Abstract
Oil palm empty fruit bunch (EFB), a rich of polysaccharide and element as potassium, is being recognized as one of the most potential kinds of lignocellulosic biomass for bioenergy and biochemical production. In this study, EFB was subjected to hydrothermal pretreatment in the absence (HT) and presence of a sulfonated bentonite catalyst (HTcat). The effect of pretreatment on enzymatic hydrolysis and anaerobic digestion was investigated. The hydrothermal pretreatments were conducted at 160–200 °C for 5–25 min, while the effect of catalytic HTcat pretreatment of EFB was studied at 180–200 °C for 25 min. The results showed that temperature and catalyst in HTcat pretreatment were the main factors that could enhance both production of glucose and biohydrogen up to 1.04–1.14- and 3.32–4.36-fold, respectively, compared with those pretreated by HT at 180–200 °C for 25 min without catalyst. The catalyst specifically enhanced hemicellulose and lignin removal from EFB. During HT pretreatment, disruption of EFB cell wall also facilitated over 70% potassium dissolution from EFB to the liquid residue at 160–190 °C for 25 min, while poorer dissolution of potassium was found at 200 °C without or with catalyst addition. The HT pretreatment successfully improved the removal of potassium from EFB and its bioconversion yield. However, the potassium forms a sticky compound with other elements and soluble organic compound, and further study is required for the valorization of the potassium and liquid residue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Palm oil production is one of the most important agro-industries in Thailand. The production of crude palm oil has increased due to the government’s policy promoting bio-diesel production and use of the oil in food. The production of palm oil, however, generates a large amount of lignocellulosic waste, including palm fiber, palm shell, and empty fruit bunch (EFB). Among these biomasses, EFB is the most abundant lignocellulosic biomass which disposes 22% of fresh fruit bunches (FFB) based on the standard biomass to FFB extraction rate [1]. Utilizing EFB as energy feedstock may promote replacement of fossil fuel and solve the environment problem coming from organic waste. However, an intensive fertilizer-rich potassium has been applied to oil palm plantation to increase production of oil palm fruit which causes much amount potassium accumulated in the structure of the plant cell wall. EFB is plentiful but much less desired as a base boiler fuel than palm fiber and shell, since its abundant potassium content causes fouling [2] that increases the frequency of shutdowns for maintenance. EFB, however, is rich in polysaccharides like glucan at about 33.5–41.2% [3,4,5] that can be anaerobically digested to generate biohydrogen or hydrolyzed to glucose, which in turn can be fermented to get ethanol or other industrial chemicals via biotechnological processing.
Sugar and biohydrogen production from lignocellulosic biomass requires two basic steps, namely pretreatment followed by hydrolysis or digestion. Pretreatment in the first step disrupts the biomass matrix, and an enzymatic hydrolysis or subsequent anaerobic digestion constitutes the next step to degrade the biomass into sugars or biohydrogen, respectively. The main problem in sugar and biohydrogen production from biomass is the need to destroy the cell wall and make the polysaccharides (i.e., cellulose and hemicellulose) accessible to microorganisms. Therefore, pretreatment is needed to enable hydrolysis or anaerobic digestion with high yield.
A number of studies have reported on various physicochemical pretreatments to enhance the digestibility of EFB, such as organosolv pretreatment [6], acid pretreatment [4], and N-methylmorpholine-N-oxide pretreatment [3]. Most recently, lignocellulose pretreatment using water as pretreating agent in subcritical state has shown superior results compared with others. In the absence of catalyst, hydrothermal pretreatment has been proven to maximize physical changes and minimize the hydrolysis of cellulose. This has led to minimal sugar degradation products during pretreatment, and thus, the pretreated cellulose is more reactive for subsequent enzymatic hydrolysis to yield maximum glucose concentration [7, 8]. In the present study, the pretreatment of EFB is hydrothermal with water in subcritical state [7, 9]. It has been reported that at temperatures above 100 °C, the dielectric constant of water decreases while its ionic product increases during subcritical water extraction. From 100 up to 200 °C, the dielectric constant of water decreases, to the level of methanol at room temperature [10], and beyond 200 to 300 °C, water may act as an acid or a base catalyst because of the H3O+ and OH− ions having concentrations many orders of magnitude higher than at ambient temperature. Apart from that, hydronium ions are generated also from acetic acid released from the hemicellulose degradation. The presence of these hydronium ions is basically autohydrolyzed the linkages of lignocellulosic materials [8]. Although subcritical water is a much improved solvent and can itself catalyze reactions that would normally require an added acid or base, hemicellulose is not completely removed from lignocellulose matrix which led to obstruct the cellulose moiety from enzymatic hydrolysis. Recently, most of the hydrothermal pretreatments were studied for the effect of alkaline addition to enhance hemicellulose and lignin removal [7, 11, 12]; however, the high cost of wastewater pretreatment of effluent or chemical recovery is required. In order to efficiently remove hemicellulose from lignocellulose feedstock, a solid acid catalyst is more attractive due to recyclability of such heterogeneous catalyst, which can significantly reduce the operating costs. A solid acid catalyst with sulfonic groups has been reported as effective for the degradation of β-1,4 glycosidic linkages in cellulose [13]. Among solid acid catalysts, sulfonated bentonite is an inexpensive choice that is simple to synthesize and reusable, for the pretreatment of lignocellulosic biomass [14].
Therefore, this study aimed to investigate the effects of retention time and temperature on EFB pretreatment. Effects of sulfonated bentonite catalyst in the hydrothermal (HT) pretreatment on enzymatic hydrolysis and anaerobic digestibility of EFB were also studied. Simultaneously, the influences of HT pretreatment on potassium dissolution from EFB to the liquid residue from pretreatment were also investigated.
2 Materials and methods
2.1 Preparation of oil palm empty fruit bunch
EFB was collected from an oil palm factory, Palm Thai Pattana Co., Ltd. in Satun province, Thailand. It was first air-dried for 1 week and reduced to about 2 mm particle size. This was dried in a hot air oven at 60 °C until constant weight, then stored in plastic bags at room temperature.
2.2 Catalyst synthesis and characterization
The sulfonated bentonite catalyst used in this study was prepared as described by Sakdaronnarong et al. [14]. Briefly, bentonite clay was calcined at 600 °C for 6 h. Then, sulfonation to introduce the OSO3H groups was done by impregnation in concentrated sulfuric acid (98%wt) at 170 °C under nitrogen flushing for 18 h. The sulfonated bentonite obtained was washed with deionized water until neutral pH and was then dried at 100 °C overnight. Elimination of sulfate ions was done by boiling in deionized water for 20 min with stirring. Finally, the solid acid catalyst was ready to use after drying at 80 °C for 12 h.
The sulfonated bentonite catalyst synthesized was characterized. Specific surface and porosity were determined by Brunauer–Emmett–Teller (BET) measurements (Quantachrome AUTOSORB 1-AG, USA), and the pore size distribution was determined using the Barrett–Joyner–Halenda (BJH) method. The functional groups on sulfonated bentonite catalyst were characterized by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) (Thermo Scientific Nicolet 6700, USA) over the range 4000–400 cm−1 with 4-cm−1 resolution, and each recorded spectrum was the average of three spectra with 100 scans.
2.3 Hydrothermal pretreatment
Hydrothermal pretreatment (HT) was carried out at BioResource and BioRefinery Laboratory, Mahidol University, Thailand. An HT reactor with 500 mL working volume was used throughout this study. Batches of 30 g EFB were each subjected with 300 mL of deionized water (solid-to-liquid ratio of 1:10) in the HT reactor vessel. The batch reactor was heated to 160–200 °C for 5–25 min. Effect of sulfonated bentonite catalyst addition (HTcat) in hydrothermal pretreatment was investigated when 0.5%wt of catalyst was added for the HT pretreatment between 180 to 200 °C for 25 min. The solid fraction (approximately 40–60%wt based on raw material) was separated by filtration on a filter paper in a Büchner funnel and the wet sample was kept in a deep freezer at − 20 °C for future use. Prior to enzymatic hydrolysis and anaerobic digestion, in order to produce sugars and biohydrogen, respectively, the sample was thawed and the moisture content was analyzed to calculate the dry weight of material. The liquid fraction (approximately 70–90% based on fresh deionized water added) was collected for analysis of furfural and hydroxymethylfurfural (HMF) contents by high-performance liquid chromatography (HPLC) and of potassium content by inductively couple plasma (ICP). Each pretreatment was conducted in triplicate.
2.4 Enzymatic hydrolysis assay
The enzymatic hydrolysis experiments of the solid fraction from pretreatment of EFB were performed according to Sakdaronnarong et al. [15]. The samples were hydrolyzed with Accellerase 1500 (Genencor, USA) at an enzyme loading of 110 FPU/g substrate (dry basis) in 50 mM sodium acetate buffer (pH 5.0) at 50 °C for 72 h. All the enzymatic hydrolysis experiments were carried out in triplicate.
Total reducing sugar (TRS) concentration in the hydrolysate was quantified by DNS assay [16], while glucose and xylose yields were analyzed using HPLC. The enzymatic hydrolysis yields were determined using Eqs. (1) and (2).
2.5 Biohydrogen production
Biohydrogen production of pretreated EFB with HT pretreatment (160–200 °C) and HTcat (180–200 °C) for 25 min was carried out in triplicate in sealed batch reaction vessels. The total reaction volume was 60 mL containing 0.5 g pretreated EFB on dry basis. The ratio of inoculum to substrate was 3:1 on the total solids basis. The inoculum was sludge taken from a full-scale anaerobic digester treating wastewater at a concentrated rubber latex factory in Songkhla province, Thailand. Prior to the experiments, the inoculum was heated to 80 °C for 1 h. Then, the inoculum to substrate mixtures was supplemented with 10% (v/v) of buffer solution (50 g/L NaHCO3) and 1% (v/v) of a medium containing macro- and microelements. The composition of this nutrient and trace element solution [17] was as follows:
Nutrient solution: NH4Cl, 1.4 g/L; K2HPO4, 1.25 g/L; MgSO4.H2O, 0.5 g/L; CaCl2.2H2O, 0.05 g/L; yeast extract, 0.5 g/L; trace element solution, 5 mL/L.
Trace element solution: FeCl2.4H2O, 2000 mg/L; H3BO3, 50 mg/L; ZnCl2, 50 mg/L; CuCl2.2H2O, 38 mg/L; MnCl2.4H2O, 500 mg/L; (NH4)6Mo7O24.4H2O, 50 mg/L; AlCl3.6H2O, 90 mg/L; CoCl2.6H2O, 2000 mg/L).
The pH of the mixture was adjusted to 5.5 ± 0.5 using small quantity of 10% HCl. Effective volume of 60 mL was made up with distilled water. The batches were incubated at 55 ± 0.5 °C while shaking at 150 rpm. Blanks containing the same amount of inoculum without substrate were used. Daily biohydrogen generation was measured by graduate glass syringes [18]. The analysis of gas composition was performed using gas chromatography (7820A Agilent Technologies) with a thermal conductivity detector and ShinCarbon ST Micropacked column (1.00 mm × 2 m), using argon as the carrier gas.
2.6 Analytical methods
The chemical composition of EFB was analyzed according to Goering and Van Soest [19]. The neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were analyzed. The contents of cellulose, hemicelluloses, and lignin were determined according to Eqs. (3), (4), and (5), respectively.
Potassium content in the solid and liquid samples was quantified using ICP Optical Emission Spectrometer, Optima 4300 DV, Perkin Elmer Instrument, USA. Elemental contents in precipitate from HT pretreatment were determined by X-ray fluorescence spectrometer (XRF), PW2400, PHILIPS, Netherlands. Microstructures of untreated and pretreated EFB were analyzed by scanning electron microscopy (SEM), model Quanta 400, FEI, Czech Republic.
The concentrations of sugar species, furfural, and HMF were measured with an HPLC (Water e2695, USA), equipped with a refractive index detector using 300 mm × 7.8 mm Aminex HPX-87H Ion Exclusion Column (Bio-Rad, USA), operated at 40 °C with 0.005 M H2SO4 as the mobile phase at 0.6 mL/min flow rate.
2.7 Statistical analysis
The Minitab statistical software version 16 was used for statistical analyses of enzymatic hydrolysis and biohydrogen yields, including regression analysis and analysis of variance (ANOVA). Multiple means were compared with one-way ANOVA followed by the Tukey method for post hoc comparison. Mathematical regression model of cellulose recovery from the different temperature and RT of HT pretreatment of EFB was calculated using Regression Toolbox, MS Excel 2016. The threshold level for significance was set at p ≤ 0.05.
3 Results and discussion
3.1 Characteristics of catalyst
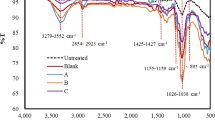
Sulfonation introduced sulfonic groups into the bentonite structure. From BET analysis, it was found that porosity, pore diameter, and specific surface area of sulfonated bentonite catalyst were 1.289 × 10−2 cm3/g, 1.506 nm, and 14.18 m2/g, respectively. Compared with native bentonite clay which have porosity of 0.212 × 10−2 cm3/g, pore diameter of 1.820 nm, and specific surface area of 10.48 m2/g, sulfonated bentonite has greatly higher porosity and surface area but lower in pore diameter. The results are in accordance with the acid activation of bentonite clay for adsorption application [20]. The FTIR spectrum of sulfonated bentonite catalyst (Fig. 1) shows the synthetic catalyst had a peak in the range 1030–1070 cm−1 for SO3− stretching [21]. It has been reported that the sulfonic group SO3H is effective in the acid-catalyzed degradation of β-1,4 glycosidic linkages in hemicellulose and cellulose [13].
3.2 Chemical composition of solid pretreated EFB sample
Compositional changes in EFB after pretreatment with HT and HTcat were compared. The compositions are presented in Table 1. The results show that hemicellulose and lignin removal improved with increasing temperature and retention time, while cellulose was preserved. The regression Eq. 6 represents the influences of temperature (T, °C) and retention time (RT, min) on cellulose recovery in pretreated EFB.
Temperature was the main factor determining cellulose recovery (p < 0.05). As shown in the regression model, an increase of pretreatment temperature significantly enhanced cellulose recovery relative to an increase of RT. The reason is that the temperature substantially influenced the degradation of hemicellulose and lignin fractions from EFB which led to an enhanced cellulose recovery. In contrast, an increase of retention time insignificantly influenced the cellulose recovery since the crystalline structure of cellulose resisted to the hydrothermal degradation relative to amorphous hemicellulose [22]. The best hemicellulose and lignin reductions were 15.6–17.5% and 6.0–8.2%, respectively, from those in untreated EFB, obtained with HT at the highest tested temperature of 200 °C for 5–25 min.
Better removal of hemicellulose and lignin was observed for the HT pretreatment with sulfonated bentonite catalyst (HTcat). It was found the acidified heterogeneous catalyst significantly contributed to hemicellulose and lignin removal for all the pretreatment temperature between 180 and 200 °C as shown by the significant levels (p < 0.05). Cleavage of β,1–4 glycosidic bonds within hemicellulose and in some amorphous cellulose is mainly due to acid catalysis. Simultaneously, acid-catalyzed depolymerization of lignin took place by the cleavage of ester bonds [23] and ether bonds [24] in lignin. As shown in Table 1, the best pretreatment that provided the most cellulose, and the least hemicellulose and lignin remaining in pretreated EFB, was HT pretreatment at 200 °C for 15 min, while HTcat pretreatment at 200 °C for 25 min gave similar chemical composition of the pretreated EFB. Although cellulose recovery from HT pretreatment at 200 °C for 15 min and HTcat pretreatment at 200 °C for 25 min were not significantly different, the released fermentable sugars from enzymatic hydrolysis of pretreated EFB from these conditions were substantially different which was discussed in the next section. Further increase in pretreatment duration seemingly increased lignin content. This may be caused by formation of pseudo-lignin, a lignin-like polymer as a humin structure made from condensation of carbohydrate degraded, onto cellulose surfaces during acid-catalyzed depolymerization at elevated temperature [25]. However, the performance in enzymatic hydrolysis of pretreated EFB, in terms of total reducing sugar, glucose, and xylose yields, needs to be considered.
3.3 Enzymatic hydrolysis of pretreated EFB
The enzymatic hydrolysis of pretreated EFB was tested for sugar yield with HT and HTcat pretreatments, assessing the effects of temperature and retention time of pretreatment. Figure 2 shows sugar yield of untreated EFB, which was 11.4% TRS, 10.68% glucose, and 3.62% xylose. Harsher pretreatment could improve both total reducing sugar and glucose yields by degrading the lignocellulose matrix, increasing biomass porosity and specific surface [26], increasing cellulose swelling, and reducing cellulose crystallinity [27]. As shown in Fig. 2, the most suitable HT condition was 200 °C for 25 min, which provided the highest TRS and glucose yields of 76.0% and 57.1%, respectively.
In order to identify the main factors in the HT pretreatment, regression models of TRS and glucose yields with temperature and retention time of HT as regressors were fit, and are shown in Table 2. Temperature was the dominant factor affecting TRS yield, whereas glucose yield was significantly influenced by both temperature and retention time.
Sulfonated bentonite catalyst was applied in HT pretreatment to enhance the enzymatic yields, at HT temperatures from 180 to 200 °C for 25 min that are the most favorable for glucose production. The results in Table 3 show that the sulfonated bentonite as catalyst improved the glucose yield by about 4.5–6.1 enhancement ratio (ER) while poorer glucose yields were obtained without the catalyst (4.3–5.3). The acidic sites on the catalyst acted at the β-1,4 glycosidic bonds of glucose subunits in hemicellulose and amorphous cellulose, enhancing cellulose degradation in subsequent enzyme hydrolysis. This increased specific surface and porosity of the pretreated EFB, which also enhanced enzymatic hydrolysis yields from those of untreated EFB [28]. Moreover, bentonite clay is composed of mostly SiO2, Al2O3, Fe2O3, MgO, CaO, and Na2O with their ratios depending on the source [29]. This kind of heterojunction metal oxide catalyst could absorb cellulose, which can improve catalysis at the active sites of the catalyst i.e. sulfonic groups [30].
3.4 Biohydrogen potential of pretreated EFB
The biohydrogen yields are shown in Fig. 3. The untreated EFB gave a biohydrogen yield of 0.31 ± 0.06 L H2/kg-VS. The analysis of variance (ANOVA) with Tukey post hoc test revealed significant (p < 0.05) increases of biohydrogen yield, and 1.59 ± 0.12 L H2/kg-VS was obtained at 190 °C HT for 25 min that reduced hemicellulose and lignin contents by 17.68% and 5.19%, respectively (Table 1). Harsh pretreatment conditions may increase the accessible sample by degradation of hemicellulose and lignin [31] in the pretreated EFB, leading to weakened shielding effects and the opening of additional pores in the biomass microstructure. However, no significant change (p > 0.05) in biohydrogen yield was observed when elevating the temperature further from 190 to 200 °C for 25 min. Autohydrolysis during HT could be driven by acetic acid [32, 33], which is a by-product from acetyl group degradation; this mild condition could moderately disrupt microstructure of EFB.
When sulfonated bentonite catalyst was applied in HT at 180 to 200 °C, significantly higher biohydrogen yields were found (p < 0.05) than without catalyst, at all pretreatment temperatures. The sulfonated bentonite catalyst effectively improved enzymatic hydrolyzability of cellulose, and the hydrolysis phase is typically the one that limits anaerobic digestion of lignocellulosic biomass. Moreover, the solid catalyst promoted disruption of EFB cell walls as evident in the SEM images (Fig. 4). The biohydrogen yield of EFB was therefore improved by catalyzing the HT reactions.
The effectiveness of HTcat pretreatment at elevated temperatures increased the content of cellulose by removing hemicellulose and lignin, which for obvious reasons increased the biohydrogen yield. Possibly, not only the sulfonated catalyst accelerated hydrolysis of EFB in the hydrothermal pretreatment, but also acetyl was degraded to acetic acid at elevated temperatures. Moreover, the removal of hemicellulose from EFB could considerably enhance accessibility of cellulose to cellulase enzymes, via increased specific surface and porosity [28, 34], and this increased both sugar and biohydrogen yields for the HTcat pretreated EFB. The biohydrogen yield reached its maximum at 8.09 ± 0.38 L/kg-VS with HTcat 200 °C for 25 min, which was greatly improved by about fourfold from that without catalyst. Although the sulfonated bentonite catalyst was clearly able to enhance anaerobic digestibility of pretreated EFB, inhibitors including furfural and HMF (Table 4) obstruct utilization of the pre-hydrolysate from HT pretreatment.
3.5 Potassium changes in EFB caused by HT pretreatment
In addition to cellulose, hemicellulose, and lignin, EFB is rich in minerals. Potassium, the main mineral element in EFB, is an integral part of live plants. It sustains the osmotic pressure in plant cells and is the most abundant cation in cytoplasm [35]. Potassium in EFB is mostly water-soluble and may be removed by aqueous dissolution.
HT pretreatment potentially removed not only hemicellulose and lignin fractions partly from the biomass, but also minerals. Our results show that after HT pretreatment at 160–200 °C for 25 min, the potassium content in EFB (initially high at 19800 mg/kg) decreased since it was readily dissolved and removed (Table 4). Pretreated solid EFB had potassium reduced by over 90% at temperatures above 160 °C, indicating that leaching was assisted by the disruption of fiber structure, particularly exposing cytoplasm where most of the potassium is located [35].
Effects of sulfonated bentonite catalyst in HT at 180 to 200 °C on potassium removal were also investigated. The results reveal that potassium after HTcat was higher than with HT. In fact, alkali metals, such as Na, Li, and K, acting as electron donors have excess mobile electrons [36] and thus could bind with hydroxyl groups or even ethers and esters, i.e., carbonyl, carboxyl, and other groups in products from cellulose, after acid-catalyzed depolymerization of EFB polysaccharides. Therefore, the potassium dissolved from EFB during HTcat might re-associate with cellulose in harsh conditions with comparatively high temperature and long reaction time.
The potassium removed from EFB was mostly dissolved (75.5–94.7%) except with HT pretreatment at 200 °C. The potassium dissolution was only 46.9% at 200 °C HT. The results are similar for 200 °C HT and HTcat. The large differences in potassium reduction and potassium dissolution reveal that the removal was only partly by dissolution.
Precipitation of a sticky compound in the filtrate was evident for both HT and HTcat pretreatment (Fig. 5) during storage. XRF analysis of the precipitate for HT at 200 °C for 25 min showed the elements K, Ca, Cl, Fe, Si, S, P, and Mg. Potassium was the dominant element (14.1% w/w of precipitate) followed by 1.33% Ca, 1.18% Cl, and 0.57% Fe, while overall, the analyzed minerals contributed 17.8% of total weight. This indicates a large organic fraction in the precipitate. During thermal preprocessing, the abundant potassium in EFB may either form compounds with other elements present [2] or with the soluble organic fraction by condensation. Unfortunately, this current study could not directly quantify the amount of precipitate in the liquid residue. In order to estimate the amount of potassium in the precipitate, the mass balance was used by subtracting from the initial potassium in feedstock, the dissolved potassium, and the remaining potassium in pretreated EFB (solid recovery after pretreatment was 56.9%). The precipitate yield was approximately 73.6 g/kg-feedstock with 14.1% K for HT at 200 °C for 25 min. Thus, the potassium removed was mainly in the precipitate (52.4%) while the soluble fraction of it was 46.9%.
A few studies have reported on utilization of liquid residue from hydrothermal pretreatment of EFB, such as production of hydrogen [37], lignin [38], and fertilizer [39]. It is interesting that potassium in EFB was converted into water-soluble products in the residue from pretreatment. This liquid residue contained a high amount of potassium, suggesting its reuse as a crude fertilizer that could be a co-product from biohydrogen production, in order to maximize the utilization of EFB with the least waste to dispose. Such liquid fertilizer used in oil palm plantations could improve profitability of oil palm cultivation. However, Nurdiawati et al. [39] reported that the liquid residue has potentially toxic substances derived from lignin and sugar during the HT pretreatment, and these can cause fertilizer burn and seed damage reducing Germination Index to 0. In our study, the liquid residue from HT pretreatment was more suitable than that from HTcat for organic fertilizer applications, since HT filtrate did not have furfural and HMF, and had less sticky compound precipitate. However, the sticky precipitate from liquid residue could clog pipes and foul storage vessels, causing technical problems in processing it to an organic fertilizer. Thus, further studies of the liquid residue are still required to extract value from it.
4 Conclusion
The present work shows that significant HT pretreatment with sulfonated bentonite catalyst effectively improved enzymatic hydrolysis and anaerobic digestibility giving increased glucose and biohydrogen yields from cellulose-rich pretreated EFB. Potassium-rich liquid residue with low furans from pretreatment could be potentially valorized as fertilizer, a co-product from sugar or biohydrogen production in pursuit of zero waste discharge. While technical feasibility of bioenergy production was enhanced, a higher value use of the liquid residue will be key for its techno-economic feasibility. Our results should advance the finding of applicability of liquid residue for plant cultivation as well as understanding of its reactivity in the environment.
References
Loh SK (2017) The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers Manag 141:285–298. https://doi.org/10.1016/j.enconman.2016.08.081
Konsomboon S, Pipatmanomai S, Madhiyanon T, Tia S (2011) Effect of kaolin addition on ash characteristics of palm empty fruit bunch (EFB) upon combustion. Appl Energy 88(1):298–305. https://doi.org/10.1016/j.apenergy.2010.07.008
Purwandari FA, Sanjaya AP, Millati R, Cahyanto MN, Horváth IS, Niklasson C, Taherzadeh MJ (2013) Pretreatment of oil palm empty fruit bunch (OPEFB) by N-methylmorpholine-N-oxide (NMMO) for biogas production: structural changes and digestion improvement. Bioresour Technol 128:461–466. https://doi.org/10.1016/j.biortech.2012.10.088
Bouza RJ, Gu Z, Evans JH (2016) Screening conditions for acid pretreatment and enzymatic hydrolysis of empty fruit bunches. Ind Crop Prod 84:67–71. https://doi.org/10.1016/j.indcrop.2016.01.041
Abdul PM, Jahim JM, Harun S, Markom M, Lutpi NA, Hassan O, Balan V, Dale BE, Mohd Nor MT (2016) Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresour Technol 211:200–208. https://doi.org/10.1016/j.biortech.2016.02.135
Nurfahmi OHC, Jan BM, Tong CW, Fauzi H, Chen W-H (2016) Effects of organosolv pretreatment and acid hydrolysis on palm empty fruit bunch (PEFB) as bioethanol feedstock. Biomass Bioenergy 95:78–83. https://doi.org/10.1016/j.biombioe.2016.09.008
Siti Aisyah MS, Uemura Y, Yusup S (2014) The effect of alkaline addition in hydrothermal pretreatment of empty fruit bunches on enzymatic hydrolysis efficiencies. Proc Chem 9:151–157. https://doi.org/10.1016/j.proche.2014.05.018
Cybulska I, Lei H, Julson J (2010) Hydrothermal pretreatment and enzymatic hydrolysis of prairie cord grass. Energy Fuel 24(1):718–727. https://doi.org/10.1021/ef9009179
Zakaria MR, Hirata S, Hassan MA (2015) Hydrothermal pretreatment enhanced enzymatic hydrolysis and glucose production from oil palm biomass. Bioresour Technol 176:142–148. https://doi.org/10.1016/j.biortech.2014.11.027
Carr AG, Mammucari R, Foster NR (2011) A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem Eng J 172(1):1–17. https://doi.org/10.1016/j.cej.2011.06.007
Singh RD, Bhuyan K, Banerjee J, Muir J, Arora A (2017) Hydrothermal and microwave assisted alkali pretreatment for fractionation of arecanut husk. Ind Crop Prod 102:65–74. https://doi.org/10.1016/j.indcrop.2017.03.017
Xiao L-P, Bai Y-Y, Shi Z-J, Lu Q, Sun R-C (2014) Influence of alkaline hydrothermal pretreatment on shrub wood Tamarix ramosissima: characteristics of degraded lignin. Biomass Bioenergy 68:82–94. https://doi.org/10.1016/j.biombioe.2014.06.010
Qi X, Lian Y, Yan L, Smith RL (2014) One-step preparation of carbonaceous solid acid catalysts by hydrothermal carbonization of glucose for cellulose hydrolysis. Catal Commun 57:50–54. https://doi.org/10.1016/j.catcom.2014.07.035
Sakdaronnarong C, Pipathworapoom W, Vichitsrikamol T, Sema T, Posoknistakul P, Koo-amornpattana W, Laosiripojana N (2018) Integrative process for a sugarcane bagasse biorefinery to produce glucose, bio-oil and carbon microspheres. Process Saf Environ Prot 116:1–13. https://doi.org/10.1016/j.psep.2018.01.006
Sakdaronnarong C, Srimarut N, Lucknakhul N, Na-songkla N, Jonglertjunya W (2014) Two-step acid and alkaline ethanolysis/alkaline peroxide fractionation of sugarcane bagasse and rice straw for production of polylactic acid precursor. Biochem Eng J 85:49–62. https://doi.org/10.1016/j.bej.2014.02.003
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Raposo F, Banks CJ, Siegert I, Heaven S, Borja R (2006) Influence of inoculum to substrate ratio on the biochemical methane potential of maize in batch tests. Process Biochem 41(6):1444–1450. https://doi.org/10.1016/j.procbio.2006.01.012
Dechrugsa S, Kantachote D, Chaiprapat S (2013) Effects of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure. Bioresour Technol 146:101–108. https://doi.org/10.1016/j.biortech.2013.07.051
Goering HK, Van Soest PJ (1970) Forage fiber analysis (apparatus, reagents, procedures and some applications). Agriculture Handbook No. 379, USDA-ARS, Washington DC
Jovanović N, Janaćković J (1991) Pore structure and adsorption properties of an acid-activated bentonite. Appl Clay Sci 6(1):59–68. https://doi.org/10.1016/0169-1317(91)90010-7
Moriana R, Vilaplana F, Karlsson S, Ribes-Greus A (2011) Improved thermo-mechanical properties by the addition of natural fibres in starch-based sustainable biocomposites. Compos A: Appl Sci Manuf 42(1):30–40. https://doi.org/10.1016/j.compositesa.2010.10.001
Li Y, Khanal SK (2017) Bioenergy: principles and applications. Wiley Blackwell, Hoboken
Banoub J, Delmas G-H, Joly N, Mackenzie G, Cachet N, Benjelloun-Mlayah B, Delmas M (2015) A critique on the structural analysis of lignins and application of novel tandem mass spectrometric strategies to determine lignin sequencing. J Mass Spectrom 50(1):5–48. https://doi.org/10.1002/jms.3541
Sturgeon MR, Kim S, Lawrence K, Paton RS, Chmely SC, Nimlos M, Foust TD, Beckham GT (2014) A mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkages: implications for lignin depolymerization in acidic environments. ACS Sustain Chem Eng 2(3):472–485. https://doi.org/10.1021/sc400384w
Aarum I, Devle H, Ekeberg D, Horn SJ, Stenstrøm Y (2018) Characterization of pseudo-lignin from steam exploded birch. ACS Omega 3(5):4924–4931. https://doi.org/10.1021/acsomega.8b00381
Wang G, Zhang S, Xu W, Qi W, Yan Y, Xu Q (2015) Efficient saccharification by pretreatment of bagasse pith with ionic liquid and acid solutions simultaneously. Energy Convers Manag 89:120–126. https://doi.org/10.1016/j.enconman.2014.09.029
Grimaldi MP, Marques MP, Laluce C, Cilli EM, Sponchiado SRP (2015) Evaluation of lime and hydrothermal pretreatments for efficient enzymatic hydrolysis of raw sugarcane bagasse. Biotechnol Biofuels 8(1):205. https://doi.org/10.1186/s13068-015-0384-y
Zhang Z, O’Hara IM, Doherty WOS (2012) Pretreatment of sugarcane bagasse by acid-catalysed process in aqueous ionic liquid solutions. Bioresour Technol 120:149–156. https://doi.org/10.1016/j.biortech.2012.06.035
Ross CS, Shannon EV (1926) The minerals of bentonite and related clays and their physical properties1. J Am Ceram Soc 9(2):77–96. https://doi.org/10.1111/j.1151-2916.1926.tb18305.x
Sakdaronnarong C, Saengsawang A, Siriyutta A, Jonglertjunya W, Nasongkla N, Laosiripojana N (2016) An integrated system for fractionation and hydrolysis of sugarcane bagasse using heterogeneous catalysts in aqueous biphasic system. Chem Eng J 285:144–156. https://doi.org/10.1016/j.cej.2015.09.098
Imman S, Arnthong J, Burapatana V, Champreda V, Laosiripojana N (2014) Effects of acid and alkali promoters on compressed liquid hot water pretreatment of rice straw. Bioresour Technol 171:29–36. https://doi.org/10.1016/j.biortech.2014.08.022
Imman S, Arnthong J, Burapatana V, Champreda V, Laosiripojana N (2015) Influence of alkaline catalyst addition on compressed liquid hot water pretreatment of rice straw. Chem Eng J 278:85–91. https://doi.org/10.1016/j.cej.2014.12.032
Rissanen JV, Grénman H, Willför S, Murzin DY, Salmi T (2014) Spruce hemicellulose for chemicals using aqueous extraction: kinetics, mass transfer, and modeling. Ind Eng Chem Res 53(15):6341–6350. https://doi.org/10.1021/ie500234t
Sakdaronnarong C, Jiratanakittiwat K, Tangkitthanasakul T, Laosiripojana N (2017) Ionosolv pretreatment of sugarcane bagasse and rice straw assisted by catalytic hydrothermal and microwave heating for biorefining. Food Bioprod Process 105:104–116. https://doi.org/10.1016/j.fbp.2017.06.005
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annu Rev Plant Physiol 31(1):239–298. https://doi.org/10.1146/annurev.pp.31.060180.001323
Carlsson M (2015) Carbon formation in steam reforming and effect of potassium promotion: potassium dopants prevent carbon formation and aid catalyst recovery. Johnson Matthey Technol Rev 59(4):313–318. https://doi.org/10.1595/205651315X688992
Chong PS, Jahim JM, Harun S, Lim SS, Mutalib SA, Hassan O, Nor MTM (2013) Enhancement of batch biohydrogen production from prehydrolysate of acid treated oil palm empty fruit bunch. Int J Hydrog Energy 38(22):9592–9599. https://doi.org/10.1016/j.ijhydene.2013.01.154
Coral Medina JD, Woiciechowski AL, Filho AZ, Brar SK, Magalhães Júnior AI, Soccol CR (2018) Energetic and economic analysis of ethanol, xylitol and lignin production using oil palm empty fruit bunches from a Brazilian factory. J Clean Prod 195:44–55. https://doi.org/10.1016/j.jclepro.2018.05.189
Nurdiawati A, Novianti S, Zaini IN, Nakhshinieva B, Sumida H, Takahashi F, Yoshikawa K (2015) Evaluation of hydrothermal treatment of empty fruit bunch for solid fuel and liquid organic fertilizer co-production. Energy Procedia 79:226–232. https://doi.org/10.1016/j.egypro.2015.11.469
Acknowledgements
The authors would like to thank Palm Thai Pattana Co., Ltd. for oil palm empty fruit bunch and Chalong Latex Industry Co., Ltd. Songkhla, Thailand, for anaerobic inoculum. The authors would also like to recognize the support from the Biogas & Biorefinery Laboratory, Faculty of Engineering, Prince of Songkla University. We acknowledge Assoc. Prof. Dr. Seppo Karrila and the Publication Clinic, Research and Development Office, PSU, for help in manuscript proofreading.
Funding
This project was supported by the Thammasat University Research Fund under the Research University Network (RUN) initiative (Energy Cluster). B.C. also would like to acknowledge the support from Thailand Research Fund (TRF) contract no. MRG5980051. C.S. would like to thank for the support from NRCT-NSFC research grant (Fiscal year 2015-2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Charnnok, B., Sakdaronnarong, C. & Sinbuathong, N. Hydrothermal pretreatment with sulfonated bentonite catalyst enhances potassium removal and bioconversion of oil palm empty fruit bunch to sugar and biohydrogen. Biomass Conv. Bioref. 9, 389–399 (2019). https://doi.org/10.1007/s13399-018-0360-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0360-4