Abstract
Fish waste disposal is a major cause for concern for the seafood processing industries. Fish processing generates enormous quantities of waste as almost 45% of the live weight of fish is regarded as waste. Current ways of managing fish waste involves dumping in oceans, landfills, or treating them with already established strategies. Dumping these wastes without any form of treatment is far from being environmental friendly. Current utilization strategies suffer from disadvantages such as incomplete utilization of solid and liquid wastes or generation of new waste effluents that needs further processing. Therefore, there is a need to find an alternate/supplemental method of seafood utilization. Previously, we have reported the use of microwave hydrothermal carbonization (MHTC) to carbonize fish waste to hydrochar. Here, a conventional heating method such as a custom autoclave reactor is reported that could also be used to carbonize fish waste to hydrochar. Upon response surface design optimization, it was found that a maximal yield of hydrochar (~ 35%) can be achieved at a holding temperature of 180 °C and at a holding time of 120 min. We have also characterized the elemental, proximate, energy, and surface properties of hydrochar produced by conventional hydrothermal carbonization (CHTC). It was found that the quality of the hydrochar produced by MHTC is largely comparable to CHTC. This further proves that HTC could be employed to generate energy from non-lignocellulosic wastes such as fish waste while getting rid of the waste in an eco-friendly manner.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aquaculture and the seafood processing sector generate enormous quantities of waste globally. Seafood, being highly perishable, needs to be processed to extend its shelf life. Similar to most food industries, fish processing operations produce waste (fish carcasses, viscera, skin, and heads). According to one study, which considers 45% of the live weight as waste, it predicts that nearly 63.6 million metric tonnes of waste is generated globally [1]. This represents a biomass waste that is available continuously but is often underutilized. Managing the waste from seafood processing industries is a major concern not only because of the environmental problems associated with dumping of such wastes in the ocean or in landfills but also due to the problems related to excessive nutrient inputs into the immediate ecosystem [2].
Presently, the most frequently used methods for the utilization of seafood waste are the production of fish oil, fish meal, silage, or in the manufacture of organic fertilizer [3,4,5]. One of the major disadvantages that are common to most of these processes is that they do not utilize the waste completely and therefore often leave behind or generate new wastes that need further treatment. Fish meal, oil, and silage operation are the most common but have been minimally commercialized. Fish meal processing plants generate new waste effluents (blood and sticky-water effluent) that are found to contain more contaminants than waste that is produced from seafood processing industries [4, 6, 7]. There is a growing alertness over the dangers of air and water pollution from these plants and it is likely that these issues will continue to be of great concern in this era of rapid climate change. In addition to this, according to a recent study, most of the fish meal plants were found to use fresh fish (that were suitable for human consumption) rather than fish waste to produce high-quality and high-value meal [8]. Fish silage plants, despite utilizing the fish waste completely, have been associated with disagreeable odor and is therefore not preferred for use in large-scale feed formulations. Also, ensilaging produces a liquid product, which is difficult to store, transport, and handle. For these reasons, often fish meal is preferred over silage, which suffers from other disadvantages as described above. In a recent study, it has been found that fish meal and silage products have no net environmental benefits compared to incineration, composting, and landfilling of fish waste [5]. Due to the disadvantages of the currently commercialized technologies, in the past two decades, the extraction of bioactive components from fish waste has garnered increasing attention. The components that are commonly extracted from fish wastes are bioactive peptides and fatty acids such as omega-3 fatty acids. Bioactive extraction also suffers from the disadvantage that this process focuses on one specific component and not on the entire waste. Therefore, this process also leaves behind waste that needs further treatment. Taken together, due to the need for additional waste treatment methods, and to the preference of fresh fish over decaying waste fish as feedstock for some of these technologies, this leads to the underutilization of the latter. Hence, it has become necessary to develop technologies that can completely utilize the fish waste and thus serve as a stand-alone or a supplemental technology to maximize utilization of fish waste.
Fish waste being an animal waste could broadly be classified as a non-lignocellulosic biomass as these wastes do not contain any lignin, cellulose, or hemicellulose. These wastes contain complex carbohydrates, proteins, and fats. The biochemical profiles of different types of seafood are presented in Table 1, but it should be noted that these profiles are not well characterized for the different varieties of fishes. According to one study, the carbohydrate content of fishes could range from 8 to 15% depending on the type of fish [12]. On the other hand, non-lignocellulosic wastes are a rich source of proteins and fats. The protein content could range from 20 to 60% depending on the type of waste [12]. For instance, fish wastes contain 30–50%, meat wastes contain 20–25%, and shrimp wastes contain 30–45% of protein [12]. Fatty acid composition could range from 10 to 60% depending on the type of the waste [12]. These values indicate that the carbohydrate content of fish waste is not as high as in lignocellulosic biomass. A wide variety of lignocellulosic biomass due to its rich carbohydrate profile is often utilized by HTC [13]. Owing to the poor carbohydrate profile of seafood [12], this waste was previously underutilized for carbonization processes. We have recently shown that using microwave hydrothermal carbonization (MHTC), it is possible to produce hydrochar of quality that is comparable to the one produced from municipal, sewage, and poor-quality lignocellulosic wastes from seafood wastes such as fish [14] and shrimp wastes [15].
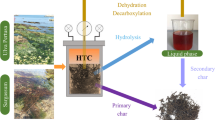
HTC is a thermochemical decomposition process which has been identified to be more suitable for the carbonization of wet biomass feedstock (≥ 50% moisture content) [16,17,18,19,20]. The process can utilize wet biomass such as fish waste directly without an energy intensive drying step that is needed for processes like pyrolysis [20]. HTC involves biomass submersion in subcritical water conditions to thermochemically convert organic components into carbonaceous solids (hydrochar) and aqueous liquid (bio-oil) rich in organic moieties with little or no gaseous products [20]. Therefore, the utilization of seafood waste to produce char and oil by hydrothermal carbonization could be an ideal solution to deal with waste management while creating additional energy resources. Previously, we had used microwaves as the medium of heat transfer to treat fish wastes [14], and here, we have employed response surface design guided optimization of process parameters of conventional HTC by using a custom autoclave HTC reactor to treat fish waste. Further, we have also characterized the energy, elemental, proximate, and morphological properties of hydrochar.
2 Materials and methods
2.1 Feedstock preparation for HTC
Fish waste comprising of heads, tails, viscera, fins, and scales from a variety of fishes including northern anchovy, salmon, and cod were obtained fresh from the local market, stored, and processed as previously described [14, 15, 21]. A complex mixture was obtained to recapitulate the heterogeneous nature of the fish waste produced from industries. The wastes were then stored at − 20 °C until use for a maximum of 2 weeks. On the day of the experiment, required amount of waste was first thawed and then homogenized in a food-grade blender until a smooth paste is obtained. From previous studies, it is known that enzyme pre-treatment is essential to successfully carbonize fish waste at holding temperatures and times considered in this study [21]. No hydrochars were produced from untreated, acid, or alkali-treated fish waste [21]. Therefore, fish waste was enzymatically pre-treated before CHTC as previously described [14, 15, 21]. Briefly, 30 g of homogenized fish waste was mixed with an enzyme cocktail (20%, w/w of each enzyme in the ratio of 1:1:1) of Viscozyme (catalog no.: V2010), lipase (catalog no.: L0777), and protease (catalog no.: P4860), and the incubation was carried out in laboratory incubator/shaker (Incushaker mini, Benchmark Scientific, USA) at ~ 40 °C with rotation at 120 rpm for a period of 6 h. After enzyme pre-treatment, a less-viscous digested fish waste was then subjected to CHTC.
2.2 Hydrothermal carbonization
Fish waste was carbonized in a customized cylindrical autoclave reactor of 150 mL capacity, made of stainless steel 316. Controlled heating was carried out by a heating mantle with a rated heating power of 350 W equipped with a thermocouple and a temperature controller. A tubular internal heater made of INCOLOY with a maximum heating power of 300 W (Omega, Canada) was employed to ensure efficient and more uniform heating. Further, thermal insulation with glass wool was employed to minimize the loss of heat to the surrounding environment (Fig. 1). Following CHTC at varying process conditions, the end product was then subjected to vacuum filtration to separate the solid fraction (i.e., wet hydrochar) from the liquid, biocrude liquor. The wet hydrochar was then oven-dried at 105 °C for 24 h to produce dry hydrochar. The yield of the hydrochar was calculated on a dry basis.
2.3 HTC optimization protocol
Based on the results from a previous study, where fish waste was carbonized using MHTC, it is known that the holding temperature and time of the HTC reaction significantly affects the yield of the hydrochar [15]. Biomass-water ratio did not have any effect on the yield of the hydrochar during MHTC [15]. This effect was further confirmed in a preliminary CHTC screening study that was conducted at 180 °C and 120 min by varying the biomass-water ratio (0.5, 1, and 1.5) (Supplementary Fig. S1). Therefore, holding time and temperature of CHTC was further optimized using response surface design. The pressure of this process was not controlled, and hence, the pressure will be guided by the temperature of the water as this reaction takes place in a closed system. As previously described, with two factors, namely the holding temperature and time, the CCD design resulted in 4 factorial points, 4 axial points, and 4 center runs [15]. Due to technical limitations, a face-centered non-rotatable design was implemented as the CHTC system has a very narrow operating temperature range. For the holding temperature, the minimal and maximal limit (− 1,1) was set to 150 and 210 °C. For the holding time, the maximal and minimal limit (− 1,1) was set to 60 and 120 min. Biomass-water ratio was kept constant at 1. A 4-center run was adapted to improve the reliability of this model. CHTC was performed in a random fashion to account for any random hidden effects that may be present. Significant effects were analyzed using ANOVA (analysis of variance) through DoE suite in JMP. The main effects and a further model building were implemented by using F test (Fisher’s test) and probability values (α = 0.05). This design resulted in a total of 12 runs. The second order linear regression model as below could be used to model the output variable, i.e., hydrochar yield (%) (βn,ε—co-efficients, xn—variables).
The experimental results from CHTC trials were fitted to the above equation using the SAS statistical software, JMP licensed to McGill University.
2.4 Chemical and energy properties of hydrochar
Elemental and proximate analyses were conducted to characterize the chemical composition of the dry hydrochar produced at varying CHTC process parameters. The elemental composition, i.e., C, H, N, and S were determined using an elemental analyzer (Fisons Instruments CHNS-O EA 1108). Fish waste dry hydrochar and raw waste samples were analyzed for moisture content, ash content, and volatile solids per ASTM International Standard protocols. First, to decipher the moisture content, 1.0 g of hydrochar was placed in a hot air oven at 105 °C until a constant weight was achieved. Weights were noted initially after 4 h and after every hour after the initial 4-h period (ASTM 871-82, 2006) [22,23,24]. Second, to measure the ash content, 1.0 g of the test sample was taken in an open crucible and placed in a muffle furnace that was heated to 600 ± 10 °C for 4 h. The weight was noted after the crucible was cooled (ASTM-D 3174-04, 2009) [22,23,24]. Finally, to measure the volatile matter, 1.0 g of test sample was placed in a muffle furnace that was heated to 950 ± 10 °C for 7 min (ASTM-D 3175-07) [22,23,24]. The total fixed carbon was determined by the difference from 100, considering the percentile amounts of moisture, volatile matter, and ash content. Energy value was determined using a Parr adiabatic bomb calorimeter (Parr Oxygen Bomb Model 1341EB, Calorimeter Thermometer Model 6772, Parr Instrument Company, Moline, IL, USA) to calculate calorific value as previously described [25, 26].
2.5 Scanning electron microscope (SEM)
The raw fish waste samples and recovered hydrochar at various operating conditions were analyzed for their surface morphology and microstructure by a Hitachi TM-3000 (Tokyo, Japan) scanning electron microscope for comparative microstructural analysis. Varying resolutions from 50× to 1500× was used to analyze the morphological structure.
3 Results and discussion
3.1 Optimization of CHTC process parameters
From previous studies, it was known that biomass-water ratio had invariable effect on the yield of hydrochar during MHTC of seafood wastes such as fish and shrimp wastes [14, 15]. Further, we confirmed that this holds true for CHTC of fish waste (Supplementary Fig. S1). Previous studies using other feedstocks have found mixed effects of biomass-water ratio on the yield of hydrochar. For instance, biomass-water ratio did not significantly affect the yield of hydrochar resulting from HTC of paper mill sludge [27]. Further, biomass-water ratio did not affect the elemental, proximate, and energy value of the resulting hydrochar [27]. HTC of sunflower stem and walnut shell has found that higher biomass-water ratios have been detrimental to the yield of hydrochar, where increasing the biomass-water ratio resulted in lower yields [28]. The hydrochar produced from walnut shell at higher biomass-water ratio possessed higher calorific value [28]. The effects of biomass-water ratio on other feedstocks such as food waste, sewage, and municipal wastes are yet to be conclusively determined. Taken together, these results suggest that biomass-water ratio may or may not affect the HTC process depending on the feedstock used. In this study, due to the insignificant effect of biomass-water ratio on the yield of hydrochar from fish waste (Supplementary Fig. S1), for further optimization and characterization studies, this parameter was not considered.
Response surface methodology (face-centered central composite design) with two independent variables (holding temperature and time) was performed with the goal of maximizing the hydrochar yield. Face-centered central composite design (Fig. 2a) was adapted in this study due to technical limitations, as CHTC equipment used in this study cannot hold the biomass at temperatures higher than 210 °C reliably. Therefore, the use of star points that could facilitate more sophisticated rotatable design could not be implemented. Further, having the same study design enabled us to compare the results obtained in this study with our previous study where MHTC of fish waste was conducted [14].
The response surface plot (Fig. 2b) shows that the hydrochar yield initially increases upon increasing the CHTC process parameters, i.e., holding temperature and time, and the yield decreases upon increasing the process parameters further. Firstly, upon examining the effect of the process parameters individually, we found that both the holding temperature (F(1,11) = 6.09; P = 0.04) and holding time (F(1,11) = 32.57; P = 0.001) significantly affected the hydrochar yield. Secondly, it was a surprise to note the lack of a significant interaction effect of holding temperature and time (F(1,11) = 1.45; P = 0.27). Thirdly, the curvature term (temperature × temperature effect) was found to exhibit a significant effect on the yield (F(1,11) = 88.39; P < 0.0001), which is not surprising after noting that the response surface of the hydrochar yield exhibited a curved surface (Fig. 2b). Such a curved surface is formed due to the fact that the hydrochar yield at the center point (180 °C and 90 min) falls on a different surface plane than the surface plane that fits the four corner points. The CCD design response surface model significantly fitted (P = 0.0005) the experimental yield as is depicted by a high value of R2 (0.96). The percentage error between the actual and predicted hydrochar yield values ranged between 0.1 and 3.6% with mean percentage error of 1.4%. However, it is noteworthy that the difference between the actual and predicted hydrochar yield was not significantly different (P = 0.50, matched-pairs t test). The quadratic model fit of the hydrochar yield is depicted by the equation below (T—temperature, t—time),
Based on surface plot as shown in Fig. 2b, for the tested process parameter space, the maximal yield of hydrochar from fish waste (~ 34.5%) was obtained by conducting CHTC at holding temperature of 180 °C and holding time of 120 min. On comparing this result to the results obtained from a previous study that employed MHTC of fish waste in a similar response surface experimental design, it was found that both MHTC and CHTC resulted in a maximal yield of hydrochar (~ 35%). However, on first impression, the results suggested that the optimized CHTC process parameters were found to be better, as the maximum hydrochar yield was obtained at a lower holding temperature (180 °C) than MHTC (200 °C), and for a similar holding time of (~ 120 min) (Supplementary data Table S4). Despite this advantage, one of the concerns that is often associated with conventional methods of heating is the longer come-up time, which increases the total experimental time. This is true in this study as the come-up time of the CHTC to reach 150, 180, and at 210 °C was found to be about 3–4 times more than that of the MHTC (150 °C: CHTC = 30.33 ± 1.20 min, MHTC = 8.67 ± 0.89 min, P = 0.0007, Student’s t test; 180 °C: CHTC = 41.33 ± 1.45 min, MHTC = 12.61 ± 0.88 min, P = 0.0005, Student’s t test; 210 °C: CHTC = 63.33 ± 1.20 min, MHTC = 16.33 ± 0.88 min, P < 0.0001, Student’s t test). These results suggest that CHTC and MHTC have advantages and disadvantages when it comes to achieving a certain yield of hydrochar. MHTC greatly reduces the come-up time, which will result in shorter process times, despite having a higher operating temperature than CHTC. In a previous comparative study where MHTC and CHTC of lignocellulosic biomass was evaluated, it was found that the hydrochar yield produced from both these processes were comparable [29]. However, the same study reported that MHTC greatly reduces the process time [29]. Further, another comparative study which evaluated the effect of CHTC and MHTC on human biowaste reported that the yield of the hydrochar were similar irrespective of the heating method [30]. These results strongly suggest that the final yield of the hydrochar produced does not vary greatly depending on the heating method employed. However, different heating medium may impose different optimal process conditions to achieve a certain yield of the hydrochar during HTC. It is possible that for some feedstocks this notion may very well not hold true. A study which evaluated the effect of feedstock categories, i.e., carbohydrates, proteins, and fatty oils, on the yield and energy properties found that yield and energy properties are dependent on the type of feedstock categories being carbonized [31]. Taken together, in order to determine which technique is better for processing fish waste, future experiments should focus on solving the energy and mass balance of the CHTC and MHTC processes. Also, one may need to take into account the material, elemental, proximate, and morphological differences in the hydrochar that is made from these processes to decide on the best process that suits their needs.
3.2 Elemental analysis of fish waste hydrochar
The trend of elemental composition of the dry hydrochar obtained from fish waste at various operating conditions of CHTC and that of raw fish waste (before enzyme pre-treatment) is shown in Fig. 3a and Supplementary data Table S1. As the holding temperature and holding time of CHTC increased from 150 °C and 60 min to 210 °C and 120 min, the amount of atomic carbon in the hydrochar obtained increased from ~ 38 to ~ 51%. The maximal atomic carbon in the hydrochar (~ 52%) is obtained at CHTC operating parameters of 210 °C and 90 min. Atomic oxygen content followed an opposite trend compared to that of the atomic carbon. With increase in holding temperature and time from 150 °C and 60 min to 210 °C and 90 min, atomic oxygen in hydrochar varied from 51 to 37%. Atomic hydrogen and nitrogen in hydrochar mildly increased upon increasing the holding temperature from 150 to 210 °C. Sulfur composition is negligible at different operating conditions of the CHTC. These results indicate that hydrochar is formed by the enrichment of atomic carbon and by the removal of atomic oxygen simultaneously, possibly by decarboxylation, demethylation, and dehydration reactions as suggested by the Van Krevelen diagram (Fig. 3b), during CHTC in a holding time and temperature-dependent manner. In our previous study, we found that MHTC of fish waste resulted in a similar trend of atomic elements as seen in the hydrochar produced by the CHTC of fish waste [14]. Similar trends in the atomic elements were observed in a variety of feedstocks including several lignocellulosic raw materials [32,33,34,35,36] and mixed waste such as sewage sludge [37] and food waste [38,39,40]. For instance, in food waste derived hydrochar, the elemental composition was found to be 38–65% carbon, 5–7% hydrogen, 0.5–6% nitrogen, 24–55% oxygen, and insignificant amounts of sulfur [40]. The higher carbon content with food waste could be attributed to the higher holding temperature used (350°C) [40]. In sewage waste derived hydrochar, the elemental composition was found to be 37–39% carbon, 5–6% hydrogen, 2–5% nitrogen, and 52–54% oxygen [37]. These collectively suggest that the hydrochar derived from fish waste during CHTC is largely comparable to other feedstocks.
At the optimal process conditions, i.e., a holding temperature of 180 °C and a holding time of 120 min, the CHTC hydrochar produced consisted of ~ 42% of atomic carbon, ~ 48% of atomic oxygen, ~ 5% of atomic hydrogen, ~ 6% of atomic nitrogen, and ~ 0.1% of atomic sulfur (Supplementary data Table S4). At similar process conditions of MHTC of fish waste, (180 °C and 120 min), the hydrochar produced consisted of ~ 48% of atomic carbon, ~ 39% of atomic oxygen, ~ 5% of atomic hydrogen, ~ 7% of atomic nitrogen, and ~ 0.4% of atomic sulfur (Supplementary data Table S4) [14]. On comparing these two results, it is clear that the elemental properties are largely similar, with percentage difference of less than 15% in every atomic element. These results strongly argue that with respect to the elemental composition of the hydrochar, either the CHTC or MHTC of fish waste results in a similar quality.
We then computed H/C and O/C atomic ratios to better understand the reaction pathways that might underlie hydrochar production and plotted the Van Krevelen diagram (Fig. 3b and Supplementary data Table S1). At holding temperature of 150 °C, upon increasing the holding time, there was an increase in the demethylation reaction, represented by a shift to the right in the O/C ratio. Similarly, upon increasing the holding temperature to 180 °C, there was an increase in the demethylation reactions at all holding times tested as depicted by the higher O/C values for the hydrochar compared to that of the raw fish waste. Such demethylation reactions are known to occur in other feedstocks [41]. It is also possible that at these temperatures other uncharacterized reactions may occur in the raw material. Further increase in temperature to 210 °C suggested that the decarboxylation reactions are clearly evident during CHTC of fish waste as indicated by lower O/C values of hydrochar compared to raw fish waste. Irrespective of the holding temperature or time, the lower H/C atomic ratio compared to raw waste depicts the occurrence of dehydration reaction during CHTC at all holding temperatures and times. These results suggest that underlying reactions during CHTC of non-lignocellulosic waste might be more complex than that of the lignocellulosic material especially at lower holding temperatures. Similarly, decarboxylation, demethylation, or dehydration reactions occur either exclusively or in combination during MHTC of fish waste [14], shrimp waste [15], and several lignocellulosic materials and mixed waste streams such as food waste, municipal, sewage, and human biowaste [29, 32,33,34, 37, 40, 42]. For instance, in food waste decarboxylation, demethylation and dehydration reaction was observed in hydrochars derived from both enzyme pre-treated and untreated food waste during HTC at temperatures 150 to 350 °C. Comparing this to our results, it is suggestive that at the least at high holding temperatures, the reactions that take place during the formation of hydrochar might be similar.
3.3 Proximate analysis of hydrochar
Proximate analysis results for the dry hydrochar produced at different operating conditions of CHTC hydrochar and raw fish waste are summarized in Fig. 4a, b. The amount of fixed carbon in the hydrochar increases with increase in holding temperature. For instance, when the temperature increases from 150 to 210 °C (at holding time of 60 min), the fixed carbon content in the hydrochar increases from 15.97 ± 1.14% to 30.89 ± 0.98%. Further at a particular holding temperature, increasing the holding time from 60 to 120 min increases the fixed carbon in the hydrochar (Fig. 4a and Supplementary data Table S2). For instance, at the holding temperature of 210 °C, the fixed carbon increases from 30.89 ± 0.98% to 35.16 ± 1.54% as the holding time increases from 60 to 120 min. On the other hand, volatile matter followed an opposite trend, where it decreases as the holding temperature and time increases during CHTC. The volatile matter decreased from 46.23 ± 1.08 to 38.76 ± 0.94% as the holding temperature and time increases from 150 °C, 60 min to 210 °C, 120 min respectively. The increase in the fixed carbon content could be attributed to the removal of the volatile matter and the mass conversion that occurs during CHTC [43]. The ash content of the hydrochar followed a trend similar to that of the volatile matter, where it decreases with increasing holding temperature and holding time. Ash content decreased from 31.54 ± 1.30 to 22.54 ± 1.90% as the holding time and temperature increase from 150 °C, 60 min to 210 °C, 120 min respectively. This trend of ash content suggests that the inorganics present in the fish waste is being leached more at a higher holding temperature in a holding time dependent manner [44]. We then computed the fuel ratio [43] (fuel ratio = fixed carbon / volatile matter) to understand the fuel characteristics of the hydrochar produced. As the holding temperature and time increases from 150 °C, 60 min to 210 °C, 120 min, the fuel ratio (Fig. 4c) increases from 0.35 to 0.91, indicating that the fuel characteristics of hydrochar improve at higher holding temperature and time. The hydrochar produced at a temperature of 210 °C and holding time of 120 min exhibits highest fixed carbon (35.15 ± 1.54%) and contains lowest volatile matter (38.76 ± 0.94%), which explains the highest fuel ratio (0.91) obtained in the tested parameter space. With respect to the feedstock used, similar trends of fixed carbon, ash content, and volatile matter have been observed in MHTC of shrimp waste [15], several lignocellulosic materials and mixed waste streams such as sewage, municipal, food, and human biowastes [29, 32,33,34, 37, 40, 42]. For instance, the proximate composition of the hydrochar derived from sewage waste was found to be 3–5% moisture, 25–39% ash, 55–75% volatile matter, and 1–6% fixed carbon.
The optimal CHTC process conditions to produce maximal yield, which is a holding temperature of 180 °C and a holding time of 120 min, result in hydrochar that comprises of ~ 26% of fixed carbon, ~ 44% of volatile matter, ~ 27% of ash content, and ~ 3% of moisture content (Supplementary data Table S4). Upon MHTC of fish waste, the hydrochar produced exhibits similar trends of increase or decrease in the fixed carbon, volatile matter, and ash content [14] (Supplementary data Table S4). At a similar operating condition, i.e., at a holding temperature of 180 °C and a holding time of 120 min, MHTC of fish waste produces hydrochar that consists of ~ 19% of fixed carbon, ~ 59% of volatile matter, ~ 20% of ash content, and ~ 2% of moisture content [14] (Supplementary data Table S4). On comparing these two hydrochars, CHTC hydrochar has ~ 36, ~ 35, and 10% more fixed carbon, ash content, and moisture content, respectively, than that of MHTC hydrochar (Supplementary data Table S4). On the other hand, CHTC hydrochar has lower volatile matter (34% less) MHTC hydrochar (Supplementary data Table S4). These results suggest that the distribution of materials during HTC may depend on the medium of heat used. Despite CHTC hydrochar having high fixed carbon content, the energy values, as reported in the next section, determined by the bomb calorimeter were indifferent. These results taken together suggest that the energy value may strongly depend not only on the carbon value but also on the volatile matter, ash, and moisture content.
3.4 Energy values of hydrochar
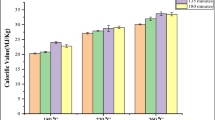
The energy value determined from analytical bomb calorimetry experiments for the fish waste dry hydrochar derived after CHTC are reported in Fig. 5a and in Supplementary data Table S2. It is evident that the calorific value increases from 21.45 ± 0.06 to 24.99 ± 0.14 MJ/kg as the CHTC holding temperature increases from 150 °C, 60 min to 210 °C, 120 min. The highest calorific value (25.64 ± 0.80 MJ/kg) was obtained from hydrochar produced at holding temperature of 180 °C and time of 90 min. The hydrochar derived from the CHTC optimal conditions (180 °C and 120 min) has an energy value of 23.92 ± 1.04 MJ/kg. From our previous study, MHTC hydrochar derived at a similar condition (180 °C and 120 min) has an energy value of 22.76 ± 0.11 MJ/kg [14]. These results indicate that the energy values of hydrochar produced from fish waste at optimal conditions in CHTC or at similar conditions in MHTC are largely similar.
The energy value of hydrochar produced from several other feedstocks including lignocellulosic [45], agricultural [46], and sewage sludge [17] has been found to correlate with atomic carbon content obtained from the elemental analysis of the hydrochar. We wondered if the carbon content in hydrochar produced from fish waste CHTC correlates with the energy value. Figure 5b indicates that the total carbon content in the hydrochar does not correlate with the calorific value as computed from bomb calorimetry. The R2 value of the best-fit line is 0.13, which is not significantly correlated to the observed calorific value (P = 0.14). However, the carbon content in the hydrochar produced from MHTC of fish waste was found to be good predictor of the heating value [14].
We then computed energy enrichment factor (EEF = calorific value of hydrochar/calorific value of raw fish waste), a parameter that measures the energy densification in hydrochar. EEF value of > 1 represents improved energy densification in hydrochar. EEF value of hydrochar produced at all tested holding temperatures (150–210 °C) and holding time (60–120 min) were above 1 (Supplementary data Table S2), indicating a good energy densification of hydrochar produced by CHTC. Similar to the energy value, the EEF factor of hydrochar from CHTC (~ 1.19) optimal conditions is largely comparable to MHTC hydrochar (~ 1.15 produced at near optimal conditions. Subsequently, we computed energy yield (energy yield = hydrochar yield × EEF) for hydrochar made at different operating conditions and the results are depicted in Fig. 6. We found that energy yield increased from 29.33 to 36.31% as the holding temperature and holding time increases from 150 °C, 60 min to 210 °C, 120 min (Fig. 6). This increase in energy yield is attributed to the increasing hydrochar yield with increasing holding temperature and time. On comparing the energy yield between CHTC and MHTC, it was found that they exhibited similar and comparable energy yield owing to similar yield and similar energy values.
Owing to the ease of use of mathematical models to determine the energy value, several models have been developed and employed for the determination of energy value based on the elemental and proximate analysis data. One such common way used to estimate the calorific value is based on the unified model developed by Channiwala and Parikh 2002, which is based on the elemental and proximate analysis of fuels that encompasses a wide variety of biomass including lignocellulosic waste such as agricultural waste, woody biomass, and also mixed waste such as sewage sludge, animal waste, and municipal waste [47]. The model equation is as below (C—carbon, H—hydrogen, S—sulfur, O—oxygen, N—nitrogen, A—ash content).
The above equation was used to compute the gross calorific values (also known as high heating value) based on the proximate analysis of the hydrochar produced from fish waste. It was noted that the gross calorific value calculated from above equation and the actual calorific value calculated from bomb calorimetry matched poorly as depicted by poor R2 value (R2 = 0.19; P = 0.42; Supplementary data Fig. S3a, b). Further, due to poor prediction, the values of percentage error ranged from 0.05 to 26%. This is an alarming overestimation of the energy value for the hydrochar derived from fish waste. These results suggest that the equation that are mostly used to estimate the energy value of mostly lignocellulosic wastes and mixed wastes may not necessarily be good to predict the energy value of the fish waste hydrochar derived from CHTC. With respect to the type of feedstock used, CHTC produced hydrochar of calorific value that is comparable to the hydrochar obtained from lignocellulosic biomass (24–30 MJ/kg) [48], food waste (15–27 MJ/kg) [40], sewage sludge (15–16 MJ/kg) [42], and human biowaste (22–25 MJ/kg) [42]. Based on the calorific value obtained from bomb calorimetry, CHTC fish waste hydrochar resembles lignite [43].
3.5 SEM analysis of hydrochar
The microstructure of CHTC fish waste hydrochar was assessed by performing SEM experiments and the micrographs of hydrochar obtained at varying operating conditions are shown in Fig. 7. The SEM micrographs reveal interesting features of the surface morphology of the hydrochar. At first glance, compared to raw fish waste, hydrochar irrespective of the operating conditions looks different. The raw material has no defined structure as it is flaky and appeared as blobs of clumped up biomass. However, at CHTC holding temperature of 150 °C, carbonization process has resulted in the transformation of the surface morphology of hydrochar as is evident from the improved surface structure of the hydrochar. In particular, higher holding times produced microspheres that are typical of hydrochar produced from lignocellulosic wastes [49]. However, upon increasing the holding temperature of the CHTC process from 150 to 180 and 210°C, the microspheres formed during carbonization are more clearly visible. Interestingly, microspheres were formed separately and also were observed on top of the plate like structures with few cracks. Such cracks are likely to be formed during the release of volatile substances during CHTC. These transformations are very interesting and thus indicate the decomposition of the monomers of the complex macromolecules that fish waste is composed of such as carbohydrates, proteins, and lipids and subsequent precipitation and growth into spheres. Different microstructures of hydrochar due to different operating conditions are also seen in other types of biomass such as lignocellulosic biomass [49]. A similar microstructure of hydrochar has been observed during MHTC of sewage sludge and human biowaste [42]. Clear microspheres were also observed in hydrochar derived from kitchen waste [50]. In our previous study, a similar and a better evolution of hydrochar surface properties evolved with increase in holding temperature and holding time [14]. Microspheres of different sizes were more prominently and frequently observed in hydrochar produced from MHTC of fish waste [14]. Taken together, these results indicate that MHTC might produce hydrochar with more microspheres which may result in better surface characteristics. MHTC has a much lower come-up time compared to CHTC, which also increases the total process time of the CHTC. These results strongly suggest that the better microspherical structure of hydrochar observed during MHTC could be potentially attributed to both the faster come-up time and a quicker total process time.
This study primarily focussed on the optimization of the hydrochar yield during CHTC of fish waste and then further characterized and compared the elemental, proximate, morphological, and energy quality of CHTC hydrochar with MHTC hydrochar. It should be noted that the liquid end product of the hydrochar is not characterized in this study. This end product should be further characterized as it may be of commercial interest due to the presence of dissolved organic compounds that occurred during HTC. The coffee-like scent of the liquid is a good indication of the presence of aromatic organic compounds. In previous studies, in other feed stocks such as food waste, the liquid end product has been found to contain many organic compounds, especially was enriched in 5-hydroxy methyl furfural [40]. The maximal solid yield recovered in this study was about ~ 35%, which suggests the rest of the matter is either present in the liquid or the gaseous phase. Therefore, in future studies a thorough characterization and quantification of components in the liquid product is needed. The gaseous products during HTC are often considered very little if any and should also be characterized in future studies [13]. This is of particular interest not only for the realistic assessment of mass balance in this process but also to evaluate the cost-benefit analysis, as liquid product might hold equal or more commercial value than the solid hydrochar.
This study has devised an efficient stand-alone or supplemental technology to completely utilize the fish wastes to produce end products that are of commercial interest. First, the yield of hydrochar obtained from a non-conventional biomass for HTC, i.e., non-lignocellulosic fish waste is largely comparable to certain lignocellulosic wastes, food wastes, and sewage wastes. Thus, this study would expand the feedstock pool that is available for HTC, which now can also include non-lignocellulosic wastes such as seafood waste and meat waste. Second, the hydrochar produced from fish waste possesses energy value and carbon content comparable to other hydrochars derived from traditionally used feedstock including certain lignocellulosic wastes, sewage, food waste, and even low-grade coals such as lignite. This suggests that hydrochar could potentially be used as a solid fuel. However, further thorough characterization of the fuel properties and emission properties are needed to conclusively determine the applicability of the hydrochar as a solid fuel. Previous studies that evaluated the co-combustion of hydrochars derived from coconut fiber and eucalyptus leaves with lignite have shown that such blends increase the energy conversion efficiency [51]. Third, hydrochars can be potentially used in a variety of fields such as carbon sequestration, catalytic, electronic, and biological applications. Field studies have shown mixed results where hydrochar has been shown to improve the carbon stability or decrease it [52]. Further, fish waste hydrochar also contains nitrogen, which may lead to the release of greenhouse gases [53]. Alternately, the nitrogen could also be beneficial as it will lead to a higher C/N ratio than most hydrochars and may potentially lead to better nitrogen availability to plants [54]. One other factor that could affect the suitability of fish waste hydrochar is the heavy metals that might potentially be present in the char. Further experimentation in the field and heavy metal characterization is necessary to assess the suitability of the hydrochar as carbon sequestering agent. Fourth, many studies have shown that HTC provides a green pathway for the synthesis of hydrochar containing micro and nanospheres [52]. These properties make hydrochar suitable for use as anode material in lithium ion batteries [52]. It has been shown that hydrochar exhibits higher cycling performance and conductivity in batteries as compared to biochar and coal [52]. The spheres in the hydrochar can also be used as support for catalysts through either impregnation of nanoparticles or through modification of surface functional groups [52]. Finally, hydrochar has also been shown to serve as potential adsorbents for the removal of heavy metals such as lead from water owing to their oxygen-rich functional groups that can selectively target positive ions [55]. It is noteworthy that the other end product, the liquid end product, could also be a good source of organic compounds that could of potential interest [40]. Therefore, HTC can provide a method to convert a low-value fish waste into two very attractive end products that may find applications not only as a source of energy or a sequestering agent but also for the production of variety of carbon materials including anodes in batteries and fuel cells.
4 Conclusions
In this study, we have optimized the CHTC of fish waste to maximize the yield of hydrochar using a response surface design. We found that the maximal yield of hydrochar was obtained at a holding temperature of 180 °C and 120 min, i.e., at a lower holding temperature compared to the MHTC of fish waste. Further, the yield of hydrochar produced at varying conditions of CHTC of fish waste is comparable to the yield of hydrochar achieved by MHTC at similar conditions. However, CHTC exhibits a come-up time that is 3–4 times more than that of the MHTC come-up time for fish waste. This suggests that MHTC might be preferable if shorter process times are key to the process engineer. Future experiments should aim to evaluate the energy and mass balance of these processes to decipher the suitability of these processes for large-scale manufacturing. This is very important as MHTC operates at a higher optimal process temperature of 200 °C, than CHTC that operates at 180 °C for carbonizing fish waste. This study adds one more proof to the new idea that the non-lignocellulosic wastes such as fish waste could be utilized for energy purposes by HTC irrespective of the heating medium used. Upon comparing the properties of hydrochar produced by CHTC and MHTC, i.e., the elemental and proximate composition, energy value, and the morphological properties, it is clear that there are no major differences. The end use of the hydrochar will also determine the medium of heating and processing conditions that one should consider in the treatment of the feedstock. For instance, if hydrochar with structurally well-formed microspheres are preferred, then MHTC has edge over CHTC. Therefore, further experiments are needed to assess additional properties of hydrochar such as porosity to assess its suitability to be adsorbent, fuel combustion properties to assess its suitability as energy source, and heavy metal and greenhouse gas emission to assess its environmental suitability. Such experiments could pave way for an engineer to make an informed choice on the technology and process requirements for HTC.
Abbreviations
- ANOVA:
-
Analysis of variance
- C:
-
Carbon
- CCD:
-
Central composite design
- CHTC:
-
Conventional hydrothermal carbonization
- DoE:
-
Design of experiment
- EEF:
-
Energy enrichment factor
- HTC:
-
Hydrothermal carbonization
- H:
-
Hydrogen
- MHTC:
-
Microwave hydrothermal carbonization
- N:
-
Nitrogen
- O:
-
Oxygen
- RFW:
-
Raw fish waste
- SEM:
-
Scanning electron microscope
- S:
-
Sulfur
References
Rai AK, Bhaskar N, Baskaran V (2015) Effect of feeding lipids recovered from fish processing waste by lactic acid fermentation and enzymatic hydrolysis on antioxidant and membrane bound enzymes in rats. J Food Sci Technol 52(6):3701–3710. https://doi.org/10.1007/s13197-014-1442-3
Shahidul Islam M, Tanaka M (2004) Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar Pollut Bull 48(7–8):624–649. https://doi.org/10.1016/j.marpolbul.2003.12.004
Hardy RW, Tacon AG (2002) Fish meal: historical uses, production trends and future outlook for sustainable supplies. Responsible Marine Aquaculture, p 311–325. https://doi.org/10.1079/9780851996042.0311
Arvanitoyannis IS, Kassaveti A (2008) Fish industry waste: treatments, environmental impacts, current and potential uses. Int J Food Sci Technol 43(4):726–745. https://doi.org/10.1111/j.1365-2621.2006.01513.x
Lopes C, Antelo LT, Franco-Uría A, Alonso AA, Pérez-Martín R (2015) Valorisation of fish by-products against waste management treatments—comparison of environmental impacts. Waste Manag 46:103–112. https://doi.org/10.1016/j.wasman.2015.08.017
Jamieson BL, Gagnon GA, Gonçalves AA (2017) Physicochemical characterization of Atlantic Canadian seafood processing plant effluent. Mar Pollut Bull 116(1):137–142
Grant MT, Corkum J, Morry C (2003) Management of wastes from Atlantic seafood processing operations. AMEC Earth & Environmental Limited TE23016, Dartmouth, p 135
Cashion T, Le Manach F, Zeller D, Pauly D (2017) Most fish destined for fishmeal production are food-grade fish. Fish Fish 18:837–844
Esteban M, Garcia A, Ramos P, Marquez M (2007) Evaluation of fruit–vegetable and fish wastes as alternative feedstuffs in pig diets. Waste Manag 27(2):193–200
Mandeville S, Yaylayan V, Simpson B (1992) Proximate analysis, isolation and identification of amino acids and sugars from raw and cooked commercial shrimp waste. Food Biotechnol 6(1):51–64
Ya T, Simpson B, Ramaswamy H, Yaylayan V, Smith J, Hudon C (1991) Carotenoproteins from lobster waste as a potential feed supplement for cultured salmonids. Food Biotechnol 5(2):87–93
Garcıa A, Esteban M, Marquez M, Ramos P (2005) Biodegradable municipal solid waste: characterization and potential use as animal feedstuffs. Waste Manag 25(8):780–787
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Titirici M-M, Fühner C, Bens O, Kern J (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2(1):71–106
Kannan S, Gariepy Y, Raghavan GV (2017) Optimization and characterization of hydrochar produced from microwave hydrothermal carbonization of fish waste. Waste Manag 65:159–168
Kannan S, Gariepy Y, Raghavan GV (2017) Optimization and characterization of hydrochar derived from shrimp waste. Energy Fuels 31(4):4068–4077
Reza MT (2011) Hydrothermal carbonization of lignocellulosic biomass. University of Nevada, Reno
Ramke H-G, Blöhse D, Lehmann H-J, Fettig J (2009) Hydrothermal carbonization of organic waste. In: Proc., Twelfth International Waste Management and Landfill Symposium, Sardinia, Italy
Lynam JG, Reza MT, Yan W, Vásquez VR, Coronella CJ (2015) Hydrothermal carbonization of various lignocellulosic biomass. Biomass Conversion and Biorefinery 5(2):173–181. https://doi.org/10.1007/s13399-014-0137-3
Lu X, Pellechia PJ, Flora JRV, Berge ND (2013) Influence of reaction time and temperature on product formation and characteristics associated with the hydrothermal carbonization of cellulose. Bioresour Technol 138:180–190. https://doi.org/10.1016/j.biortech.2013.03.163
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Biorefin 4(2):160–177
Kannan S, Gariepy Y, Raghavan V (2015) Optimization of enzyme hydrolysis of seafood waste for microwave hydrothermal carbonization. Energy Fuel 29(12):8006–8016
Doshi P, Srivastava G, Pathak G, Dikshit M (2014) Physicochemical and thermal characterization of nonedible oilseed residual waste as sustainable solid biofuel. Waste Manag 34(10):1836–1846
Pala M, Kantarli IC, Buyukisik HB, Yanik J (2014) Hydrothermal carbonization and torrefaction of grape pomace: a comparative evaluation. Bioresour Technol 161:255–262
Cao X, Ro KS, Libra JA, Kammann CI, Lima I, Berge N, Li L, Li Y, Chen N, Yang J (2013) Effects of biomass types and carbonization conditions on the chemical characteristics of hydrochars. J Agric Food Chem 61(39):9401–9411
Delaquis E, Samson R, Seguin P, Mustafa A, Martel H (2014) Impacts of improved switchgrass and big bluestem selections on yield, morphological characteristics, and biomass quality. Advances in Agriculture 2014:14. https://doi.org/10.1155/2014/192824
Mustafa AF, Thacker PA, McKinnon JJ, Christensen DA, Racz VJ (2000) Nutritional value of feed grade chickpeas for ruminants and pigs. J Sci Food Agric 80(11):1581–1588
Mäkelä M, Benavente V, Fullana A (2015) Hydrothermal carbonization of lignocellulosic biomass: effect of process conditions on hydrochar properties. Appl Energy 155:576–584
Román S, Nabais J, Laginhas C, Ledesma B, González J (2012) Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process Technol 103:78–83
Elaigwu SE, Greenway GM (2016) Microwave-assisted and conventional hydrothermal carbonization of lignocellulosic waste material: comparison of the chemical and structural properties of the hydrochars. J Anal Appl Pyrolysis 118:1–8. https://doi.org/10.1016/j.jaap.2015.12.013
Afolabi OOD, Sohail M (2017) Comparative evaluation of conventional and microwave hydrothermal carbonization of human biowaste for value recovery. Water Sci Technol 75:2852–2863
Teri G, Luo L, Savage PE (2014) Hydrothermal treatment of protein, polysaccharide, and lipids alone and in mixtures. Energy Fuel 28(12):7501–7509
Elaigwu SE, Greenway GM (2016) Chemical, structural and energy properties of hydrochars from microwave-assisted hydrothermal carbonization of glucose. International Journal of Industrial Chemistry 7:1–8. https://doi.org/10.1007/s40090-016-0081-0
Álvarez-Murillo A, Sabio E, Ledesma B, Román S, González-García CM (2016) Generation of biofuel from hydrothermal carbonization of cellulose. Kinetics modelling. Energy 94:600–608. https://doi.org/10.1016/j.energy.2015.11.024
Donar YO, Çağlar E, Sınağ A (2016) Preparation and characterization of agricultural waste biomass based hydrochars. Fuel 183:366–372. https://doi.org/10.1016/j.fuel.2016.06.108
Cordero T, Marquez F, Rodriguez-Mirasol J, Rodriguez JJ (2001) Predicting heating values of lignocellulosics and carbonaceous materials from proximate analysis. Fuel 80(11):1567–1571. https://doi.org/10.1016/S0016-2361(01)00034-5
Yin C-Y (2011) Prediction of higher heating values of biomass from proximate and ultimate analyses. Fuel 90(3):1128–1132. https://doi.org/10.1016/j.fuel.2010.11.031
Danso-Boateng E, Shama G, Wheatley AD, Martin SJ, Holdich RG (2015) Hydrothermal carbonisation of sewage sludge: effect of process conditions on product characteristics and methane production. Bioresour Technol 177:318–327. https://doi.org/10.1016/j.biortech.2014.11.096
Li L, Diederick R, Flora JRV, Berge ND (2013) Hydrothermal carbonization of food waste and associated packaging materials for energy source generation. Waste Manag 33(11):2478–2492. https://doi.org/10.1016/j.wasman.2013.05.025
Parshetti GK, Chowdhury S, Balasubramanian R (2014) Hydrothermal conversion of urban food waste to chars for removal of textile dyes from contaminated waters. Bioresour Technol 161:310–319. https://doi.org/10.1016/j.biortech.2014.03.087
Kaushik R, Parshetti GK, Liu Z, Balasubramanian R (2014) Enzyme-assisted hydrothermal treatment of food waste for co-production of hydrochar and bio-oil. Bioresour Technol 168:267–274. https://doi.org/10.1016/j.biortech.2014.03.022
Schimmelpfennig S, Glaser B (2012) One step forward toward characterization: some important material properties to distinguish biochars. J Environ Qual 41(4):1001–1013
Afolabi OOD, Sohail M, Thomas CPL (2015) Microwave hydrothermal carbonization of human biowastes. Waste and Biomass Valorization 6(2):147–157. https://doi.org/10.1007/s12649-014-9333-4
He C, Giannis A, Wang J-Y (2013) Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: hydrochar fuel characteristics and combustion behavior. Appl Energy 111:257–266. https://doi.org/10.1016/j.apenergy.2013.04.084
Reza MT, Lynam JG, Uddin MH, Coronella CJ (2013) Hydrothermal carbonization: fate of inorganics. Biomass Bioenergy 49:86–94. https://doi.org/10.1016/j.biombioe.2012.12.004
Chen W-H, Ye S-C, Sheen H-K (2012) Hydrothermal carbonization of sugarcane bagasse via wet torrefaction in association with microwave heating. Bioresour Technol 118:195–203. https://doi.org/10.1016/j.biortech.2012.04.101
Basso D, Patuzzi F, Castello D, Baratieri M, Rada EC, Weiss-Hortala E, Fiori L (2016) Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag 47:114–121
Channiwala SA, Parikh PP (2002) A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 81(8):1051–1063. https://doi.org/10.1016/S0016-2361(01)00131-4
Kang S, Li X, Fan J, Chang J (2012) Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Ind Eng Chem Res 51(26):9023–9031
Peterson SC, Appell M, Jackson MA, Boateng AA (2013) Comparing corn stover and switchgrass biochar: characterization and sorption properties. J Agric Sci 5(1):1
Zhang F, Wu Q (2012) Functional materials development from kitchen waste. Procedia Environ Sci 16:70–74
Liu Z, Quek A, Hoekman SK, Srinivasan M, Balasubramanian R (2012) Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour Technol 123:646–652
Hu B, Wang K, Wu L, Yu SH, Antonietti M, Titirici MM (2010) Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Mater 22(7):813–828
Kammann C, Ratering S, Eckhard C, Müller C (2012) Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J Environ Qual 41(4):1052–1066
Gajić A, Koch H-J (2012) Sugar beet (Beta vulgaris L.) growth reduction caused by hydrochar is related to nitrogen supply. J Environ Qual 41(4):1067–1075
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sust Energ Rev 45:359–378
Acknowledgements
The authors are grateful to Dr. Valerie Orsat for providing access to the FTIR equipment. The authors would like to acknowledge the “Elemental Analysis Service” at the University of Montreal and Dr. Arif Mustafa for the help with the bomb calorimetry experiments. The authors would like to acknowledge Dr. Darwin Lyew and Dr. Ramesh Murugesan for their help in consultations during the course of the research.
Funding
This work was supported by operating grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to GSVR and it was also supported by the Faculty for the Future grant by Schlumberger Foundation to SK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors are affiliated to the McGill University and have filed for a patent for the method described in this study. The authors declare that they do not have any non-financial competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 295 kb)
Rights and permissions
About this article
Cite this article
Kannan, S., Gariepy, Y. & Vijaya Raghavan, G.S. Optimization of the conventional hydrothermal carbonization to produce hydrochar from fish waste. Biomass Conv. Bioref. 8, 563–576 (2018). https://doi.org/10.1007/s13399-018-0323-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0323-9