li K, Lv C, Chen CM (2016) Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J Mater Chem A 4(5):1637–1646
Wu D, Cheng J, Wang T, Liu P, Yang L, Jia D (2019) A novel porous N- and S-self-doped carbon derived from chinese rice wine lees as high-performance electrode materials in a supercapacitor. ACS Sustain Chem Eng 7(14):12138–12147
Zhao G, Li Y, Zhu G, Shi J, Lu T, Pan L (2019) Biomass-based N, P, and S self-doped porous carbon for high-performance supercapacitors. ACS Sustain Chem Eng 7(14):12052–12060
Mao L, Zhang Y, Hu Y, Ho KH, Ke Q, Liu H, Hu Z, Zhao D, Wang J (2015) Activation of sucrose-derived carbon spheres for high-performance supercapacitor electrodes. RSC Adv 5(12):9307–9313
Wang J, Ding B, Xu Y, Shen L, Dui H, Zhang X (2015) Crumpled nitrogen-doped graphene for supercapacitors with high gravimetric and volumetric performances. ACS Appl Mater Interfaces 7(40):22284–22291
Maiti S, Das AK, Karan SK, Khatua BB (2015) Carbon nanohorn-graphene nanoplate hybrid: an excellent electrode material for supercapacitor application. J Appl Polym Sci 132(25):42118
Fan LQ, Zhong J, Wu JH, Lin JM, Huang YF (2014) Improving the energy density of quasi-solid-state electric double-layer capacitors by introducing redox additives into gel polymer electrolytes. J Mater Chem A 2(24):9011–9014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, T.N., Le, P.A. & Phung, V.B.T. Facile green synthesis of carbon quantum dots and biomass-derived activated carbon from banana peels: synthesis and investigation. Biomass Conv. Bioref. 12, 2407–2416 (2022). https://doi.org/10.1007/s13399-020-00839-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00839-2