Abstract
Macroalgae are considered to be one of the rich lignocellulosic biomass materials. Aquatic biomass has gained more attention to biofuels generation in recent years due to its renewable, abundant, and environmentally friendly aspects. Macroalgae are photosynthetic organisms that are found in both marine and freshwater environments. These are considered as a third-generation feedstock for the production of biofuels since they have the ability to synthesize a high amount of lipids, proteins, and carbohydrates. This research study aimed to evaluate the potential of bioethanol production from macroalgae (Rhizoclonium sp.) biomass. The fermentation process was applied in the research by two-way separate hydrolysis and fermentation (SHF). Algae biomass undergoes a pretreatment process to release necessary sugars for yeast digestion. The fermentation process was carried at 30 to 35 °C in the incubator. Finally, the percentage of ethanol was estimated by the ebulliometer. Fermentation was enhanced by immobilization of yeast, which showed the highest concentration of ethanol (65.43 ± 18.13 g/l) after 96 h of fermentation and can be reused for several times for fermentation. Moreover, these study results confirmed that freshwater macroalgae biomass is a suitable and susceptible raw material for bioethanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Renewable resources of energy are a part of the Asian region’s fight against climate changes, at the same time they contribute to economic growth, increasing the number of employed people and provide energetic safety [1]. The utilization of renewable energy is significantly increasing, together with energy security concerns, efforts to mitigate the environmental impact of fossil fuels, and upgrading in living standards and renewable technologies [2]. Bioethanol is a renewable liquid fuel that is expected to be most widely used around the globe as ethanol, which can be produced from abundant supplies of starch and cellulose biomass [3]. A large number of bioethanol production countries in the world are Brazil, the USA, and Canada. Ethanol fuel is another alternative that comes to use the power of the world in the areas of transport, vehicles, and various chemical components in the industry [4].
Ethanol can be produced from biological processes. They are arising from the plant by the fermentation process to convert polysaccharide starch into monosaccharide sugar. Furthermore, convert from sugar to alcohol, by using the enzyme, or some chemicals hydrolysis followed by fermentation [5]. Then, convert it to pure alcohol by distillation and separation of water. Their immobilization in an active state may increase the application of enzymes for industrial purposes. Enzyme immobilization offers technical and economic advantages, such as cost-reduction of biocatalysts (as they can be reused many times), easy separation from reaction mixtures, and the possibility of using higher enzyme activity per volume in the reactor, compared with soluble enzyme preparations [6], which can be widely used for the components and applications in various industries. Including the application was that it could be used as a mixed proportion additive for current fuel to reduce the imported oil from abroad.
The production of ethanol mostly depends on the primary raw material. These raw materials mainly contain sugars, such as sugarcane and molasses rich in sugar content. The other raw materials, such as cassava and corn powder, are considered as lignocellulosic biomass. However, the raw material is the used food crops to produce ethanol [7]. This may not be sufficient for ethanol production in the future. Therefore, it is interesting to bring other raw materials (i.e., lignocellulosic biomass) to produce ethanol. Lignocellulosic biomass was mainly available from agricultural waste such as rice straw, sugarcane bagasse, maize, bark, and weed, including algae, which are aquatic plants. This biomass can be hydrolyzed by several enzymes to produce fermentable sugars for subsequent biofuel production [8, 9].
Macroalgae, an abundant and carbon-neutral renewable resource, are now considered as third-generation biomass biofuel that can be used in bioenergy production. It grows in any aquatic environment and generates lignin-free biomass material [6]. Generally, macroalgae grow faster compared with terrestrial crops and do not compete with the agricultural land area for mass cultivation. Also, macroalgae contain high carbohydrate content, which is rich in polysaccharides and lignocellulosic biomass [10].
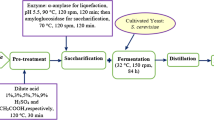
In general, bioethanol production from biomass involves the following steps such as pretreatment, enzymatic hydrolysis, fermentation, and distillation [11]. The saccharification of macroalgae is an essential step prior to ethanol fermentation. In the renewable energy sector, macroalgae are promising biomass to produce ethanol because the algae biomasses are containing enough nutrients such as in protein, lipid, and carbohydrates; these carbons can digest sugars and ferment them into ethanol. This study aimed for various physical, chemical, and biological pretreatments of macroalgae Rhizoclonium sp. to produce bioethanol by using immobilized Saccharomyces cerevisiae via traditional fermentation protocol.
2 Materials and methods
2.1 Macroalgae collection and biomass preparations
Freshwater macroalgae (Rhizoclonium sp.) was harvested at Mae Taeng District, Chiang Mai Province, Thailand (Latitude: 19° 09′ 17.63″ N, Longitude: 98° 58′ 43.12″ E) and transported to the School of Renewable Energy, Maejo University, Sansai, Chiang Mai 50290 within 2 h for identification and analysis. Harvested algae were dried by a hot air dry oven at 50 °C, for 48 h. The dried biomass was powdered by blender about 1–2 mm size. Algae were growing environment, sample area measuring, harvesting, drying, and powdered biomass are shown in Fig. 1. A microscopic photograph of Rhizoclonium sp. (at × 100 magnification) is displayed in Fig. 2.
2.2 Preparation of medium (yeast extract peptone dextrose medium)
General methods in yeast genetics are specified using yeast extract peptone dextrose (YPD) medium for cultivating Saccharomyces cerevisiae and other yeasts. Yeasts grow well on a minimal medium containing only dextrose and salts. The addition of protein and yeast cell extract hydrolysates allows for faster growth of yeast cells [12]. YPD medium was prepared for 1 L, which was enriched with the media components, as shown in Table 1.
2.3 Preparation of immobilized yeast
Yeasts are a beneficial fungal strain, Saccharomyces cerevisiae TISTR 5020, and was obtained from Faculty of Science Laboratories, Maejo University, Sansai, Chiang Mai, Thailand. These yeasts were cultivated in a liquid YPD medium (10 g/l yeast extract, 20 g/l peptone, and 20 g/l dextrose) on the shaker at 150 rpm for 48 h at room temperature. Modified method of the immobilization prescribed by Kwang Ho Lee’s and Williams’s method was utilized [13], the reaction of yeast (Saccharomyces cerevisiae cells) with sodium alginate (0.02 M) to calcium chloride solution (0.05 M) procured. Saccharomyces cerevisiae cells were immobilized in calcium alginate beads. Immobilized yeast was generated from yeast solution containing sodium alginate, which was dropped into a prechilled CaCl2 solution. Finally, immobilized yeast cells were washed with sterilized distilled water and kept in the refrigerator at 4 °C for further use: yeast preparation, calcium alginate beads preparation, free-immobilized cell, and fermentation system are presented in Fig. 3.
2.4 Determination of total sugar by phenol-sulfuric method
The standard curve of sugar was prepared using the serial concentration of glucose solution (0–250 μg/ml) in distilled water. Five hundred micrograms of each concentration was transferred to the test tube and added with 500 μl of 5% Phenol solution. The mixtures were shaken and followed by the addition of 2.5 ml conc. sulfuric acid (H2SO4). All mixtures were homogenized by vortex and subsequently stand for 10 min. The absorbance (490 nm) of the reaction mixture was measured. Finally, the relation between A490 and glucose concentration was plotted. Determination of total sugar in samples, sugar concentration in sample solution, was determined as the method described above. The reaction mixture composed of 500 μl of the sample solution, 500 μl of 5% Phenol solution, and 2.5 ml conc. H2SO4.
2.5 Determination of reducing sugar by dinitrosalicylic acid method
The standard curve preparation of reducing sugar was prepared using the serial concentration of glucose or mannose or xylose solution (0–1000 μg/ml) in distilled water. Five hundred microliters of each concentration was filled into the test tube and added with 500 μl of dinitrosalicylic acid method (DNS) solution and subsequently boiled for 15 min. Followed by cooling and added using 4 ml of distilled water was performed. After homogenizing the reaction mixture, the absorbance at 540 nm was measured. The relation between glucose concentration and A540 was plotted. The amount of reducing sugar in the sample solution, the 500 μl of sample solution, was determined with the method as described above, similar to standard curve preparation. After the A540 measurement, reducing sugar concentration was calculated by comparing it with the standard curve [14].
2.6 Statistical analysis
Data were reported as mean ± SE from triplicate observations. Significant differences between means were analyzed. All statistical analyses were performed using SPSS Version 20.0. A correlation was assumed significant when P < 0.05
3 Results and discussion
3.1 Pretreatment and enzymatic hydrolysis
Like cellulosic ethanol, bioethanol production from algae requires four major unit operations, including pretreatment, hydrolysis, fermentation, and distillation. In order to produce sugars from the algae biomass, pretreatment is designed to help separate cellulose, hemicellulose, and lignin so that the complex carbohydrate molecules in the algae cell can be broken down by enzyme-catalyzed hydrolysis (water addition) into their simple constituent sugars [15]. Then, the fermentable sugars can be fermented into ethanol by ethanol-producing microorganisms and finally recover and purify the ethanol to meet fuel specifications. Additionally, some separated solids can be recovered and utilized as fuel to provide process heat and electricity at an alcohol production facility [16]. Implementation analyzes and summarizes the results of the project evaluation of bioethanol production from algae. Three pretreatments such as biological, chemical, and biochemical pretreatment were investigated. Finally, the best pretreatment was selected, which provides the highest concentration of reducing sugar. Measurement was done by analyzing the concentration of reducing sugar, total sugar, and ethanol in g/l after fermentation at 48 h for 6 days.
The S. cerevisiae is a yeast; it has been traditionally used for the production of ethanol by Trichoderma Reesei. It forms white bolls and develops a pale yellow-colored colony. It enters a steady phase in two days, and it has rapid growth. This fungus produces several enzymes like cellulase and hemicellulase enzyme; media for this fungal culture must be changed in every alternative day [3, 17]. The pretreatment results are shown in Fig. 4. The results obtained the total sugar by chemical, biological, and biochemical pretreatment and were 19.87 ± 0.35 g/l, 17.81 ± 0.20 g/l, and 21.01 ± 1.25 g/l, respectively. The achieved reducing sugar by chemical, biological, and biochemical pretreatment were 3.89 ± 0.16 g/l, 8.53 ± 0.21 g/l, and 5.03 ± 0.67 g/l, respectively.
The enzyme hydrolysis results are shown in Fig. 4. The obtained total sugar by chemical, biological, and biochemical pretreatment was 78.95 ± 3.95 g/l, 91.23 ± 1.72 g/l, and 88.20 ± 1.57 g/l, respectively. The obtained reducing sugar by chemical, biological, and biochemical pretreatment was 47.22 ± 1.73 g/l, 27.22 ± 1.88 g/l, and 34.03 ± 3.54 g/l, respectively. In this study, chemical pretreatment was selected because after enzyme hydrolysis, the result of the amount of reducing sugar was higher than biological and biochemical pretreatment, mostly of reducing sugar is a monosaccharide. Therefore, yeast can convert simple sugar into ethanol. Due to the recalcitrance of lignocellulose, chemical pretreatment is one of the most important methods for achieving desirable pyrolytic products. To destroy the lignocellulosic structure, decrease the thermal stability, and alter the components in the biomass, a variety of chemical treatments have been developed prior to pyrolysis, including acid and alkali pretreatments [18].
3.2 Bioethanol production
The depletion of fossil fuels attracts bioethanol as one of the most beneficial fuels due to its energy security and environmental safety over fossil fuel [8, 19, 20]. It is an environment-friendly oxygenated method as it contains 34.7% oxygen, which is absent to gasoline. The presence of oxygen in bioethanol gives 15% higher combustion efficiency over gasoline, resulting in lesser emission of particulate nitrogen oxides. Additionally, other harmful gases such as sulfur oxide and carbon monoxide being emitted by gasoline can be reduced by mixing ethanol in gasoline. These harmful gases both contribute to acid rain or going to the water and contaminate potable water sources, which causes a detrimental effect on health [4, 21]. Fermentation is the process that converts soluble sugars into alcohol by the metabolic process of microorganisms. In the absence of oxygen, some bacteria and yeast can metabolize carbohydrates such as monosaccharides and disaccharides and produce the ethanol and with the release of carbon dioxide [22]. Mostly in all refineries, the traditional yeast is used for the ethanol fermentation process. Mostly S. cerevisiae species of yeast has been used extensively in alcohol production, especially in the brewery and wine industries. This type of yeast reduces the distillation cost as it gives a high ethanol yield and high productivity and also can withstand high ethanol concentration. Immobilization of enzymes may affect enzyme activity, specificity, and selectivity and also alter its structural form. These changes may not always give positive effects to the enzyme properties. Several factors will affect the yield of biodiesel produced using an enzymatic reaction. The factors include enzyme specificity and efficiency. Furthermore, different enzymes might need different operating conditions for its optimum activity [6].
The 1st fermentation (free cell yeast) results are shown in Fig. 5. The concentrations of total sugar by chemical pretreatment were 238.25 ± 12.75 g/l, 148.95 ± 2.29 g/l, 134.91 ± 9.00 g/l, and 127.02 ± 15.46 g/l respectively. The concentration of reducing the sugar by chemical pretreatment was 105.00 ± 10.00 g/l, 50.89 ± 2.67 g/l, 32.00 ± 4.18 g/l, and 33.67 ± 2.96 g/l respectively. Conversely, concentrations of ethanol were highest 52.34 ± 16.34 g/l at the 96 h fermentation. The 1st fermentation by immobilized yeast results is shown in Fig. 6. The concentrations of total sugar by chemical pretreatment were 213.95 ± 1.90 g/l, 99.30 ± 3.83 g/l, 96.84 ± 4.21 g/l, and 72.28 ± 9.67 g/l respectively. The concentration of reducing the sugar by chemical pretreatment was 109.52 ± 5.77 g/l, 52.57 ± 2.73 g/l, 30.95 ± 8.86 g/l, and 18.19 ± 4.16 g/l respectively. Conversely, concentrations of ethanol were highest 65.43 ± 18.13 g/l at the 96 h fermentation. Immobilized cells are increasingly being used in bio-industries and may also have benefits for the brewing industry. The significant challenge for applying this technology successfully is focused on the main fermentation in combination with the secondary fermentation [23].
The 2nd fermentation by immobilized yeast results is shown in Fig. 7. The concentration of total sugar by chemical pretreatment was 218.16 ± 4.14 g/l, 129.65 ± 10.79 g/l, 123.77 ± 4.20 g/l, and 118.77 ± 2.93 g/l respectively. The concentration of reducing sugar by chemical pretreatment was 104.29 ± 5.71 g/l, 66.29 ± 7.16 g/l, 77.52 ± 12.46 g/l, and 60.48 ± 4.65 g/l respectively. Conversely, concentrations of ethanol were highest 39.26 ± 7.85 g/l at the 48 h fermentation. The fluidized bed bioreactor with immobilized yeast technology has a great potential for ethanol fermentation of stalk juice of sweet sorghum [24]. Immobilization of cells containing specific enzymes has further advantages such as elimination of long and expensive procedures for enzymes separation and purification and it is vital to expand their application by enabling easy separation and purification of products from reaction mixtures and efficient recovery of the catalyst [25].
From the literature survey, the majority of research investigating the production of bioethanol has focused on brown algae, probably due to its relative abundance and ease of cultivation. Particular species that have received attention are Saccharina latissima, Laminaria hyperborean, Laminaria digitata, and Saccharina japonica [10, 26,27,28,29]. The conversion of macroalgal biomass to bioethanol is not as simple as the conversion of some biomass types such as sugarcane and corn because of the complex carbohydrates contained within the biomass [2]. Some of the unique polysaccharides found in brown seaweeds, such as mannitol and laminarin, require specific enzymes to convert these polysaccharides to simple sugars that can be used by yeast to produce bioethanol. An ethanol-producing bacterium called Zymobacter palmae has been reported as having the ability to ferment many different types of sugars, including mannitol [30]. The same study, however, also confirmed that Z. palmae was unable to use laminarin. Further work, however, conducted with an alternative yeast Pichia angophorae showed that this particular species appeared to use both the laminarin and mannitol content of the biomass for ethanol production [31].
Using P. angophorae a maximum ethanol recovery of 0.43 g/g substrate was recorded and 0.38 g/g mannitol for Z. palmae. Using the same strain of yeast (P. angophorae) [32] managed to produce 167 methanol/kg (132 g/g) of Laminaria digitata using the enzyme laminrase for pretreatment. Another research group managed to recover high yields of ethanol by discovering a method to use DNA from a bacterium called Vibrio splendidus alongside Escherichia coli allowing the conversion of alginate in Saccharina japonica to ethanol [7,8,9, 14]. The group managed to recover an ethanol yield of 0.281 g/gbiomass. Table 2 shows the bioethanol yields recovered from various studies for a number of different species and processing methods [28,29,30,31]. The two-stage hydrolysis and fermentation of the invasive red algae Gracilaria salicornia were investigated, finding a recovery of 79.1 gethanol/kgbiomass [35]. The potential for ethanol recovery following agar removal from Gracilaria verrucosa was investigated. The researchers used enzymatic hydrolysis with cellulase and ß-glucosidase to release the sugars in the biomass yielding 0.87 gsugars/gcellulose.
The sugars were fermented using S. cerevisiae, a common yeast, to recover 0.43 gethanol/gsugars. Ulva has received the most research for bioethanol production in terms of green macroalgae, as it is one of the most common species of green macroalgae. Ulva is a relatively fast-growing macroalga and, therefore, has been considered as a potential source of bioenergy [36]. Several studies have considered bioethanol production, enzymatically hydrolyzed a batch of Ulva fasciata, sampled locally in Hawaii, using a cellulase enzyme [37, 38]. The hydrolysate was fermented with S. cerevisiae yielding the equivalent of 126 l of ethanol per ton of biomass. The researchers believed this to be about 43% of the potential of the biomass, given the characteristics. Similar experiments have been conducted by the Danish Technological Institute looking at bioenergy recovery from Ulva lactuca. The biomass was hydrolyzed with a number of commercial enzymes and fermented with S. cerevisiae. The greatest yield produced was 0.141 g ethanol g dry biomass. From Table 2, it shows a comparison of bioethanol yields (gEthanol/gBiomass) between another species and Rhizoclonium sp. It was found that Rhizoclonium sp. was producing bioethanol yields and was much higher than other algal species using free cell yeast (0.523), first time immobilized yeast (0.654), and second time (reused) immobilized yeast (0.393) in this study.
The most initial total sugar and reducing sugar of fermentation by free cell yeast, the 1st immobilized yeast, and the 2nd immobilized yeast decreasing are indicated in Table 3. The concentration of ethanol by the 1st immobilized yeast maximum increase of 65.43 ± 18.13 g/l was higher than free cell yeast (52.34 ± 16.34 g/l) after 96 h of fermentation. Moreover, it can reuse immobilized yeast for the second fermentation and was 39.26 ± 7.85 g/l after 48 h of fermentation.
4 Conclusion
Algae are green aquatic plants multiplying naturally using photosynthesis, which is rich in lignocellulosic biomass. Algae act as a promising source for biofuel due to rapid growth and lignocellulosic nature Rhizoclonium sp. was used in this investigation. The result of pretreatment and enzyme hydrolysis process by three methods indicate that chemical pretreatment has a concentration of reducing sugar more than biological and biochemical pretreatment (47.22 ± 1.73 g/l). The concentration of ethanol in g/l between fermentation comparison was completed by free cell yeast and immobilized yeast. It was found that cell immobilization in beads offers important advantages, such as ease of cell separation from the medium, a decrease in costs due to cell reuse in subsequent reaction cycles, and a reduced possibility of contamination. Furthermore, the results of cell immobilization showed a stable final ethanol concentration. Immobilized yeast has the highest concentration of ethanol (65.43a ± 18.13 g/l) after 96 h of fermentation and can be reused for the second fermentation and reduces the expenses for bioethanol production. Consequently, algae (Rhizoclonium sp.) can be considered as a potential feedstock for promising and efficient bioethanol production.
References
Bhuyar P, Sathyavathi S, Math RK (2020) Production of bioethanol from starchy tuber (Amorphophallus commutatus) and antimicrobial activity study of its extracts. African J Biol Sci 2:70–76
Annam Renita A, Sreedhar N, Magesh Peter D (2014) Optimization of algal methyl esters using RSM and evaluation of biodiesel storage characteristics. Bioresour Bioprocess 1:1–9
Bhuyar P, Yusoff MM, Ab Rahim MH, Sundararaju S, Maniam GP, Govindan N (2020) Effect of plant hormones on the production of biomass and lipid extraction for biodiesel production from microalgae Chlorella sp. J Microbiol Biotechnol Food Sci 9:4. https://doi.org/10.15414/jmbfs.2020.9.4.671-674
Ramaraj R, Tsai DDW, Chen PH (2014) An exploration of the relationships between microalgae biomass growth and related environmental variables. J Photochem Photobiol B Biol 135:44–47
Ramaraj R, Unpaprom Y (2019) Optimization of pretreatment condition for ethanol production from Cyperus difformis by response surface methodology. 3. Biotech 9:1–9
Norjannah B, Ong HC, Masjuki HH, Juan JC, Chong WT (2016) Enzymatic transesterification for biodiesel production: a comprehensive review. RSC Adv 6:60034–60055
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Vu PT, Unpaprom Y, Ramaraj R (2017) Evaluation of bioethanol production from rice field weed biomass. Emergent Life Sci Res 3:42–49
Vu PT, Unpaprom Y, Ramaraj R (2018) Impact and significance of alkaline-oxidant pretreatment on the enzymatic digestibility of Sphenoclea zeylanica for bioethanol production. Bioresour Technol 247:125–130
Saengsawang B, Bhuyar P, Manmai N, Ponnusamy VK, Ramaraj R, Unpaprom Y (2020) The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel 274:117841
Bhuyar P, Rahim MH, Yusoff MM, Gaanty Pragas Maniam NG (2019) A selective microalgae strain for biodiesel production in relation to higher lipid profile. Maejo Int J Energy Environ Commun 1:8–14
Ramaraj R, Tsai DDW, Chen PH (2014) Freshwater microalgae niche of air carbon dioxide mitigation. Ecol Eng 68:47–52
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar PV, Alam M, Kumar R, Sastry M (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett 1:515–519
Ramaraj R, Unpaprom Y (2019) Enzymatic hydrolysis of small-flowered nutsedge (Cyperus difformis) with alkaline pretreatment for bioethanol. Maejo Int J Sci Technol 13:110–120
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Bhuyar P, Sundararaju S, Rahim MH, Ramaraj R, Maniam GP, Govindan N (2019) Microalgae cultivation using palm oil mill effluent as growth medium for lipid production with the effect of CO2 supply and light intensity. Biomass Convers Biorefine. https://doi.org/10.1007/s13399-019-00548-5
Anjum S, Abbasi BH, Shinwari ZK (2016) Plant-mediated green synthesis of silver nanoparticles for biomedical applications: challenges and opportunities. Pak J Bot 48:1731–1760
Kumar R, Strezov V, Weldekidan H, He J, Singh S, Kan T, Dastjerdi B (2020) Lignocellulose biomass pyrolysis for bio-oil production: a review of biomass pretreatment methods for production of drop-in fuels. Renew Sust Energ Rev 123:109763. https://doi.org/10.1016/j.rser.2020.109763
Zabed H, Sahu JN, Suely A, Boyce AN, Faruq G (2017) Bioethanol production from renewable sources: current perspectives and technological progress. Renew Sust Energ Rev 71:475–501
Bhuyar P, Tamizi NA, Rahim MH, Pragas G, Maniam NG (2019) Desalination of polymer and chemical industrial wastewater by using green photosynthetic microalgae, Chlorella sp. Maejo Int J Energy Environ Commun 1:9–19
Wannapokin A, Ramaraj R, Whangchai K, Unpaprom Y (2018) Potential improvement of biogas production from fallen teak leaves with co-digestion of microalgae. 3. Biotech 8:1–18
Sarris D, Papanikolaou S (2016) Biotechnological production of ethanol: biochemistry, processes and technologies. Eng Life Sci 16:307–329
Willaert R, Nedovic VA (2006) Primary fermentation of beer with immobilized yeast—a review on flavour formation and control strategies. J Chem Technol 81(8):1353–1367
Zhao XQ, Bai FW (2009) Yeast flocculation: new story in fuel ethanol production. Biotechnol Adv 27(6):849–856
Stolarzewicz I, Białecka-Florjańczyk E, Majewska E, Krzyczkowska J (2011) Immobilization of yeast on polymeric supports. Chem Biochem Eng Q 25(1):135–144
Bhuyar P, Rahim MHA, Sundararaju S, Ramaraj R, Maniam GP, Govindan N (2020) Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef Univ. Aust J Basic Appl Sci 9:9. https://doi.org/10.1186/s43088-019-0031-y
Soumiya S, Baskar G (2017) Macroalgae: source for sustainable production of biodiesel. Int J Ind Eng 1:1–7
He Z, Saw WL, Lane DJ, van Eyk PJ, de Nys R, Nathan GJ, Ashman PJ (2020) The ash-quartz sand interaction behaviours during steam gasification or combustion of a freshwater and a marine species of macroalgae. Fuel 263:116621
Tipnee S, Ramaraj R, Unpaprom Y (2015) Nutritional evaluation of edible freshwater green macroalga Spirogyra varians. Emer Life Sci Res 1(2):1–7
Adeniyi OM, Azimov U, Burluka A (2018) Algae biofuel: current status and future applications. Renew Sust Energ Rev 90:316–335
Yenjit P, Issarakraisila M, Intana W, Chantrapromma K (2010) Fungicidal activity of compounds extracted from the pericarp of Areca catechu against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol Technol 55:129–132
Oliveira DA, Angonese M, Ferreira SRS, Gomes L (2017) Food and bioproducts processing nanoencapsulation of passion fruit by-products extracts for enhanced antimicrobial activity. Food Bioprod Process 104:137–146
Manmai N, Unpaprom Y, Ramaraj R (2020) Bioethanol production from sunflower stalk: application of chemical and biological pretreatments by response surface methodology (RSM). Biomass Convers Biorefine. https://doi.org/10.1007/s13399-020-00602-7
Vaithanomsat P, Apiwatanapiwat W, Chumchuent N et al (2011) The potential of coconut husk utilization for bioethanol production. Kasetsart J (Nat Sci) 45:159–164
Mishra A, Kavita K, Jha B (2011) Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr Polym 83:852–857
Sivaprakash G, Mohanrasu K, Ananthi V, Jothibasu M, Nguyen DD, Ravindran B, Chang SW, Nguyen-Tri P, Tran NH, Sudhakar M, Gurunathan K, Arokiyaraj S, Arun A (2019) Biodiesel production from Ulva linza, Ulva tubulosa, Ulva fasciata, Ulva rigida, Ulva reticulate by using Mn2ZnO4 heterogenous nanocatalysts. Fuel 255:115744
Shankar K, Kulkarni NS, Jayalakshmi SK, Sreeramulu K (2019) Saccharification of the pretreated husks of corn, peanut and coffee cherry by the lignocellulolytic enzymes secreted by Sphingobacterium sp. ksn for the production of bioethanol. Biomass Bioenergy 127:105298
Miezah K, Obiri-Danso K, Kádár Z, Heiske S, Fei-Baffoe B, Mensah M, Meyer AS (2017) Municipal solid waste management in a low income economy through biogas and bioethanol production. Waste Biomass Valori 8:115–127
Acknowledgments
The authors gratefully acknowledged the Program in Biotechnology, Energy Research Center, School of Renewable Energy, Maejo University, Chiang Mai, Thailand, for the research facilities to accomplish this experimental study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khammee, P., Ramaraj, R., Whangchai, N. et al. The immobilization of yeast for fermentation of macroalgae Rhizoclonium sp. for efficient conversion into bioethanol. Biomass Conv. Bioref. 11, 827–835 (2021). https://doi.org/10.1007/s13399-020-00786-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00786-y