Abstract
Carbon dots (CDs), with high photostability, tunable excitation and emission wavelength, excellent biocompatibility, and environmental friendliness, attract great interest because of their promising applications in various fields. The synthesis of CDs from renewable biomass is especially attractive due to its sustainable and cost-efficient feature. However, one of the major problems for this route is the low yield of CDs. To overcome this issue, we propose a facile, universal, and highly effective synthesis method to prepare fluorescent CDs from various biomass hydrothermal carbons (HTCs). As compared with the current methods for synthesizing biomass derived CDs, our approach offers ultrahigh yield (42.5% based on the weight of biomass and 99.0% based on the weight of HTC) that is much higher than those obtained from other methods. The physiochemical properties of the as-prepared CDs can be tuned by reaction temperature. Furthermore, the PL quantum yield is high up to 16.6%, and is correlated with the surface chemical groups and conjugated π-domains of the carbon core in CDs. The fluorescence of CDs is effectively and selectively quenched by Cu2+, which enables CDs applying as fluorescent Cu2+ nanoprobe with a linear range of 0–30 μmol L−1 and a detection limit of 85 nmol L−1. Due to simple operation, mild condition, and low cost of the process, this method have a great potential in the large-scale synthesis and application of CDs from biomass.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon dots (CDs), the quasi-spherical nanoparticles with size less than 10 nm, are a novel class of nano carbons. Owing to their excellent biological properties and photochemical performances, rich photoluminescence (PL), and tunable surface chemistry, CDs attract great interest in bioimaging, biosensing, optronics, catalysis, and energy devices (solar cells, batteries and super-capacitors) (Li et al. 2013a; Ran et al. 2013; Yuan et al. 2017; Zhu et al. 2013). CDs are classified into several categories, namely, tiny sized carbon nanoparticles, graphene quantum dots, graphitic carbon quantum dots, amorphous carbon dots, and polymer dots (Gao et al. 2017). Correspondingly, the synthetic methods for CDs are generally divided into top-down and bottom-up approaches. Top-down method including laser ablation (Hu et al. 2009), acid hydrolysis (Ye et al. 2013), discharge (Bao et al. 2011), and electrochemical oxidation (Zhao et al. 2008), usually produces CDs from bulk carbon materials, such as graphite, CNTs, and carbon black. However, this method generally requires expensive equipment and materials, as well as harsh conditions. Bottom-up method, synthesizing CDs from abundant and low-cost organic molecules, involves hydrothermal (Zhu et al. 2013), microwave pyrolysis (Jaiswal et al. 2012; Yuan et al. 2018), and solvothermal (Yuan et al. 2017), exhibiting low cost, eco-friendliness, and easy chemical modification.
Hydrothermal synthesis is widely adopted due to the green process, easy operation, and mild reaction conditions (Li et al. 2013b; Wang et al. 2014a; Wang and Hu 2014). The properties of prepared CDs mainly depend on the types of precursors such as small molecules, synthetic polymers, and biomass (He et al. 2011; Li et al. 2015; Liu et al. 2018a, b; Xiong et al. 2017). Up to now, great efforts have been made to develop green CDs through hydrothermally treating various biomass resources containing paper ash (Wei et al. 2013), willow bark (Qin et al. 2013), wheat straw (Yuan et al. 2015), grass (Liu et al. 2012), protein (Wu et al. 2013; Zhang et al. 2012b), chitosan (Wang et al. 2016a), fiber (Yang et al. 2016), and lignin (Chen et al. 2016). However, the production yields are low. For example, Chen et al. (2016) prepared lignin-derived CDs with low yield of 0.8–12.06 wt% through hydrothermal treatment. Yang et al. (2012) synthesized fluorescent carbon nanoparticles by hydrothermally carbonizing chitosan with a low yield of 7.8%. In fact, most of biomass is converted into solid carbon product (hydrothermal carbon) during hydrothermal process, instead of CDs in the suspension.
In this paper, a universal, low-cost, and high-efficiency route was developed for high-yield synthesis of fluorescent CDs from HTC, which is completely different from the traditional method to obtain CDs from hydrothermal suspension. It is found that HTC can be transferred into CDs by low-concentration sodium hydroxide solution and oxygen treatment. The yields of CDs are extremely high (67–99%), which is much higher than those of CDs from the hydrothermal suspension. The as-prepared CDs show excellent photoluminescence properties with high quantum yield (QY), excitation-dependent PL behaviour, up-conversion properties, and long-term photostability. Furthermore, the CDs are used as a fluorescent sensing material for selective detection of Cu2+. This new synthetic method is also highly effective for various biomass resources including hemicelluloses, lignin, cellulose, and chitosan, and thus opens up new way for the operable and highly value-added application of HTC and the industrial synthesis and application of CDs.
Materials and methods
Materials
Hemicelluloses, lignin, chitosan, and α-cellulose were purchased from Aladdin (China). Sodium hydroxide (ACS, 97%) and hydrochloric acid (36–38%) were from Guangzhou Chemical Reagent (China). FeCl3, FeCl2, KCl, MgCl2, CuCl2, CoCl2, BaCl2, ZnCl2, NiCl2, CrCl3, AlCl3, and CaCl2 were purchased from Tianjin Damao Chemical Reagent (China). Quinine sulfate dehydrate (AR, 99.0%, Biotech) was purchased from Aladdin (China). All the reagents were of analytical grade and used directly without any further purification.
Preparation of hydrothermal carbon (HTC)
In a typical preparation, 1.5 g biomass (hemicelluloses, lignin, chitosan, and cellulose) was dispersed in ultrapure water (30 mL) by stirring. The mixture was transferred to stainless steel autoclaves (SLM-50). Then the chamber was put into a furnace after sealing. The hydrothermal reaction was carried out in the furnace under 600 rpm at 240 °C for 6 h. After cooling to room temperature, the hydrothermal carbon was collected by vacuum filtering with a 0.22 μm microporous membrane and thoroughly washed with deionized water, and then dried in a vacuum oven at 80 °C overnight.
Synthesis of CDs
50 mg of the resultant hydrothermal carbon was added into 50 mL Teflon-lined stainless-steel autoclave (SLM-50) with 25 mL of 0.1 M NaOH. The chamber was sealed, and then O2 was blown into the autoclave to remove air and keep the pressure at 1 MPa. After that, the autoclave was heated at 160 °C (or 100 °C, 120 °C, 140 °C, and 180 °C) for 1 h with stirring (1000 rpm). After being naturally cooled to room temperature, the obtained yellow solution containing water-dispersion CDs was neutralized by HCl and filtered through a 0.22 μm microporous membrane. Then, the filtrate was dialyzed in a dialysis bag (retaining molecular weight: 500 Da) in deionized water for 5 days. Finally, the solution was freeze-dried to obtain the purified CDs for characterization and property measurements. Samples denoted as CDs100, CDs120, CDs140, CDs160 and CDs180 were synthesized at 100 °C, 120 °C, 140 °C, 160 °C and 180 °C, respectively.
Characterization
Transmission electron microscopy (TEM) and the high-resolution transmission electron microscopy (HR-TEM) images were obtained using a JEM-2100F at 200 kV. Samples for TEM measurements were prepared by placing a drop of the colloidal solution of CDs on a carbon-coated copper grid and then dried at room temperature. The morphologies of the HTCs were observed using scanning electron microscopy (SEM, ZEISS Merlin, Germany). X-ray diffraction (XRD) patterns were monitored by a Bruker D8 diffractometer using Cu Kα radiation (λ = 0.15418 nm) as an X-ray source. Raman spectra were recorded by a Raman spectrometer (LabRAM ARAMIS – Horiba Jobin–Yvon, France) operating with 532 nm laser. X-ray photoelectron spectra (XPS) were recorded by X-ray Photoelectron Spectrometer (K-Alpha+, Thermo fisher Scientific) with an exciting source of Al·Kα (1486.6 eV, 6 mA × 12 kV). The FT-IR spectra were recorded by a FT-IR spectrophotometer (TENSOR27, Germany). The tested sample was obtained by mixing CDs and KBr power and pressing. Elemental analysis was carried out by using a Vario EL cube instrument (Elementar, Geman), and C and H contents were tested. Solid-state cross polarization/magic angle spinning (CP MAS) 13C NMR experiments was performed on a Bruker Avance III 400 MHz (9.4 T) spectrometer with a 4 mm zirconia rotor as sample holder spinning at MAS rate of 5 kHz. The chemical shift reference for 13C was tetramethylsilane at 0 ppm. UV–visible absorption spectra of CDs were recorded by using a South Korea S3100 UV–Vis spectrophotometer. Fluorescence spectra were recorded by the HORIBA Scientific FluoroMax-4 fluorescence spectrophotometer.
Fluorescence QY measurement
Quantum yield was measured according to the established procedure by using quinine sulfate in 0.10 M H2SO4 solution as a reference. The quinine sulfate (QR = 0.54) dissolved in 0.1 M H2SO4 (refractive index η = 1.33) and CDs were mixed in deionized water (η = 1.33). The absorbance was measured by a South Korea S3100 UV–vis spectrophotometer. The PL emission intensity was measured by PL spectrometer (HORIBA Scientific FluoroMax-4) at 350 nm excitation wavelength. The quantum yield was calculated using the following equation:
where Q is the quantum yield, I is the measured integrated emission intensity, A is the absorbance intensity and η is the refractive index of the respective solvents. The subscript R refers to the reference fluorophore (quinine sulfate). In order to minimize re-absorption effects, the absorbance in the 10 mm fluorescence cuvette were kept under 0.05 at the excitation wavelength of 350 nm.
Photostability and pH stability of CDs solution
The CDs solution was exposed under a 300 W xenon (Xe) lamp (Ceaulight, CEL-HXF300) for various time spans to investigate their photostability. HCl (0.1 M) or NaOH (0.1 M) was used to adjust the pH of the aqueous solution of resultant CDs.
Procedure for Cu2+detection
Typically, 3 mL CDs solution (0.01 mg/mL) and 1 mL Cu2+ solution with different concentrations (20–2000 μM) were mixed and shaken at room temperature for 5 min. The fluorescence quenching spectra were immediately recorded at an excitation wavelength of 360 nm. To evaluate the detection selectivity, various metal ions such as K+, Al3+, Mg2+, Ca2+, Cr3+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, and Ba2+ were individually added into CDs solutions.
Results and discussion
Synthesis of CDs
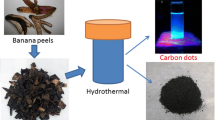
As shown in Fig. 1, the various biomass including hemicelluloses, lignin, cellulose and chitosan were hydrothermally treated for producing a small amount of CDs in supernatant and more HTCs. Then, the green fluorescent CDs were prepared from biomass-HTCs via a mild treatment of NaOH/O2 solution in this experiment. In the process of hydrothermal treatment, the biopolymers are hydrolyzed and dehydrated into various small molecules including furfural aldehydes, ketones, and formic acid. The subsequent polymerization and condensation among these compounds produce the black solid product (HTC, Fig. 1) (De and Karak 2013; Sevilla and Fuertes 2009). Firstly, we used hemicelluloses as a biomass precursor to optimize the synthesis process. The SEM image in Fig. 2a shows that the HTC from hemicelluloses is composed of spherical particles with a narrow size distribution (~ 1.2 um). Then, the resultant HTC was quickly disintegrated into CDs with intensive fluorescence emission by NaOH/O2 solution treatment for 0.5–1.0 h. The conversion rate of CDs from the HTC is ultrahigh, ca. 73.5% (CDs160, 160 °C, 1.0 h) based on the weight of HTC. Therefore, NaOH/O2 treatment of HTC is a highly effective strategy to obtain CDs.
The typical TEM images (Fig. 2b, c) show that the CDs160 is uniform with a narrow size distribution of 1.6–4.1 nm in diameter (average diameter of 2.9 nm, Fig. 2d). HR-TEM (Fig. 2c) reveals clear lattice fringes of CDs with a spacing of 0.21 nm, which can be attributed to the (100) diffraction planes of graphite (Zhang et al. 2012a). The XRD patterns shown in Fig. 2e demonstrate the graphic carbon of CDs160 with wide peak centered at 2θ = 24.9°, while a broad peak at 2θ = 21.5° is corresponding to the (002) peak in the patterns of HTC, indicating a high content of amorphous structure. The shifting peak correlates with the crystal transition from amorphous carbon to graphic carbon. However, the G band and D band representing graphic and amorphous carbon in Raman spectra is weak (Fig. S1, EMS) due to the fluorescent interference of CDs.
The FT-IR of CDs160 and HTC are showed in Fig. 2f. The strong band at 3437 cm−1 indicates the presence of –OH groups. The peaks at 2927 and 1384 cm−1 are attributed to the stretching and bending vibrations of C–H. The strong peaks at 1590 and 1450 cm−1 are ascribed to the stretching vibration of aromatic C=C. The peaks at 870 and 762 cm−1 are related to the in-plane and out-plane deformations of aromatic C–H (Wang et al. 2011). Therefore, the CDs and HTC contain aromatic skeletons, revealing that aromatization takes places in hydrothermal carbonization. The aromatic structure is also a typical unit exists in lignin from pulp fibers. NaOH/O2 mixture is usually used in pulp bleaching for decomposing and removing lignin. Here, NaOH/O2 solution is used to decompose HTC into pieces and finally obtained CDs. The CDs160 shows no peak at 1261 cm−1 (C–O–C) but a new peak at 1760 cm−1 (C=O), indicating the ether linkage is broken and more oxygen-containing functional groups (carboxyl, ester, or carbonyl) are formed during the NaOH/O2 treatment of HTC. The apparent reduce of peak at 2927 cm−1 also shows the decrease of alkyl group in side chain after the oxidation. The elemental analysis (Table 1) demonstrates a significant decrease in carbon content from 72.7 to 37.4% and an increase in oxygen content from 21.9 to 58.6%, when the HTC was converted to CDs160.
The XPS survey spectra of HTC and CDs (Fig. 3a and 3d) show two typical peaks at 284.7 eV for C 1s and 531.4 eV for O 1s. The C/O atomic ratios of HTC and CDs160 are 1.53 and 0.62, respectively (Table S1, EMS). Thus, the elemental and XPS analysis again confirm the cleavage of ether bonds and oxidation of hydroxyl. The de-convolved high-resolution C1s spectra (Fig. 3b, e) show three C-types including C–C/C=C (284.8 eV), C–O (286.5 eV), and C=O (288.3 eV) (Qian et al. 2014). As shown in Table S1 (EMS), the atomic percentage of C–O for CDs160 (8.88%) is lower than that of HTC (13.18%), indicating that ether cleavage occurs during the treatment of NaOH/O2. And the high atomic percentage of C=O for CDs160 (18.86%) indicates that the oxygen functional groups like aldehyde, ketone, ether and carboxyl are formed. In the O1s spectra (Fig. 3c, f), two peaks at 531.5 eV and 533.0 eV are attributed to C=O and C–O groups respectively (Liu et al. 2017). These results also indicate that the ether linkages and hydroxyls are partially oxidized by NaOH/O2 and then produce oxygen-containing groups.
In order to clarify the formation mechanism of CDs in the hydrothermal process of NaOH/O2, the evolution of carbon chemical environment was further studied by 13C CP MAS NMR (Fig. 4). The peaks in 10–50, 127–160, and 170–210 ppm are attributed to sp3 C–H, sp2 carbon resonate of aromatic carbon, and carbonyl carbon, respectively (Baccile et al. 2009; Falco et al. 2011). The distinct peak at 128 ppm is assigned to carbon atoms in aromatic rings, indicating the existence of aromatic structure in both HTC and CDs (Titirici et al. 2008). After NaOH/O2 treatment, peaks at 158 ppm (O–C=CH) and 208 ppm (C=O group in ketones and aldehydes) decline apparently, while the intensity of peak at 175 ppm (C=O group in carboxylic acid) in the oxidized products (CDs) show a significant increase of (Baccile et al. 2009; Falco et al. 2011). In addition, FT-IR and XPS demonstrate the broken of ether linkage. Therefore, the unsaturated C=O and the C–O linked with unsaturated C=C are attacked during the NaOH/O2 treatment.
With molecular oxygen as an oxidant and sodium hydroxide as an active agent, the phenolic lignin containing side chain or ring conjugated carbonyl structure is effectively oxidized through nucleophilic or electrophilic reaction on the unsaturated carbonyl or carbanions. As shown in Fig. 2f, the peak at 1230 cm−1 attributed to C–O in phenol is tiny visible. Therefore, the phenolic structure containing side chain (linked by ether bond) or ring conjugated carbonyl structure in HTC can be oxidized in NaOH/O2 treatment. Then the HTC is decomposed into small pieces (i.e. CDs) with carboxylic acid on the surface. Moreover, the decrease intensity of peaks in region of 10–50 ppm (C–H) reveals the break of side chain, which is also found in FT-IR analysis.
Luminescence properties of CDs
The as-synthesized CDs160 dispersion exhibits bright blue-green fluorescence under irradiation with 365 nm UV light (Fig. 5a). In addition, the absorption spectra of CDs160 show broad absorption bands at 218 nm and 261 nm. Combining results from XRD (graphic carbon), FT-IR (rich C=C), and XPS (rich C=O groups), the absorption bands can be attributed to the E-band and B-band of the π–π* transition of the well-defined conjugate aromatic structures in the carbon core, respectively (Liu et al. 2017). The result indicates that CDs160 has electronic states with a well-defined conjugate aromatic structure, giving rise to the large energy band (Thiyagarajan et al. 2016).
a UV–vis absorption of CDs160. Inset: the photograph of the CDs160 suspension under sunlight (left) and UV light of 365 nm (right). b PL emission spectra excited by various incident lights progressively from 320 to 400 nm with an increment of 20 nm. c PL excitation spectra excited by various incident lights progressively from 400 to 500 nm with an increment of 20 nm. d Up-conversion PL emission spectra of CDs160 excited by various incident lights progressively from 760 to 940 nm with an increment of 60 nm. e Photostability test of the CDs160 suspension under continuous irradiation with Xe light. f pH-dependent PL at pH = 2–12 for CDs160
Furthermore, CDs160 shows a typical excitation-dependent fluorescence property (Fig. 5b). The emission peak gradually red-shifts from 443 to 489 nm when the excitation wavelength increases from 320 to 400 nm. The maximum emission wavelength locates at 460 nm under the excitation of 360 nm. The excitation-dependent behavior is usually attributed to the size distribution and surface chemistry, or different emissive traps (salvation effect) (Zhu et al. 2015). Due to uniform size of CDs160, the excitation-dependent behavior of CDs160 may result from the defects produced by the oxygen functional groups on the surface. CDs160 also shows emission-dependent PL behavior, which means that the excitation peak gradually red-shifts from 342 to 379 nm when the emission wavelength increases from 400 to 500 nm with an increment of 20 nm (Fig. 5c). The strongest PL excitation peak appears at 360 nm under 460 nm emission, which is consistent with the PL emission spectrum with a strong emission peak at 460 nm (Fig. 5b). Furthermore, CDs160 exhibits obvious up-conversion emission (anti-Stokes type emission) under the wavelength from 760 to 940 nm, as shown in Fig. 5d. The up-conversion PL behavior can be attributed to the simultaneous absorption of two or more photons (multi-photon activation process).
The stability of luminescent nanomaterials is an important factor to assess their applications. It is found that the fluorescence intensity of CDs160 only slightly decreases to 97.7% of its initial intensity after 11 h irradiation, indicating that CDs160 has excellent photo stability (Fig. 5e). Furthermore, the pH-stability was also investigated in a wide range of pH values (Fig. 5f). CDs160 exhibits a pH-dependent PL behavior. The intensity of blue-green emission only slightly decreases at high and low pH values, but stays steady in pH ranging from 3 to 9. The effect of pH on the emission stability of CDs160 is contributed to the protonation/deprotonation of carboxyl groups, which is also observed for other carboxyl-containing CDs (Ding et al. 2016; Wang et al. 2014b).
The effect of decomposition temperature on the yield and fluorescence of CDs
In order to investigate the effect of synthesis conditions on the yield and fluorescence of CDs, the reaction time, temperature, and sodium hydroxide concentration were optimized (hemicelluloses derived HTC as a representative). As shown in Table 1, reaction time and sodium hydroxide concentration have little effect on the yield and fluorescence of CDs. On the contrary, reaction temperature plays an important role in synthesizing CDs. The yield of CDs is high up to 94.4% (based on HTC) at 100 °C, and decreases to 63.6% when temperature increasing to 180 °C. The QY of CDs, however, significantly increases from 2.2 to 16.6% when temperature rises from 100 to 180 °C. The elemental content (Table 1) indicates that CDs has a higher content of oxygen and lower content of carbon with increasing decomposing temperature.
The results of XPS survey spectra (Fig. S2 and Table S2, EMS) show that a higher temperature (180 °C, CDs180) leads to decrease in C–C/C=C percentage but increase in C=O percentage, suggesting that C–C/C=C in CDs are partly broken at high temperature, producing more oxygen-containing groups (C=O) on the surface of CDs. Therefore, the tunable PL QY might be attributed to the surface states of CDs that are sensitive to temperature. A yield high up to 73.5% based on the weight of HTC (22.7% based on the weight of biomass) is superior to those of CDs from other methods including hydrothermal treatment (Alam et al. 2015), microwave (Huang et al. 2017), acid oxidation (Ding et al. 2018), and carbonization (Hsu et al. 2012; Wang et al. 2016b, 2017), as shown in Table 2. Furthermore, the QY of 16.6% is also higher than or comparable to those of biomass-derived CDs from other methods (Table 2).
The chemical structures of these CDs are further confirmed by the FT-IR spectra (Fig. S3, EMS). With the increase of temperature, the bending vibration of C–H bond at 1380 cm−1 becomes weaker, while the vibration peak of aromatic skeleton at 1440 cm−1 is more significant, revealing the increase of conjugate carbon (–C=C–) in the carbon core. The peak at 2θ = 23.9° in the XRD pattern of CDs100 gradually moves to higher degree with higher temperature (Fig. S4, EMS), suggesting that the carbon core is more regular and compact with raising temperature. Therefore, the increasing QY of CDs with rising reaction temperature is attributed to larger unitary emission center caused by combination of oxygen-containing groups (C=O) and conjugated carbon atoms (Hu et al. 2015).
The average sizes are 2.3 nm, 1.3 nm, 1.9 nm, and 2.4 nm for CDs100, CDs120, CDs140, and CDs180, respectively, as shown in Fig. 6a–d. The PL spectra of CDs100, CDs120, CDs140, and CDs180 all show typical excitation-dependent fluorescence property (Fig. 6e–h). The peak of PL emission does not show obvious shift when the size of the CDs changes from 1.3 to 2.4 nm (Fig. S5), indicating that the origin of blue-green emission is attributed to the special edge states formed from hybridization of the carbon backbone, the carboxyl and carbonyl groups (C=O) at the edge of the CDs (Wang et al. 2014b) rather than the quantumn size effect (Li et al. 2010). A larger unitary emission center with growing temperature results in higher PL strength but no significant change in the emission peak positions of PL, due to the unaltered emission center of these CDs.
Detection of Cu2+
Figure 7a displays the fluorescence intensities of the CDs160 solutions with different metal ions at 460 nm. Cu2+ could quench the fluorescence of CDs more effectively as compared with other metal ions. The fluorescence emission spectra of CDs at different Cu2+ concentrations are shown in Fig. 7b. Upon excitation at 360 nm, the emission intensity decreases with increasing concentration of Cu2+. Efficient fluorescence quench at a low Cu2+ concentration can still be observed. Moreover, no significant change in the peak wavelength or shape of the emission spectrum is found, suggesting a strong interaction between Cu2+ and surface functionalities (hydroxyl or carboxyl groups) on the CDs (Zhang et al. 2019). The fluorescence emission intensity of CDs-Cu2+ system decreases monotonously with an increase in the concentration of Cu2+ within 400 μmol L−1 (Fig. 7c). The effect of Cu2+ on the CDs fluorescence intensity was evaluated and a linear correlation was obtained with an equation of, I0/I = 1.0438 + 0.0651[Cu2+] at the range of 5–30 μM. The fluorescence quench can be modeled by a Stern–Volmer equation (I0/I = 1 + KSV[Cu2+]), with KSV = 65100 M−1 and r = 0.984 (the inset in Fig. 7). The detection limit for Cu2+ was as low as 85 nM based on three times of the standard deviation rule (LOD = 3σ/k, n = 10), satisfying the sensitivity requirement of Cu2+ detection for drinking water (20 μM) (Fitzgerald 1998). These results imply that the CDs fluorescence probe has potential application in Cu2+ detection.
a The difference in PL intensity of CDs160 dispersion between the blank and solutions containing different metal ions (excitation at 360 nm; [Mn+] = 100 μM, fluorescence intensities of CDs160 were recorded at 460 nm in different ions). b PL spectra of CDs160 dispersion in the presence of different Cu2+concentrations (from top to bottom: 0–450 μM). c Stern–Volmer plot for the quenching of CDs160 PL by Cu2+ (excitation at 360 nm; I0 and I are CDs160 fluorescence intensities at 460 nm in the absence and presence of Cu2+, respectively
Our approach can also be applied in synthesizing CDs from various biomass resources with both high yield and QY. For example, the CDs were prepared from HTC (240 °C, 6 h) derived from lignin, cellulose, and chitosan at 160 °C for 1 h. As shown in Fig. 8, these HTCs (Fig. 8a–c) with spherical particles or schistose and fragmentary structure are decomposed to uniformly-dispersed CDs with size of about 2 nm and clear lattice fringes (Fig. 8d–f). Figure 8g–i show that the PL emission spectra of CDs is typical excitation-dependent. Fig. S6 (EMS) shows the UV–Vis absorbance of these CDs dispersions. The broad absorption bands at 200–300 nm are attributed to π–π* transition of the carbon core and n–π* transition of C=O. The QYs of CDs from lignin, cellulose, and chitosan derived carbons are 10.0%, 13.4% and 11.7%, with yields of 67.0%, 90.2%, and 99.0% based on the weight of HTC (42.5%, 34.0%, and 33.5% based on the weight of biomass, Table 2), respectively. Therefore, this approach is effective and universal for large-scaly producing CDs from various biomass resources.
Conclusion
In summary, we develop an environment-friendly, simple, universal, and highly-effective route for high-yield production of fluorescent CDs from low-cost and renewable biomass hydrothermal carbons with low-concentration NaOH/O2 solution. As compared with the current methods, our approach offers ultrahigh CDs yields for various biomass resources. The as-prepared CDs exhibit excellent optical properties, including high QY, up-conversion performance, and photostability. The QY of CDs, determined by the surface states and conjugated structure of the carbon core, can be easily tailored by changing the reaction temperature. The CDs can be used as a fluorescence probe for Cu2+ detection with low concentration. Thus, our work provides a new way for the low-cost and green synthesis of CDs from renewable resources.
References
Alam AM, Park BY, Ghouri ZK, Park M, Kim HY (2015) Synthesis of carbon quantum dots from cabbage with down- and up-conversion photoluminescence properties: excellent imaging agent for biomedical applications. Green Chem 17:3791–3797. https://doi.org/10.1039/c5gc00686d
Baccile N, Laurent G, Babonneau F, Fayon F, Titirici M, Antonietti M (2009) Structural characterization of hydrothermal carbon spheres by advanced solid-state MAS 13C NMR investigations. J Phys Chem C 113:9644–9654. https://doi.org/10.1021/jp901582x
Bao L, Zhang ZL, Tian ZQ, Zhang L, Liu C, Lin Y, Qi B, Pang DW (2011) Electrochemical tuning of luminescent carbon nanodots: from preparation to luminescence mechanism. Adv Mater 23:5801–5806. https://doi.org/10.1002/adma.201102866
Chen W, Hu C, Yang Y, Cui J, Liu Y (2016) Rapid synthesis of carbon dots by hydrothermal treatment of lignin. Materials 9:184. https://doi.org/10.3390/ma9030184
De B, Karak N (2013) A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana Juice. RSC Adv 3:8286–8290. https://doi.org/10.1039/c3ra00088e
Ding H, Yu SB, Wei JS, Xiong HM (2016) Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 10:484–491. https://doi.org/10.1021/acsnano.5b05406
Ding Z, Li F, Wen J, Wang X, Sun R (2018) Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem 20(6):1383–1390. https://doi.org/10.1039/c7gc03218h
Falco C, Baccile N, Titirici MM (2011) Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem 13:3273–3281. https://doi.org/10.1039/C1GC15742F
Fitzgerald DJ (1998) Safety guidelines for copper in water. Am J Clin Nutr 67:1098S–1102S. https://doi.org/10.1093/ajcn/67.5.1098S
Gao J, Zhu M, Huang H, Liu Y, Kang Z (2017) Advances, challenges and promises of carbon dots. Inorg Chem Front 4:1963–1986. https://doi.org/10.1039/c7qi00614d
He X, Li H, Liu Y, Huang H, Kang Z, Lee ST (2011) Water soluble carbon nanoparticles: hydrothermal synthesis and excellent photoluminescence properties. Colloids Surf B Biointerfaces 87:326–332. https://doi.org/10.1016/j.colsurfb.2011.05.036
Hsu PC, Shih ZY, Lee CH, Chang HT (2012) Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem 14:917–920. https://doi.org/10.1039/c2gc16451e
Hu SL, Niu KY, Sun J, Yang J, Zhao NQ, Du XW (2009) One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J Mater Chem 19:484–488. https://doi.org/10.1039/b812943f
Hu Y, Yang J, Jia L, Yu JS (2015) Ethanol in aqueous hydrogen peroxide solution: hydrothermal synthesis of highly photoluminescent carbon dots as multifunctional nanosensors. Carbon 93:999–1007. https://doi.org/10.1016/j.carbon.2015.06.018
Huang Q, Lin X, Zhu JJ, Tong QX (2017) Pd-Au@carbon dots nanocomposite: facile synthesis and application as an ultrasensitive electrochemical biosensor for determination of colitoxin DNA in human serum. Biosens Bioelectron 94:507–512. https://doi.org/10.1016/j.bios.2017.03.048
Jaiswal A, Ghosh SS, Chattopadhyay A (2012) One step synthesis of C-dots by microwave mediated caramelization of poly(ethylene glycol). Chem Commun 48:407–409. https://doi.org/10.1039/c1cc15988g
Li H, He X, Kang Z, Huang H, Liu J, Lian S, Tsang CHA, Yang X, Lee ST (2010) Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew Chem Int Ed 49:4430–4434. https://doi.org/10.1002/anie.200906154
Li H, Liu R, Lian S, Liu Y, Huang H, Kang Z (2013a) Near-infrared light controlled photocatalytic activity of carbon quantum dots for highly selective oxidation reaction. Nanoscale 5:3289–3297. https://doi.org/10.1039/c3nr00092c
Li W, Zhang Z, Kong B, Feng S, Wang J, Wang L, Yang J, Zhang F, Wu P, Zhao D (2013b) Simple and green synthesis of nitrogen-doped photoluminescent carbonaceous nanospheres for bioimaging. Angew Chem Intl Ed 52:8151–8155. https://doi.org/10.1002/anie.201303927
Li H, Liu J, Guo S, Zhang Y, Huang H, Liu Y, Kang Z (2015) Carbon dots from PEG for highly sensitive detection of levodopa. J Mater Chem B 3:2378–2387. https://doi.org/10.1039/c4tb01983k
Liu S, Tian J, Wang L, Zhang Y, Qin X, Luo Y, Asiri AM, Al-Youbi AO, Sun X (2012) Hydrothermal treatment of grass: a low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv Mater 24:2037–2041. https://doi.org/10.1002/adma.201200164
Liu W, Li C, Sun X, Pan W, Yu G, Wang J (2017) Highly crystalline carbon dots from fresh tomato: UV emission and quantum confinement. Nanotechnology 28:485705. https://doi.org/10.1088/1361-6528/aa900b
Liu J, Li D, Zhang K, Yang M, Sun H, Yang B (2018a) One-step hydrothermal synthesis of nitrogen-doped conjugated carbonized polymer dots with 31% efficient red emission for in vivo imaging. Small 14:1703919. https://doi.org/10.1002/smll.201703919
Liu W, Xu S, Guan S, Liang R, Wei M, Evans DG, Duan X (2018b) Confined synthesis of carbon nitride in a layered host matrix with unprecedented solid-state quantum yield and stability. Adv Mater 30:1704376. https://doi.org/10.1002/adma.201704376
Mehta VN, Jha S, Basu H, Singhal RK, Kailasa SK (2015) One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens Actuators B Chem 213:434–443. https://doi.org/10.1016/j.snb.2015.02.104
Ng YH, Chin SF, Pang SC, Ng SM (2017) The luminescence profile of carbon dots synthesized from α-cellulose under different acid hydrolysis conditions. Opt Mater 70:50–56. https://doi.org/10.1016/j.optmat.2017.05.014
Peng H, Travas-Sejdic J (2009) Simple aqueous solution route to luminescent carbogenic dots from carbohydrates. Chem Mater 21:5563–5565. https://doi.org/10.1021/cm901593y
Qian Z, Ma J, Shan X, Feng H, Shao L, Chen J (2014) Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform. Chem Eur J 20:2254–2263. https://doi.org/10.1002/chem.201304374
Qin X, Lu W, Asiri AM, Al-Youbi AO, Sun X (2013) Green, low-cost synthesis of photoluminescent carbon dots by hydrothermal treatment of willow bark and their application as an effective photocatalyst for fabricating Au nanoparticles–reduced graphene oxide nanocomposites for glucose detection. Catal Sci Technol 3:1027–1035. https://doi.org/10.1039/c2cy20635h
Ran X, Sun H, Pu F, Ren J, Qu X (2013) Ag nanoparticle-decorated graphene quantum dots for label-free, rapid and sensitive detection of Ag+ and biothiols. Chem Commun 49:1079–1081. https://doi.org/10.1039/c2cc38403e
Sevilla M, Fuertes AB (2009) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47:2281–2289. https://doi.org/10.1016/j.carbon.2009.04.026
Shen J, Shang S, Chen X, Wang D, Cai Y (2017) Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater Sci Eng C Mater Biol Appl 76:856–864. https://doi.org/10.1016/j.msec.2017.03.178
Shi L, Li Y, Li X, Wen X, Zhang G, Yang J, Dong C, Shuang S (2015) Facile and eco-friendly synthesis of green fluorescent carbon nanodots for applications in bioimaging, patterning and staining. Nanoscale 7:7394–7401. https://doi.org/10.1039/c5nr00783f
Suryawanshi A, Biswal M, Mhamane D, Gokhale R, Patil S, Guin D, Ogale S (2014) Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on-off-on probe for Ag+ ions. Nanoscale 6:11664–11670. https://doi.org/10.1039/c4nr02494j
Thiyagarajan SK, Raghupathy S, Palanivel D, Raji K, Ramamurthy P (2016) Fluorescent carbon nano dots from lignite: unveiling the impeccable evidence for quantum confinement. Phys Chem Chem Phys 18:12065–12073. https://doi.org/10.1039/c6cp00867d
Titirici MM, Antonietti M, Baccile N (2008) Hydrothermal carbon from biomass: a comparison of the local structure from poly-to monosaccharides and pentoses/hexoses. Green Chem 10:1204–1212. https://doi.org/10.1039/B807009A
Wang Y, Hu A (2014) Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2:6921–6939. https://doi.org/10.1039/c4tc00988f
Wang X, Qu K, Xu B, Ren J, Qu X (2011) Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J Mater Chem 21:2445–2450. https://doi.org/10.1039/c0jm02963g
Wang L, Wang Y, Xu T, Liao H, Yao C, Liu Y, Li Z, Chen Z, Pan D, Sun L, Wu M (2014a) Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat Commun 5:5357. https://doi.org/10.1038/ncomms6357
Wang L, Zhu SJ, Wang HY, Qu SN, Zhang YL, Zhang JH, Chen QD, Xu HL, Han W, Yang B, Sun HB (2014b) Common origin of green luminescence in carbon nanodots and graphene quantum dots. ACS Nano 8:2541–2547. https://doi.org/10.1021/nn500368m
Wang C, Xu Z, Cheng H, Lin H, Humphrey MG, Zhang C (2015) A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature. Carbon 82:87–95. https://doi.org/10.1016/j.carbon.2014.10.035
Wang L, Li B, Xu F, Shi X, Feng D, Wei D, Li Y, Feng Y, Wang Y, Jia D, Zhou Y (2016a) High-yield synthesis of strong photoluminescent N-doped carbon nanodots derived from hydrosoluble chitosan for mercury ion sensing via smartphone APP. Biosens Bioelectron 79:1–8. https://doi.org/10.1016/j.bios.2015.11.085
Wang Z, Yu J, Zhang X, Li N, Liu B, Li Y, Wang Y, Wang W, Li Y, Zhang L, Dissanayake S, Suib SL, Sun L (2016b) Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: a comprehensive utilization strategy. ACS Appl Mater Interfaces 8:1434–1439. https://doi.org/10.1021/acsami.5b10660
Wang Z, Liu J, Wang W, Wei Z, Wang F, Gong P, Wang J, Li N, Liu B, Zhang Z, Wang W, Sun L (2017) Photoluminescent carbon quantum dot grafted silica nanoparticles directly synthesized from rice husk biomass. J Mater Chem B 5:4679–4689. https://doi.org/10.1039/c7tb00811b
Wei J, Shen J, Zhang X, Guo S, Pan J, Hou X, Zhang H, Wang L, Feng B (2013) Simple one-step synthesis of water-soluble fluorescent carbon dots derived from paper ash. RSC Adv 3:13119–13122. https://doi.org/10.1039/c3ra41751d
Wu ZL, Zhang P, Gao MX, Liu CF, Wang W, Leng F, Huang CZ (2013) One-pot hydrothermal synthesis of highly luminescent nitrogen-doped amphoteric carbon dots for bioimaging from Bombyx mori silk—natural proteins. J Mater Chem B 1:2868–2873. https://doi.org/10.1039/c3tb20418a
Xiong Y, Schneider J, Reckmeier CJ, Huang H, Kasak P, Rogach AL (2017) Carbonization conditions influence the emission characteristics and the stability against photobleaching of nitrogen doped carbon dots. Nanoscale 9:11730–11738. https://doi.org/10.1039/c7nr03648e
Xu H, Yang X, Li G, Zhao C, Liao X (2015) Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples. J Agric Food Chem 63:6707–6714. https://doi.org/10.1021/acs.jafc.5b02319
Xu M, Huang Q, Sun R, Wang X (2016) Simultaneously obtaining fluorescent carbon dots and porous active carbon for supercapacitors from biomass. RSC Adv 6:88674–88682. https://doi.org/10.1039/c6ra18725k
Yang Y, Cui J, Zheng M, Hu C, Tan S, Xiao Y, Yang Q, Liu Y (2012) One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem Commun 48:380–382. https://doi.org/10.1039/c1cc15678k
Yang G, Wan X, Su Y, Zeng X, Tang J (2016) Acidophilic S-doped carbon quantum dots derived from cellulose fibers and their fluorescence sensing performance for metal ions in an extremely strong acid environment. J Mater Chem A 4:12841–12849. https://doi.org/10.1039/c6ta05943k
Ye R, Xiang C, Lin J, Peng Z, Huang K, Yan Z, Cook NP, Samuel ELG, Huang CC, Ruan G, Ceriotti G, Raji ARO, Martí AA, Tour JM (2013) Coal as an abundant source of graphene quantum dots. Nat Commun 4:2943. https://doi.org/10.1038/ncomms3943
Yuan M, Zhong R, Gao H, Li W, Yun X, Liu J, Zhao X, Zhao G, Zhang F (2015) One-step, green, and economic synthesis of water-soluble photoluminescent carbon dots by hydrothermal treatment of wheat straw, and their bio-applications in labeling, imaging, and sensing. Appl Surf Sci 355:1136–1144. https://doi.org/10.1016/j.apsusc.2015.07.095
Yuan F, Wang Z, Li X, Li Y, Tan Z, Fan L, Yang S (2017) Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv Mater 29:1604436. https://doi.org/10.1002/adma.201604436
Yuan JM, Zhao R, Wu ZJ, Li W, Yang XG (2018) Graphene oxide quantum dots exfoliated from carbon fibers by microwave irradiation: two photoluminescence centers and self-assembly behavior. Small 14:1703714. https://doi.org/10.1002/smll.201703714
Zhang YQ, Ma DK, Zhuang Y, Zhang X, Chen W, Hong LL, Yan QX, Yu K, Huang SM (2012a) One-pot synthesis of N-doped carbon dots with tunable luminescence properties. J Mater Chem 22:16714–16718. https://doi.org/10.1039/c2jm32973e
Zhang Z, Hao J, Zhang J, Zhang B, Tang J (2012b) Protein as the source for synthesizing fluorescent carbon dots by a one-pot hydrothermal route. RSC Adv 2:8599. https://doi.org/10.1039/c2ra21217j
Zhang DW, Papaioannou N, David NM, Luo H, Gao H, Tanase LC, Degousée T, Samori P, Sapelkin A, Fenwick O, Titirici MM, Krause S (2018) Photoelectrochemical response of carbon dots (CDs) derived from chitosan and their use in electrochemical imaging. Mater Horiz 5:423–428. https://doi.org/10.1039/c7mh00784a
Zhang Y, Gao Z, Yang X, Chang J, Liu Z, Jiang K (2019) Fish-scale-derived carbon dots as efficient fluorescent nanoprobes for detection of ferric ions. RSC Adv 9:940–949. https://doi.org/10.1039/c8ra09471c
Zhao QL, Zhang ZL, Huang BH, Peng J, Zhang M, Pang DW (2008) Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem Commun 41:5116–5118. https://doi.org/10.1039/b812420e
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed 52:3953–3957. https://doi.org/10.1002/anie.201300519
Zhu Y, Wu Z, Jing M, Hou H, Yang Y, Zhang Y, Yang X, Song W, Jia X, Ji X (2015) Porous NiCo2O4 spheres tuned through carbon quantum dots utilised as advanced materials for an asymmetric supercapacitor. J Mater Chem A 3:866–877. https://doi.org/10.1039/c4ta05507a
Acknowledgments
This work is supported by Fundamental Research Funds for the Central Universities (2019PY13), Guangdong Natural Science Funds for Distinguished Young Scholar (2017A030306029 and 2016A030306027), and Natural Science Foundation of Guangdong Province (2016A030313487).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Jing, S., Peng, X. et al. Synthesizing green carbon dots with exceptionally high yield from biomass hydrothermal carbon. Cellulose 27, 415–428 (2020). https://doi.org/10.1007/s10570-019-02807-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02807-0