Abstract
One of the biggest challenges hindering the successful commercialisation of biofuels is the high dosages of enzymes still required to hydrolyse lignocellulose into fermentable sugars. Enzyme recycling has been recognised as a promising approach to alleviate this problem by reducing enzyme costs and improving the profitability of the bioconversion process. However, developing an effective recycling system is a challenging process and requires an in-depth understanding of enzyme-substrate interactions. In this context, this study investigated the adsorption, desorption and re-adsorption of a cellulase mixture (CelMix) to various lignocellulosic components to gain insights into enzyme-substrate interactions, in order to develop an effective enzyme recycling strategy. Lignin-rich residues exhibited a higher adsorption capacity for CelMix (~ 38% adsorption) compared to Avicel cellulose (15.6% adsorption). The recovery of CelMix from lignin, steam pre-treated Eucalyptus and cellulose by various chemicals and alkaline washing was examined. Alkaline washing with Tris-HCl buffer (pH 9.0; 0.05 M) was the most effective method for promoting enzyme desorption (90.7% activity) and retained a substantial amount of hydrolytic activity after elution. However, minor activity loss was observed due to irreversible binding, which was further confirmed by SDS-PAGE analysis. Using this information, the feasibility of recovering the enzymes from the solid fraction after enzymatic hydrolysis of steam pre-treated Eucalyptus was evaluated by two different approaches: (i) re-adsorption of the entire hydrolysed insoluble biomass fraction (no desorption) to fresh biomass (recycling approach 1-RA1) and (ii) re-adsorption of alkaline elution desorbed enzymes from hydrolysed biomass to fresh biomass (recycling approach 2—RA2). The recycling performance of RA1 and RA2 reached > 95% of the initial sugar liberation for three continuous rounds, whilst successful reduction in enzyme loading by 50% and 40% for RA1 and RA2, respectively, was achieved. Therefore, this study shows that enzyme recycling presents a simple and effective pathway for increasing enzyme productivity, improving the economic feasibility of fermentable sugar production for biofuel.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Renewable energy derived from lignocellulosic biomass has increasingly been recognised as an ideal option for creating a sustainable bioeconomy, as it serves as a promising alternative to petroleum-based transportation fuels [1,2,3]. Enzymes play a critical role in the conversion of lignocellulosic biomass into sugar platforms; however, the high cost of enzymes required to achieve efficient biomass saccharification impedes the successful commercialisation of bio-refinery technologies [4,5,6]. Although various pre-treatment methods have been employed as a way to deconstruct the intact biomass structure and increase cellulose accessibility, large enzyme quantities or long hydrolysis times are still required to reach economic conversion levels [3, 7, 8]. Therefore, it is crucial to develop cost-effective processes that can increase the catalytic productivity of enzymes, while reducing the enzyme loading required to achieve efficient cellulose saccharification [9, 10].
One of the most pursued routes for reducing enzyme costs and improving the profitability of the bioconversion process is through enzyme recycling [5, 10, 11]. Theoretically, if enzymes are still active after hydrolysing a substrate, it should be possible to recover and re-utilise their remaining activities in multiple rounds of hydrolysis [12]. As enzymes are constantly adsorbing, desorbing and re-adsorbing on to the substrate during hydrolysis, enzymes can be recovered from the solid or liquid phase [6, 10, 13].
Although enzyme recycling may seem to be a relatively simple approach to reduce the economic processes of lignocellulosic biofuels, it is a complex process that requires in-depth knowledge of enzyme-substrate complexations and the different factors that may influence these interactions. Although notable efforts have been made to try and better understand the enzyme-substrate interactions, the exact mechanisms of adsorption-desorption are yet to be elucidated [14, 15]. Most studies have used model substrates and purified enzymes to try better understand these interactions, which unfortunately is not a true representation of the real interactions that take place between complex enzyme mixtures and lignocellulosic substrates [16]. In addition, recycling the enzymes adsorbed to the solid residue of various pre-treated lignocellulosic substrates has not been well studied, and the results reported in literature are often contradictory and elusive [6, 17, 18].
Thus, using this information as our platform, we developed two different strategies for recycling enzymes associated with the insoluble solid residue, after enzymatic hydrolysis of steam pre-treated Eucalyptus, i.e., (i) recycling the entire insoluble solid fraction (no desorption) (recycling approach 1—RA1) and (ii) recycling the desorbed enzymes via elution (recycling approach 2—RA2). The recycling performance of the two approaches was evaluated and compared by re-using a significant amount of hydrolytic activity from the insoluble solid fraction after hydrolysis in an attempt to successfully reduce the enzyme loading (to reach a given level of sugar production). This study presents potential pathways for improving the economic feasibility of biofuel production on a commercial scale.
2 Methods and materials
2.1 Enzymes and commercial substrates
The enzyme cocktail, CelMix, used in this study was developed by our lab in a previous study [19]. The enzyme cocktail was composed of Egl 68%, Cel7A 17%, Cel6A 6% and βgl1 9%. It must be noted though that although Poplar and Acacia were the hardwoods used in the study conducted by Malgas et al. [19], our enzyme cocktail optimisation studies confirmed that the same enzyme cocktail was required for optimal Eucalyptus hydrolysis (data not shown). Protease from Bacillus licheniformis, Avicel PH-101, carboxymethyl-cellulose (CMC), para-nitrophenyl substrates; p-nitrophenyl-β-D-glucopyranoside (pNPG) and p-nitrophenyl-β-D-cellobioside (pNPC), Tris base, PEG 2000, TritonX, Tween 20 and Tween 80 were purchased from Sigma-Aldrich (South Africa).
2.2 Eucalyptus and its pre-treatment via steam explosion
The lignocellulosic substrate used in this study was Eucalyptus spp., which was kindly supplied by Mondi Ltd., Durban (South Africa). Prior to pre-treatment, the wood chips were oven dried (50 °C) for 24 h and then passed through a TRIMTECH garden vacuum blower (mulch speed fixed at 6). The wood chips were then subjected to steam explosion using a 2-l StakeTech III steam gun (Stake Technologies, Norvall, ON, Canada) in the Forest Products Biotechnology/Bioengineering Laboratory at the University of British Columbia (Canada), as previously described by Bura et al. [20].
2.3 Preparation of isolated lignin samples
The lignin isolation procedure was adapted from a method previously published [21]. Lignin was obtained from extensively enzymatically hydrolysed steam exploded Eucalyptus (800 mg, dry weight). The hydrolysis was conducted using a mixture of Novozyme Cellic® CTec2 and Novozyme Cellic® HTec in a 4:1 protein ratio at a total enzyme loading of 3 mg/ml in 0.1 M sodium citrate buffer (pH 5.0) and incubated at 37 °C. After a 48-h hydrolysis period, the mixture was centrifuged (5488×g for 10 min), the supernatant was decanted, and the solid substrate washed with distilled water to remove any unbound enzymes and sugars. The residual solid was further treated with a protease (0.5 mg/ml) in sodium citrate buffer (pH 5.0, 0.1 M) and incubated at 37 °C, mixing at 25 rpm for 24 h. Thereafter, the mixture containing protease was heat de-activated by incubating the sample at 100 °C for 10 min, followed by centrifugation (5488×g, 10 min) and decanting the supernatant. The remaining solid residue was washed and alkaline treated with Tris-HCl (pH 9.0; 0.05 M) for 2 h, followed by the addition of 15% HCl in order to precipitate dissolved lignin. The obtained lignin was washed with distilled water to remove residual proteins and sugars. After drying the residue at 50 °C for 18 h, the resulting material had a low protein content and was assumed to be a pure lignin fraction.
2.4 Analytical methods
2.4.1 Compositional analysis of steam pre-treated Eucalyptus
The wood biomass was characterised using a modified sulphuric acid method by the National Renewable Energy Laboratory-NREL at the Forest Products Biotechnology Group, University of British Columbia, Canada. To estimate the Klason lignin content, the mass of the solid residue was measured after drying overnight at 105 °C. The filtrate was analysed with regard to acid-soluble lignin (ASL) and monosaccharide content using UV–Vis spectroscopy and Dionex (Sunnyvale, CA) HPLC (ICS-3000) equipped with an AS 50 auto sampler, ED50 electrochemical detector, GP 50 gradient pump and anion exchange column (Dionex CarboPac PA1), respectively.
2.4.2 Protein determination
Total protein in solution was measured by the Bradford method [22]. Bovine serum albumin (BSA) was used as a suitable protein standard. The amount of protein adsorbed to the substrate was determined as the difference between the protein concentration of the cellulases in solution before and after incubation with substrate [21]. The interference of the surfactants with the formation and colour of the dye-protein complex was accounted for by including suitable controls, i.e. (supernatant + Bradford reagent) − (surfactant + Bradford reagent).
2.4.3 Determination of cellulase activity
CMCase, Avicelase and glucosidase activities were determined using CMC, pNPC and pNPG as described in protocols by Malgas et al. [19, 23].
2.5 Adsorption experiments
Cellulase adsorption was performed by allowing the CelMix to interact with the various lignocellulosic components (Avicel, extracted lignin from steam pre-treated Eucalyptus and the whole steam pre-treated Eucalyptus). The different materials were selected to assess the difference in the adsorption dynamics of CelMix on various lignocellulosic biomass components. Incubations were performed at a 2% (w/v) substrate consistency with CelMix loaded at 6.875 mg/g of biomass. The reactions were conducted at 37 °C, with mixing at 25 rpm for 1 h so that a stable adsorption equilibrium could be reached [24, 25]. After incubation, the samples were centrifuged at 5488×g for 10 min and the liquid containing the unbound enzymes (adsorption supernatant) was collected and stored at 4 °C for SDS-PAGE analysis, quantification of enzymatic activity and protein estimation. All experiments were performed in triplicate and the adsorption experiments themselves were repeated twice for more accurate results. Samples lacking enzyme or substrate were used as enzyme and substrate controls, respectively.
2.6 Desorption of cellulases by various surfactants and alkaline media
Various chemicals (Triton X100, PEG 2000, Tween 20 and Tween 80) and Tris-HCl (pH 9.0; 0.05 M) were added to the biomass (individually) after solid-liquid separation (after adsorption) of reactions set up as described in Sect. 2.5 to determine the most effective method for desorbing cellulases from the solid residue. Tris-HCl (pH 9.0; 0.05 M) was determined to be the most effective desorption treatment and was selected for subsequent experiments. After enzyme adsorption (as described in Sect. 2.1), the solid residue was subjected to an alkaline wash treatment (Tris-HCl pH 9.0; 0.05 M) at room temperature, mixing at 25 rpm for 3 h. After incubation, the samples were centrifuged at 5488×g for 10 min and the supernatants were collected and stored at 4 °C for SDS-PAGE analysis, quantification of enzymatic activity and protein estimation. The solid fraction obtained after alkaline elution was then re-suspended in the same volume of supernatant recovery in 0.1 M sodium citrate buffer (pH 5.0).
2.7 SDS-PAGE
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using an 8% resolving gel and a 4% stacking gel as described by Laemmli [26]. The supernatant and solid fractions (Avicel cellulose, extracted lignin and steam exploded Eucalyptus) after enzyme desorption with Tris-HCl, as well as the control (complete cellulase mixture) were mixed with sample buffer and boiled for 5 min before being loaded into the gel wells.
2.8 Enzyme recycling strategies
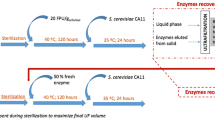
Two different enzyme recycling approaches were carried out (as illustrated in Fig. 1) in order to assess their capability/efficiency for recycling the enzymes adsorbed to the solid residue after enzymatic hydrolysis of steam pre-treated Eucalyptus as described by Lindedam et al. [17]. Enzyme recyclability was determined by measuring the amount of enzyme activity adsorbed to the insoluble solid residue and the ability of the enzymes to hydrolyse fresh steam pre-treated Eucalyptus for multiple hydrolysis cycles.
Schematic diagram of the two different recycling approaches investigated in this work. Approach 1 (RA1): re-adsorption of the entire un-hydrolysed insoluble solid fraction to fresh biomass and approach 2 (RA2): enzyme desorption via alkaline elution (Tris-HCl, pH 9.0, 0.05 M) followed by re-adsorption of desorbed enzyme to fresh biomass. In each cycle, fresh steam pre-treated Eucalyptus biomass was added at 2% (w/v). The enzyme loading in cycle 1 was 6.875 mg/g substrate. Different enzyme dosages were added (0%, 40%, 50%, and 60%) in cycle 2. In cycle 3, RA1 and RA2 were supplemented with 50% and 60% fresh enzyme, respectively
2.8.1 Enzymatic hydrolysis
Hydrolysis experiments were carried out in 400-μl reaction volumes, containing 2% (w/v) biomass suspended in sodium citrate buffer (pH 5.0; 0.1 M). After 24 h of hydrolysis at 50 °C, the supernatant was removed to measure the amount of reducing sugars (as glucose equivalents) using a modified 3,4 dinitrosalicylic acid (DNS) protocol [27].
2.8.2 Enzyme recycling of the solid residue by re-adsorption onto fresh substrate
Enzyme recycling was performed by recycling the solid residue after enzymatic hydrolysis of steam pre-treated Eucalyptus (1st round) to the next round of hydrolysis to make sure that the unhydrolysed solids containing adsorbed enzymes could be recycled at the same time [6]. After 24 h, the samples were centrifuged (5488×g; 10 min) and the hydrolysed solids with adsorbed enzymes were mixed with fresh biomass using the same 2% (w/v) solid loading. Various protein dosages (protein mass basis) of fresh CelMix (0%, 40%, 50%, 60%) were supplemented to the reaction mixture for the next round of hydrolysis in order to achieve constant production of sugars during enzyme recycling. These concentrations were chosen and optimised according to a study by Yuan et al. [6], which was based on the amount/concentration of enzyme that was lost during rounds of hydrolysis.
2.8.3 Enzyme recycling of the solid residue by alkaline washing onto fresh substrate
Enzyme recycling was performed by recycling the desorbed enzymes (by Tris-HCl, pH 9.0; 0.05 M) from the solid residue after enzymatic hydrolysis (1st round) to the next round of hydrolysis as described in Sect. 2.6. After alkaline elution, the supernatant (desorbed enzymes) was added to fresh biomass, using the same 2% (w/v) solid loading. Various protein dosages of fresh CelMix (0%, 40%, 50%, 60%) were supplemented to the reaction mixture for the next round of hydrolysis in order to achieve constant production of sugars during enzyme recycling.
3 Results and discussion
3.1 Pre-treatment and chemical composition of Eucalyptus
Eucalyptus was pre-treated by steam explosion which is a favourable method for treating lignocellulosic biomass due to its simplicity and cost-effectiveness [7, 28]. The chemical composition analysis showed that steam pre-treated Eucalyptus contained 48.9% acid insoluble lignin, 47.01% glucan and 4.81% xylan. It is evident that lignin was not removed after steam pre-treatment, as a large portion of lignin was still present in the biomass (48.9%). According to literature, steam explosion does not remove lignin from the substrate, instead, the pre-treament modifies the biomass structure and can even enrich the amount of lignin from 15 to 30% to > 40% due to condensation [28, 29].
3.2 Enzyme adsorption
Enzyme adsorption on to the solid surfaces is a critical step in the enzymatic hydrolysis of lignocellulose, during which cellulases can productively bind to cellulose or non-productively bind to other cell wall polymers [11, 24, 30]. Different pre-treated substrates possess different binding accessible sites for various cellulase components, which affect their adsorption-desorption characteristics [13, 31]. Consequently, this has resulted in many controversial results with respect to enzyme recycling, which makes it important for us to understand enzyme adsorption-desorption characteristics of a particular pre-treated substrate prior to designing a recycling system [30, 32].
In this work, the adsorption of CelMix on Avicel cellulose, lignin extracted from steam exploded Eucalyptus and the whole steam pre-treated Eucalyptus was evaluated in an attempt to better understand the influence of different lignocellulosic components on enzyme adsorption. Approximately 15.56%, 37.65% and 38.73% of the total CelMix adsorbed to Avicel cellulose, extracted lignin and steam pre-treated Eucalyptus, respectively (Fig. 2a). High cellulase adsorption on lignin has been reported in numerous studies [25, 33, 34]. It is proposed that cellulases adsorb unproductively to lignin through electrostatic, hydrophobic and hydrogen bonding [25, 32]. As a result, the presence of lignin in lignocellulose is believed to be detrimental for efficient cellulose hydrolysis and is not ideal when recycling enzymes in the liquid phase.
Since these adsorption studies are representative of the total CelMix containing different cellulases (protein quantification), it is impossible to confirm which enzymes in the CelMix had predominantly bound to the various substrates and which enzymes were free in the supernatant. As previously mentioned, different enzymes have different binding affinities for different substrates, which play a major role in their adsorption behaviour [13, 31]. Thus, specific cellulase activity assays (CMCase, pNPGase and pNPCase) were carried out in order to assess the adsorption characteristics of the individual enzymes in the cellulase mixture. The percentage of bound activity for each cellulase component was measured by the difference in the activity levels in the CelMix control and the unbound fraction as described previously [24]. Figure 2b shows that endo-glucanase (EG) bound more extensively on to all three substrates, as the CMCase activity in the supernatant was determined to be 74%, 63% and 65%, for Avicel, lignin and steam pre-treated Eucalyptus, respectively. According to MacKenzie et al. [35] and Palonen et al. [33], EG has a higher affinity for lignin compared to cellobiohydrolase (CBH), due to its open conformation active site, rendering more aromatic residues to lignin that results in a hydrophobic interaction between the enzyme and lignin. Our results were in agreement with Mackenzie et al. [35] and Palonen et al. [33]—CBHs bound to a lesser degree than EG (supernatant 1) to the three substrates. This was evident by the higher enzyme activities (pNPC activity) present in the supernatant, i.e. 90.5% (Avicel), 77.6% (lignin) and 84% (steam exploded Eucalyptus). For glucosidase activity, pNPG activity assays were performed and the activities in the supernatants were determined to be 90.7% (Avicel), 85.4% (lignin) and 87.0% (steam exploded Eucalyptus), which revealed that β-glucosidase (βgl) bound to a lesser extent than both cellulases, CBH and EG. These results were in accordance with studies performed by Pareek et al. [36], Sipos et al. [37] and Varnai et al. [38], who reported that βgl adsorbed to a lesser extent during the hydrolysis of Avicel and different lignocellulosic substrates. They proposed that the low affinity for βgl for lignocellulosic substrates is because it lacks a CBM, which facilitates adsorption to the insoluble substrate. Since the enzymes had different binding affinities for the different lignocellulosic components, their enzyme activity distribution profiles (required to synergistically hydrolyse cellulose) after adsorption was expected to be different than the complete CelMix (100%). Thus, their distribution profiles were assessed and are represented in Fig. 3. It was evident that after enzymes had interacted with the different lignocellulosic components, the distribution of enzyme activity profiles was different compared to the complete CelMix (100%). The original CelMix mixture (no adsorption) contained the major enzyme activities, i.e. 75% EG activity, 16% glucosidase activity and 9% CBH activity (Fig. 3a). After enzyme adsorption, the supernatant displayed a slight decrease in EG activity (71%) and an increase in both glucosidase (18%) and CBH activity (11%) for Avicel (Fig. 3b). The same pattern was observed after enzyme adsorption to extracted lignin with the supernatant containing 70% EG activity, 20% glucosidase activity and 10% CBH activity (Fig. 3c). Only 70% EG activity, 19% glucosidase activity and 11% CBH activity remained in the supernatant after adsorption to steam pre-treated Eucalyptus (Fig. 3d). Although only slight differences were observed with respect to the original CelMix mixture compared to un-adsorbed enzymes in the supernatant after enzyme adsorption, the results clearly indicate that EG had a higher binding affinity for the substrates (decreased CMCase activity) compared to CBHs and βgl (which was in agreement with the results attained in Fig. 2b).
3.3 The effect of various reagents on cellulase desorption
By taking advantage of the high adsorption affinity of CelMix to the substrate and considering that the bound enzymes are the key enzymes required for cellulose hydrolysis, recycling the adsorbed enzymes by re-adsorption onto fresh substrate would be a promising approach for reducing enzyme costs [18]. Thus, an alternative effective approach for recycling the adsorbed enzymes is through desorption and reuse of cellulases, which requires an understanding of the adsorption characteristics of cellulases [31, 39]. Amongst the various detergents assessed in this study (Tween 20, Tween 80, Triton X-100, PEG 2000 and Tris-HCl (pH 9.0; 0.05 M)), Tris-HCl (pH 9.0; 0.05 M) was determined to be the most effective at facilitating enzyme desorption, with a 90.67% desorption efficiency (Table 1). This was similar to the results reported by Zhu et al. [40], who found that desorption was highly favoured at pH 7 to 10, indicating the potential of using in situ pH adjustment to monitor cellulase adsorption-desorption. Significant desorption was also found with PEG 2000, which had a desorption efficiency of 81.37%, while TritonX, Tween20, Tween80 had minor effects on cellulase desorption (< 40% desorption efficiency) (Table 2).
We then determined how much initially adsorbed protein could be recovered from the solid residues, after alkaline elution with Tris-HCl (pH 9.0; 0.05 M). For extracted lignin and steam pre-treated Eucalyptus, 93.94% and 94.62% of the initially adsorbed protein were recovered, respectively, whereas 86.8% was recovered from Avicel (Table 2). These results demonstrate the potential application of Tris-HCl as an efficient desorption reagent for recovering bound enzymes from both cellulose and lignin-rich substrates and was selected for subsequent experiments. The initial (control) and recovered enzymes from Tris-HCl desorption were further analysed by using SDS-PAGE, by quantitatively visualising the amount of protein in the supernatant after alkaline desorption from the substrates (Fig. 4). The high intensity of the bands in lane B represents the control (100% CelMix). In lanes C, E and G, bands represent the supernatant containing the desorbed enzymes. By observing the differences in band intensities between the initial solution (CelMix), and recovered protein after alkaline elution, the effectiveness of alkaline desorption with Tris-HCl could further be evaluated. As expected, the bands present in lanes C, E and G (desorbed enzymes) had a lower intensity compared to CelMix control (lane B), due to the fact that CelMix is representative of the initial enzyme dosage (100%). However, the presence of these bands (lanes C, E and G) confirmed that Tris-HCl was effective at facilitating cellulase desorption from all three substrates. It must be noted though that minor quantities of CelMix remained bound onto the solid residue (more so for lignin and steam pre-treated Eucalyptus than Avicel) after alkaline elution (lanes D, F and H, Fig. 4), particularly proteins with sub-molecular masses between 50 and 75 kDa. This may be attributable to non-reversible binding of some cellulases to lignin [32].
The desorption (recovery) of cellulases from Avicel, extracted lignin and steam pre-treated Eucalyptus. Desorption was performed by washing the solid residues with Tris-HCl (pH 9.0; 0.05 M) at 25 °C for 3 h. A Precision Plus protein unstained standard; B original CelMix (0.5 mg/ml); C, E and G desorbed enzymes (supernatant wash) from Avicel, lignin and steam exploded Eucalyptus, respectively; D, F and H solid residue (after enzyme desorption) of Avicel, lignin and steam exploded Eucalyptus, respectively
Although desorption studies and SDS-PAGE analyses revealed that Tris-HCl was effective at promoting enzyme desorption, examining the effect of pH change on enzyme activity is vital when designing a feasible recycling system [41]. Currently, there is limited information on the hydrolytic activities of cellulases desorbed from residual biomass after alkaline elution [39, 41, 42]. Therefore, we wanted to determine whether enzyme activity could be recovered after desorption. The results revealed that 67.4% (Avicel), 66.7% (lignin) and 69.6% (steam exploded Eucalyptus) of CMCase activity could be recovered after alkaline elution. For pNPCase activity, 67.4% (Avicel), 41% (lignin) and 41.2% (steam exploded Eucalyptus) activity could be recovered, whereas for pNPGase activity, 70% (Avicel), 75% (lignin) and 73% (steam exploded Eucalyptus) activity could be recovered from the solid residues. Because not all enzyme activities could be recovered from alkaline elution, we were able to confirm our previous assumptions, which stated that some enzymes become denatured during the course of hydrolysis and/or bound non-reversibly to the residual biomass (as seen in the SDS-PAGE gel in Fig.4).
3.4 Comparison between enzyme recycling systems: RA1 an RA2
Both recycling approaches were performed by hydrolysing steam pre-treated Eucalyptus for 24 h (1st hydrolysis round). Generally, most of the biomass was hydrolysed within the first 24-h hydrolysis period, and a significant amount of enzymes adsorb to the substrate during this time. In addition, the advantages of performing hydrolysis for only 24 h are that it allows for shorter hydrolysis periods which ultimately minimises loss of enzyme activities [6]. For each hydrolysis round, the same amount of fresh biomass and buffer as that in the first hydrolysis cycle was added to each recycling approach. In addition, various CelMix protein mass loadings were added to the reaction mixture for each hydrolysis round (0%, 40%, 50% and 60%) in order to replenish enzyme activities. According to Xue et al. [12], if the original enzyme dosage is used in each hydrolysis cycle, the amount of sugars produced will increase as a result of enzyme accumulation. Thus, we supplemented various enzyme dosages to each cycle, in an attempt to make up the same initial enzyme loading as the original CelMix, in order to produce similar quantities of sugar as those attained in the first hydrolysis round.
As shown in Fig. 5a, the reducing sugars released after the first round of hydrolysis is represented as 100% (based on relative activity), and the amount of reducing sugars released in the second hydrolysis round was compared to the 100% relative activity in round 1. The results showed that without the supplementation of fresh CelMix, the amount of reducing sugars decreased by 65.1% and 42.2% for the mixture containing desorbed enzymes and unhydrolysed solids (no desorption) achieved by the first round, respectively. It was evident that in the second hydrolysis round, both recycling approaches required the addition of fresh CelMix. In order to statistically (p > 0.05) achieve similar amounts of reducing sugars to those attained in the first round of hydrolysis (100% enzyme loading), supplementing the solid residue (RA1) with approximately 40–50% fresh enzyme was sufficient enough for the second hydrolysis rounds, whereas a higher dosage (60%) of fresh enzyme was required when recycling the desorbed enzymes (RA2). Since our adsorption studies show that approximately 40% of enzymes adsorb to steam pre-treated Eucalyptus after 1 h (Fig. 2a), it would make sense that the 2nd cycle would require fresh enzyme dosages in order to achieve similar amounts of reducing sugars to those attained in the first hydrolysis round. Moreover, based on the assumption that the enzyme loading for the first hydrolysis round is 100%, we postulated that approximately 60% fresh enzyme supplementation would be required for the unhydrolysed solids. Our results deviated slightly from this assumption, as only 40–50% of fresh enzyme was required to achieve similar amounts of reducing sugars to those obtained in round 1. According to Yuan et al. [6], more adsorption sites on lignin become accessible during the course of hydrolysis. Thus, a plausible explanation for the slight deviations observed in this study could be that more enzymes adsorbed to the substrate (> 40%) during the 24-h hydrolysis course compared to that adsorbed to the substrate after 1 h; thus, less fresh enzyme was required. With respect to the desorbed enzymes, the higher dosage of fresh enzyme (60%) compared to the enzyme dosage required for unhydrolysed solids may be attributable to enzyme activity loss during alkaline elution, or from irreversibly binding to the solid residue after alkaline elution [33, 42]. The results in Table 1 confirm that some enzyme activities were lost during the alkaline wash process, which further supports this assumption. Another round of hydrolysis (24 h) and enzyme recycling cycle (round 3) was performed, whereby 50% and 60% fresh enzyme were supplemented to the unhydrolysed solid (no desorption) and supernatant containing the desorbed enzymes, respectively. Figure 5 b shows that both recycling approaches could liberate similar amounts of reducing sugars in the 2nd and 3rd cycle to that attained in the 1st hydrolysis cycle, and that both recycling approaches were capable of reducing the enzyme dosage for steam pre-treated Eucalyptus degradation, i.e. 50% (RA2) and 40% (RA1). With respect to conversion rates and reduction in enzyme dosage, RA1 performed slightly better than RA2, indicating that increasing the solid loading in each cycle did not have adverse effects on enzyme hydrolysis efficiency, although we expected that lignin accumulation and the increase in solid loading in each cycle would have a negative effect on hydrolysis. Kristensen et al. [43] and Visser et al. [5] reported that these negative effects are generally only observed at solid loadings above 8% (w/v). Because only 3 cycles were performed in this study, the solid loading would not have exceeded 8% (w/v). Thus, we would only be able to confidently confirm the adverse effects of lignin build up on cellulase performance if additional hydrolysis/recycling cycles were performed. It must be noted though that high solid concentrations (> 20% w/v) are required in an industrial setting; thus, additional experiments using high solid loadings would be crucial for substantiating the concept of enzyme recycling.
Evaluation and comparison of the performance of two different recycling approaches (RA1 and RA2) for recycling the enzymes associated with the insoluble solid residue after the enzymatic hydrolysis of steam pre-treated Eucalyptus (first round). a The effect of different fresh enzyme dosages on reducing sugar liberation (second round) for RA1 and RA2. b The effect of fresh enzyme loading (50% for RA1; 60% for RA2) in the third hydrolysis cycles. Each cycle was performed for 24 h, and fresh biomass (2%) was added to each hydrolysis cycle. Data points indicate the means of triplicate values ± SD
4 Conclusions
This study investigated the adsorption-desorption characteristics of a cellulase mixture (CelMix) on various lignocellulosic components to better understand enzyme-substrate interactions. The results revealed that lignin-rich residues displayed a higher adsorption capacity compared to Avicel cellulose and that alkaline washing (Tris HCl) had the greatest effect on enzyme desorption (90.67% efficiency). Based on these findings, two recycling strategies were developed in order to recover enzymes associated with the insoluble solid residue after steam pre-treated Eucalyptus hydrolysis. An evaluation of the recycling performance of two different approaches: recycling the entire un-hydrolysed solid residue (no desorption) and recycling the desorbed enzymes (via alkaline elution) for three consecutive hydrolysis rounds showed that both approaches produced good recycling efficiencies (> 95% of initial hydrolysis yield was retained after 3 cycles). Enzyme usage was decreased by 50% (RA1) and 40% (RA2), while maintaining similar glucose yields, in successive hydrolysis cycles. These results clearly indicate that it is possible to recover and recycle the insoluble solid substrate associated enzyme after biomass hydrolysis. Therefore, this method presents a simple and effective way for reducing the enzyme requirement for biomass hydrolysis, thereby contributing to the overall improvement in the economic feasibility of biofuel production. However, results may vary between different substrates and pre-treatments, and enzyme-substrate interactions may be influenced at high substrate consistency. It is therefore recommended that further studies be performed to confirm the industrial application of this method.
References
Hong Y, Nizami AS, Bafrani MP, Saville BA (2013) Impact of cellulase production on environmental and financial metrics for lignocellulosic ethanol. Biofuels Bioprod Biorefin 7(3):3
Jagadevan S, Banerjee A, Banerjee C, Guria C, Tiwari R, Baweja M, Shukla P (2018) Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotech Biofuel 11(185) https://doi.org/10.1186/s13068-018-1181-1
Zhang H, Wei W, Zhang J, Huang S, Xie J (2018) Enhancing enzymatic saccharification of sugarcane bagasse by combinatorial pretreatment of Tween 80. Biotechnol Biofuel 11:309
Leis B, Held C, Bergkemper F, Dennemarck K, Steinbauer R et al (2017) Comparative characterization of all cellulolosomal cellulases from Clostridium thermocellum reveals high diversity in endoglucanase product formation essential for complex activity. Biotechnol Biofuel 10:240
Visser EM, Leal TF, de Almeida MN, Guimaraes VM (2015) Increased enzymatic hydrolysis of sugarcane bagasse from enzyme recycling. Biotechnol Biofuel 8:5
Yuan Y, Zhai R, Li Y, Chen X, Jin M (2018) Developing fast enzyme recycling strategy through elucidating enzyme adsorption kinetics on alkali and acid pretreated corn stover. Biotechnol Biofuel 11(316)
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4(7)
Lu Y, Yang B, Gregg D, Saddler JN, Mansfield SD (2002) Cellulase adsorption and an evaluation of enzyme recycle during hydrolysis of steam-exploded softwood residues. Biochem Appl Biotechnol 98-100:641–654
Kumar V, Shukla P (2019) Extracellular xylanase production from T. lanuginosus VAPS24 at pilot scale and thermostability enhancement by immobilization. Process Biochem 71:53–60
Weiss N, Borjesson J, Pedersen LS, Meyer A.S (2013) Enzymatic lignocellulose hydrolysis: improved cellulase productivity by insoluble solids recycling. Biotechnol Biofuel 6(5)
Gomes D, Rodrigues AC, Domingues L, Gama M (2015) Cellulase recycling in biorefineries- is it possible? Appl Microbiol Biotechnol 99:4131–4143
Xue Y, Jameel H, Park S (2011) Strategies to recycle enzymes and their impact on enzymatic hydrolysis for bioethanol production. Bioresour 7(1)
Van Dyk JS, Pletschke BI (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes- factors affecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480
Hu J, Mok YK, Saddler JN (2018) Can we reduce the cellulase enzyme loading required to achieve efficient lignocellulose deconstruction by only using the initially absorbed enzymes? ACS Sustain Chem Eng 6:6233–6239
Shang Y, Su R, Huang R, Yang Y, Qi W, Li Q, He Z (2014) Recycling cellulases by pH-triggered adsorption-desorption during the enzymatic hydrolysis of lignocellulosic biomass. Appl Microbiol Biotechnol 98(12):5765–5774
Pribowo A, Arantes V, Saddler JN (2012) The adsorption and enzyme activity profiles of specific Trichoderma reesei cellulase/xylanase components when hydrolyzing steam pretreated corn stover. Enzym Microb Technol 50(3):195–203
Lindedam J, Haven MO, Chylenski P, Jorgensen H, Felby C (2013) Recycling cellulases for cellulosic ethanol at industrial relevant condition: potential and temperature dependency at high solid processes. Bioresour Technol 148:180–188
Qi B, Chen X, Su Y, Wan Y (2011) Enzyme adsorption and recycling during hydrolysis of wheat straw lignocellulose. Bioresour Technol 102:2881–2889
Malgas S, Chandra R, van Dyk JS, Saddler JN, Pletschke BI (2017) Formulation of an optimized synergistic enzyme cocktail, HoloMix, for effective degradation of various pre-treated hardwoods. Bioresour Technol 245:52–65
Bura R, Bothast RJ, Mansfield SD, Saddler JN (2003) Optimization of SO2-catalyzed steam pretreatment of corn fiber for ethanol production. Appl Biochem Biotechnol 106:319–333
Siqueira G, Arantes V, Saddler JJ, Ferraz A, Milagre AMF (2017) Limitation of cellulose accessibility and unproductive binding of cellulases by pretreated sugar cane bagasse lignin. Biotechnol Biofuel 10(176)
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Malgas S, van Dyk SJ, Pletschke BI (2015) β-Mannanase (Man26A) and α-galactosidase (Aga27A) synergism - a key factor for the hydrolysis of galactomannan substrates. Enzym Microb Technol 70:1–8
Hu G, Heitmann JA Jr, Rojas OJ, Pawlak JJ, Argyropoulos DS (2010) Monitoring cellulase protein adsorption and recovery using SDS-PAGE. Ind Eng Chem Res 49:8333–8338
Zheng Y, Zhang S, Miao S, Su Z, Wang P (2013) Temperature sensitivity of cellulase adsorption on lignin and its impact on enzymatic hydrolysis of lignocellulosic biomass. J Biotechnol 166:135–143
Laemmli UK, Amos LA, Klug A (1976) Correlation between structural transformation and cleavage of the major head protein of T4 bacteriophage. Cell 7(2):191–200
Miller GI (1959) Use of dinitrosalicylic acid reagent for the determination of reducing sugars. Anal Chem 72:426–428
Tu M, Zhang X, Paice M, McFarlane P, Saddler JN (2009) The potential of enzyme recycling during the hydrolysis of a mixed softwood feedstock. Bioresour Technol 100:6407–6415
Saini JK, Saini T, Tewari L (2014) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3. Biotech 5(4):337–353
Machado DL, Neto JM, da Cruz Pradella JG, Bonomi A, Rabelo AC et al (2015) Adsorption characteristics of β-glucosidase on Avicel, steam pretreated sugarcane bagasse and lignin. Biotechnol Appl Biochem 62(5):681–689
Baig KS, Turcotte G, Doan H (2016) Adsorption of cellulose enzymes on lignocellulosic materials and influencing factors: a review. Int J Waste Resour 6(3)
Jørgensen H (2016) Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod Biorefin 11(1):150–167
Palonen H, Tjerneld F, Zacchi G, Tenkanen M (2004) Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol 107:65–72
Seo DH, Fujita H, Sakoda A (2011) Effects of non-ionic surfactant, Tween 20, on adsorption/desorption of saccharification enzymes onto/from lignocelluloses and saccharification rate. Adsorption 17(5):813–822
Mackenzie LF, Sulzenbacher G, Divne C, Jones TA, Woldike HF et al (1998) Crystal structure of the family 7 endoglucanase 1 (Cel7B) from Humicola insolens at 2.2 a resolution and identification of the catalytic nucleophile by trapping of the covalent glycosyl-enzyme intermediate. Biochem J 335:409–416
Pareek N, Gilgren T, Jonsson LJ (2013) Adsorption of proteins in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour Technol 148:70–77
Sipos B, Dienes D, Schleicher A, Perazzini R, Crestini C et al (2010) Hydrolysis efficiency and enzyme adsorption on steam pretreated spruce in the presence of poly-(ethylene glycol). Enzym Microb Technol 47:84–90
Varnai A, Viikari L, Marjamaa K, Silka-aho M (2011) Adsorption of monocomponent enzymes in enzyme mixture analysed quantitatively during hydrolysis of lignocellulose substrates. Bioresour Technol 102:1220–1227
Rodrigues AC, Felby C, Gama M (2014) Cellulase stability, adsorption/desorption profiles and recycling during successive cycles of hydrolysis and fermentation of wheat straw. Bioresour Technol 156:164–116
Zhu Z, Sathitsuksanoh N, Zhang YHP (2009) Direct quantitative determination of adsorbed cellulase on lignocellulosic biomass with its application to study cellulose desorption. Analyst 134(11):2267–2272
Yanfei L, Sun Z, Ge X, Zhang J (2016) Effects of lignin and surfactant on adsorption and hydrolysis of cellulases on cellulose. Biotechnol Biofuel 9(20)
Du R, Li X, Tantai X, Liu Z, Yang J et al (2012) Controlled adsorption of cellulase onto pretreated corncob by pH adjustment. Cellulose 19:371–380
Kristensen J, Felby C, Jorgensen H (2009) Determining yields in high solids enzymatic hydrolysis of biomass. Appl Biochem Biotechnol 156:127–132
Acknowledgements
We gratefully acknowledge the assistance received from Prof J.N. Saddler and Dr. R. Chandra at the Forest Products Biotechnology Group, University of British Columbia, Canada for performing steam explosion pre-treatment and composition analysis on the Eucalyptus.
Funding
Financial support was from the National Research Foundation (NRF) of South Africa and Rhodes University (Sandisa Imbewu Grant). Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and therefore the NRF does not accept any liability in regard thereto.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Adsorbed CelMix enzymes were key for efficient biomass hydrolysis.
• Alkaline washing with Tris-HCl at pH 9.0 was most effective for enzyme desorption.
• Enzyme loadings were reduced by ~ 45% by recycling insoluble fraction bound enzymes.
• Recycling insoluble fraction bound enzymes shows potential for industrial application.
Rights and permissions
About this article
Cite this article
Thoresen, M., Malgas, S. & Pletschke, B.I. Enzyme adsorption-desorption and evaluation of various cellulase recycling strategies for steam pre-treated Eucalyptus enzymatic degradation. Biomass Conv. Bioref. 12, 265–274 (2022). https://doi.org/10.1007/s13399-020-00670-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00670-9