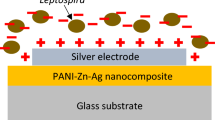
Abstract
The use of Ag–Pd bimetallic nanoparticles was reported as an enhanced sensing material in electrochemical sensing application. The composite of bimetallic nanoparticles with conducting polymer has attracted an interest in microbial sensor. Ag–Pd bimetallic nanoparticles embedded in polyaniline (PANI) system was synthesized using sol–gel method and deposited on glass substrate by spin coating technique with various concentrations of Ag and Pd. The films properties were studied using XRD, TEM and AFM to analyze the internal structure and surface morphology. Sensor performance was carried out using current–voltage (I–V) measurement and electrochemical impedance spectroscopy (EIS) to monitor the presence of Escherichia coli bacteria in liquid medium. From XRD analysis, the face-centered cubic of Ag–Pd bimetallic crystal were found at lattice (1 1 1) and (2 0 0). The crystallite size and lattice parameter were found to be decrease as concentration of Pd increased. TEM analysis shows the particles of Ag–Pd bimetallic in spherical shape with 10–20 nm in diameter. AFM analysis show the surface roughness of PANI–Ag–Pd thin films decrease as the concentration of Pd increased. From I–V and EIS analysis, the prototype sensor of PANI–Ag–Pd nanocomposite thin films performed well with high sensitivity when Pd concentration is increasing.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Safety concerns about food [1], drinking water [2], health surveillance and clinical diagnosis related to microbial pathogens [3] have attracted worldwide attention to develop microbial sensor. Gram-negative bacteria are a leading cause of food-borne illnesses such as urinary tract infections, hemorrhagic colitis, inflammation, bloody diarrhea, peritonitis and occasionally hemolytic uremic syndrome [4, 5]. Escherichia coli (E. coli) O157:H7 is one of the types of gram-negative bacteria which can easily be spread through contaminated raw food and drinks, domestic animals and contaminated environment. The hazard of this type of bacteria require for the rapid and convenient methods to avoid life threatening.
In recent study on microbe sensor, PANI is one of the conducting polymers which have widely being applied as sensing material. The intrinsically electrical features of PANI make it suitable for immobilization of biomolecules [6, 7]. Composite consisting PANI and metal nanoparticles enhance the characteristics for sensor application. The composite of PANI and metal nanoparticles not only retained the original respective intrinsic performances, but also exhibited coadjutant effect that is beneficial for improving the properties of PANI matrix in composites [8]. Ag and Pd are the transition metals which have potentials in sensing application. In recently years, Pd nanoparticles have been used in sensing application especially for bio components detection such as l-cysteine [9], vanillin [10], nifedipine [11] and glucose [12]. Some previous researcher also used Pd nanoparticles in gas sensing to detect hydrogen [13] and carbon monoxide [14]. The addition of Ag nanoparticles produces Ag–Pd bimetallic nanoparticles which have high sensitivity and selectivity due to the intrinsic effects of nanocomposite. Other characteristic such as the linear range, reproducibility and long-term stability were good to detect bio component.
Various methods have been used in previous study to synthesize Ag–Pd thin films such as magnetron sputtering [15], simultaneous electroless plating [16] and electron beam vacuum evaporation [13]. Sol–gel method has been used in several researches to synthesize Ag and Pd nanoparticle thin films separately [17, 18]. Sol–gel method can be considered as the most useful and influential method to prepare thin films [19, 20]. This method easily allows the preparation of nanocomposite materials such as inorganic matrices in which a metal phase could be highly dispersed [18]. One of the deposition techniques from sol–gel method which commonly being used is dip coating because it produce uniform surface of films and the films thickness can be easily controlled [21]. Apart from that, sol–gel spin coating technique are also being selected because of its low cost, low temperature annealing, control in compositions and nanostructures and feasibility in large scale synthesis on a variety of substrates [22].
Several reports on E. coli detection by impedance analysis have been studied by previous researchers such as Basu et al. [23], Chowdhury et al. [24], Ruan et al. [25] and Settu et al. [26]. Basu et al. [23] fabricated graphene based E. coli sensor using impedance spectroscopy to characterize the change in impedance, as a function of E. coli concentration on graphene surface. From the result, a sensitivity of 60% is achieved for an E. coli concentration of 4.5 × 107 CFU mL−1 [23]. Chowdhury et al. [24] found out that the Au/PANI/Glu/antibody sensor had successfully detected as small concentration as 102 CFU mL−1 of E. coli O157:H7 with the upper detection limit of 107 CFU mL−1. Ruan et al. [25] developed an E. coli sensor based on the surface immobilization of antibodies onto ITO electrode chips. The biosensor could detect the target bacteria with a detection limit, 6 × 103 CFU mL−1. A linear response in the electron-transfer resistance for the concentration of E. coli cells was found between 6 × 104 and 6 × 107 CFU mL−1 [25]. An interdigitated gold microelectrode-based impedance sensor was developed by Settu et al. [26] to detect E. coli in human urine samples for urinary tract infection (UTI) diagnosis and their sensor is capable of detecting a wide range of E. coli concentration, 7 × 100–7 × 108 CFU mL−1 in urine sample with high sensitivity. In this research, we developed the detection method to be a simple fabrication but accurate performance device, using low cost synthesis material and method. Electrochemical sensor is an easy device to produce commercialization especially for diagnosis and treatment in medical field, quality control in industrial field and also for environmental monitoring. The sensor performance is measured as sensitivity unit which is the ratio between the output signal and measured property. Sensitivity indicates how much the sensor’s output changes when the input quantity being measured changes. Electrochemical sensor provides electrical signal output. Amperometric and impedimetric sensors are two of the types of electrochemical sensor. In amperometric sensor, output current (I) is measured when the potential (V) is applied between working and reference electrodes. In impedimetric sensor, monitoring of variation in electrical properties arising from biorecognition events at the surfaces of modified electrodes [27].
For microbe sensing application, the performance of Ag–Pd bimetallic nanoparticles based electrochemical sensor is not been reported yet. Based on the potential of Pd nanoparticles in antimicrobial application [28], we investigated the capability of Pd nanoparticles in sensor performance to detect microbe. In this study, we fabricated a prototype E. coli sensor based on PANI–Ag–Pd nanocomposite thin films using I–V measurement and impedance analysis from EIS. The composition of Ag–Pd was varied to study the influence of addition and reduction of Ag and Pd on the sensitivity performance.
2 Experimental Details
2.1 Reagents and Materials
Silver nitrate (AgNO3), palladium (II) nitrate (Pd(NO3)2), aniline, polyvinyl alcohol (PVA) and nitric acid (HNO3) were purchased from Sigma-Aldrich and R&M Chemicals. All aqueous solutions were prepared with deionized water. Silver target for sputtering were purchased from Kurt J. Lesker. Sample of E. coli O157:H7 with concentration of 108 CFU/mL were supplied from Microbiology Laboratory, School of Bioscience and Biotechnology, Universiti Kebangsaan Malaysia.
2.2 Sample Preparation
A total of 0.5 g of AgNO3 and Pd(NO3)2 powder was dissolved in 40 mL deionized water before they were added in PVA solution. The solution of PVA-Ag-Pd nanocomposite was stirred and heated at 70 °C until the colour change to dark brown. The solution becomes PANI–Ag–Pd nanocomposite by adding aniline and 1.0 M of nitric acid. PANI–Ag–Pd nanocomposite was produced by polymerization process in the presence of metal particles. PANI–Ag–Pd nanocomposite was spin-coated onto glass substrates to be thin film. The substrates were spinned at 2000 rpm for 15 s. PANI–Ag–Pd nanocomposite thin films were annealed in a tube furnace with maximum temperature of 300 °C for 1 day. A 100 nm layer of silver electrode in comb-structure shape was sputtered on the PANI–Ag–Pd films using magnetron sputtering. Copper wires were soldered to the silver electrode terminals as the connection to the measuring equipment. The experimental procedure has been illustrated in a schematic diagram as shown in Fig. 1.
Schematic diagram of experimental work. a PANI–Ag–Pd nanocomposite thin film was deposited on glass substrate. Then the as-deposited films were analyzed using XRD, TEM and AFM. b A comb-structured silver electrode was deposited on the PANI–Ag–Pd thin film. c Sensor performance was conducted by dipping the sensor device into deionized water and E. coli O157:H7 solution and connected to I–V and impedance analysis software
2.3 Sample Characterization
The thin films were characterized by X-ray diffraction (XRD) spectroscopy, transmission electron microscopy (TEM) and atomic force microscopy (AFM). Bruker model D8 XRD diffractometer was used to identify the elements and their structure, to determine the degree of crystallinity and to obtain the crystallite size. Phillip CM12 Transmission Electron Microscopy was used to study the internal structure and to obtain the particles size. AFM was used to analyze the surface morphology which is the surface roughness of the films.
2.4 E. coli Detection Method
In this study, the performance of E. coli detection was conducted using current–voltage (I–V) measurement and electrochemical impedance spectroscopy (EIS) method by using GAMRY physical electrochemical instrument model Series G300. The sensor device is connected to the instrument by wire clip based on 2-electrode system. Experiment was conducted by dipping the sensor device into deionized water and E. coli O157:H7 solution with concentration 107 and 108 CFU mL−1. The reason of using zero E. coli and other two concentrations of E. coli in the experiment is to observe the trend of I–V characteristic and impedance towards E. coli concentrations in water. For I–V measurement method, the system mode is turned to Linear Sweep Voltammetry to obtain the I–V curve of the thin film sensor. The data was measured from 1 V to 6 V with the scan rate 800 mVs−1. For EIS method, the system mode is turned to Potentiostatic EIS. Alternating current (AC) is used and applied potential 1 V across the electrodes begins from frequency 50 kHz to 1 Hz. Graph of impedance against frequency and Nyquist plot are obtained from this measurement.
2.5 Repeatability and Stability
The repeatability of PANI–Ag–Pd nanocomposite thin film sensor was evaluated by conducting a series of three experiments in measuring I–V and impedance using one same film within a week. The results are almost similar to each other and the average values have been calculated and plotted as in Figs. 5 and 7. This investigation indicated that the repeatability of the sensor film is excellent and they can be used for a week without any abrupt change in the sensitivity and stability of performance.
3 Results and Discussion
3.1 X-Ray Diffraction (XRD)
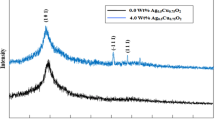
From XRD analysis, element structure, degree of crystallinity and the crystallite size can be determined. Figure 2 shows the XRD patterns of PANI–Ag–Pd nanocomposite thin films in various molar ratio of Ag–Pd. The two prominent peaks represent the face-centerd cubic of Ag–Pd bimetallic alloy which located at (1 1 1) and (2 0 0) peaks corresponding to 2θ = 39.2° and 45.6°. The high crystallinity of the samples was shown from the high and sharp peaks. The crystallinity decreases as the concentration of Pd increases. It can be seen in the pattern of PANI–Ag0.2–Pd0.8 where the intensity of the peak is lower than others. The crystallite sizes have been calculated using Scherrer equation [29]:
where D is crystallite size, κ is the Scherrer constant (0.9), λ is the X-ray wavelength (1.5406 Å), β is full width at half maximum (FWHM in radian unit) and θ is the diffraction angle. The crystallite size can be calculated by referring to prominent peak at 2θ = 39.2° with Miller Index (1 1 1). Lattice parameter, a can also be obtained from the prominent peak (1 1 1). Lattice parameter can be calculated using Eqs. 2 and 3.
where dhkl is space between two planes, λ is the X-ray wavelength (1.5406 Å) and θ is the diffraction angle at prominent peak.
where a is lattice parameter, dhkl is space between two planes and (h k l) is miller index of prominent peak.
As shown in Table 1, the crystallite size was found to be decrease as the concentration of Pd increase. The increasing of crystallite size was affected by atomic radius of the elements involved [30], where the atomic radius of Pd is smaller than Ag. This indicates that the addition of Pd concentration affects the radius of Ag–Pd crystallite; bring the size to become smaller. Lattice parameters, a are related to the 2θ where the prominent peak located at. From Table 1, a value decrease as the concentration of Pd increase because the prominent peak at (1 1 1) shifted to the left. From JCPDS (pattern: 01-072-5157), a value of AgPd is 3.977. Lattice parameter from Table 1 changes from that value and this change causes distortion in the crystal structure.
3.2 Transmission Electron Microscopy (TEM)
Figure 3 shows the TEM image of sample PANI–Ag0.2–Pd0.8 nanocomposite thin films. From the image, silver, palladium and PANI particles was observed. Since the silver and palladium nanoparticles were synthesized in the polyaniline solution, the nanoparticles got embedded into the PANI matrix. The silver and palladium nanoparticles are clearly observed spherical in shape and some particles agglomerate with different sizes in the range 10–20 nm. This indicates that nanosized inorganic particles possess a nearly spherical morphology and influence strongly the composite morphology [31]. The particle size range is corresponding with the result from AFM analysis.
3.3 Atomic Force Microscopy (AFM)
AFM analysis of PANI–Ag–Pd nanocomposite thin films can determine the surface roughness and particle size of the samples. Figure 4 shows the 3D images of AFM topography of PANI–Ag–Pd thin films surface. From the data analysis, the sample PANI–Ag0.8–Pd0.2 in Fig. 4a has the highest surface roughness (8.070 nm) and the largest particle size (17.8 nm). It is followed by PANI–Ag0.6–Pd0.4 in Fig. 4b which has 6.224 nm for surface roughness and 17.5 nm for particle size. The values of surface roughness and particles size of PANI–Ag0.4–Pd0.6 in Fig. 4c is becoming decrease which are 3.236 nm and 17.4 nm respectively and followed by PANI–Ag0.2–Pd0.8 in Fig. 4d which has 2.978 nm for surface roughness and 17.0 nm for particle size. From the trend, it can be seen that the surface roughness and particle size are affected by the molar percentage of Ag and Pd. The increase of the Pd molar percentage leads to the decreasing of PANI–Ag–Pd thin film surface roughness and decreasing of particle size. In microbial thin film sensor applications, film which has smoother surface will perform better sensitivity [32]. It is because the smooth film surface and smaller particle size make the interaction between nanoparticles and bacteria cell wall become more effective. This finding can be related to the study of antimicrobial activity by Adam et al. [28]. In their report, the nano size of Pd particles is important because the electronic structure of the nanoparticles surface will change as a function of the size and enhance the surface reactivity. In specific, nanoparticles with sizes 10 nm can attach to the surface of the bacteria cell membrane and break the normal function [28].
3.4 Sensitivity Performance
The prototype electrochemical biosensor was performed to test the sensitivity on E. coli presence. The experiment was conducted using I–V measurement to test the current change of the sensor with deionized water, E. coli 107 CFU mL−1 and E. coli 108 CFU mL−1. Figure 5 shows the I–V curve of PANI–Ag–Pd nanocomposite thin film sensor when the sensor electrodes were immersed in deionized water and E. coli solution. I–V curve data of sensor electrode in deionized water are required as our reference data since E. coli bacteria is detected in water. The measurement of current (mA) was taken in 1–6 V. From the result, when sensor was immersed in E. coli 107 CFU mL−1, the current increases higher than the current when the sensor was immersed in deionized water. The current become higher when the concentration of E. coli increases to be 108 CFU mL−1. This indicates that microbe had make interaction with metal nanoparticles. The interaction happened when E. coli cell wall attached to Ag and Pd nanoparticles. Ag nanoparticles have the ability to anchor to the bacterial cell wall and subsequently penetrate it, causing structural changes in the cell membrane such as in permeability [33]. The interaction also related to the differences in E. coli outer membrane order which are gram-negative or gram-positive. The cell wall of gram-negative bacteria contains lipids, protein and lipopolysacharrides (LPS) while the cell wall of gram-positive bacteria does not contain LPS. Lipids, protein and LPS can ensure more effective defences against biocides. E. coli which are gram-negative bacteria type possess those biomolecules and they have a unique periplasmic space that makes them more susceptible to metal nanoparticles such as Ag–Pd nanoparticles [34].
The sensitivity (S) of a sensor is described as the ratio of the magnitude of response upon exposure to the microbe (Ie) to that without exposure to the microbe (Io). Figure 6 shows the graph of sensitivity (S) on E. coli against the annealing temperature of PANI–Ag–Pd nanocomposite thin films which is calculated using Eq. 4 [35]:
where S is the sensitivity of sensor electrode on E. coli, Ie is the current when the sensor device is exposed to E. coli, and Io is the current when the sensor device is not exposed to E. coli. Figure 6 shows that the maximum sensitivity is performed by PANI–Ag0.2–Pd0.8, the Pd-rich nanocomposite thin film with sensitivity 4.69. From our previous study, Cu-rich PANI–Ag0.25–Cu0.75 performed the highest percentage of sensitivity which is 0.03 [36]. In another study using PANI–Ag–Fe nanocomposite thin films, the maximum sensitivity is performed by Ag-rich composite with sensitivity 0.14 (for non-annealed sample) and Fe-rich with sensitivity 0.04 (for annealed sample) [37]. This indicates PANI–Ag–Pd performed higher sensitivity among PANI–Ag–Cu and PANI–Ag–Fe and the Pd-rich sample more sensitive than Ag-rich sample.
The Nyquist impedance plots of the as-deposited PANI–Ag–Pd nanocomposite thin films when the films were immersed into E. coli bacteria solution are shown in Fig. 7. \(Z_{real}\) is the real part and \(Z_{imag}\) is the imaginary part of the complex impedance over the frequency range 1 Hz–50 kHz with AC amplitude of 1 V. The equivalent RC model for PANI–Ag–Pd nanocomposite thin films is shown in Fig. 8 where Rsol is the solution resistance, CCPE is a constant phase element capacitance, W is the Warburg impedance and Rct is the charge transfer resistance at PANI–Ag–Pd film interface. The equivalent circuit was considered in order to analyze the impedance spectroscopy data. In the circuit, the element W is related to the ion transfer phenomenon [38]:
The constant phase element (CPE) is used for the diffusion at low frequencies and it is defined as Eq. 6 [37]:
In Fig. 7, PANI–Ag–Pd nanocomposite thin films show the impedance data in the range of 5–20 \({\text{k}}\Omega\) for all compositions. These impedance differences in the cell system were obtained by changing the thin film samples (different compositions) in the sensor system where the films were immersed into E. coli solution.
Total impedances were obtained from the total of real impedance and imaginary impedance using Eq. 7:
PANI–Ag–Pd nanocomposite thin films were exposed to E. coli solution and the impedance values have been measured. The decrease in the impedance with increasing frequency can be attributed to capacitive component of the current [39]. After bacteria exposure, the impedance value at high frequency decreases which indicates that the decrease in charge transfer resistance dominates the total impedance value in the low frequency range [37]. From Fig. 9, the higher the Pd concentration, the lower the impedance was measured. The impedance decreases due to the smaller size of the crystallite and particle inside the nanocomposite and low roughness of the film surface which may lead to higher sensitivity. This finding is corresponding to our previous study on the nanocomposite of PANI–Ag–Cu [36]. Lower impedance decreases the resistivity in the E. coli solution and makes the interaction between metals and microbe become more effective.
4 Conclusion
PANI–Ag–Pd nanocomposite thin films based electrochemical E. coli sensor was synthesized using sol–gel spin coating method. Compositions of Ag–Pd bimetallic nanoparticles in PANI matrix were varied to study the optimum composition for the sensor to perform high sensitivity. The effects on structural and morphological properties on sensor performance were studied. XRD analysis shows the present Ag–Pd bimetallic alloys were in face-centered cubic structure and the crystallite sizes were found the smallest in samples PANI–Ag0.2–Pd0.8. TEM shows the image of Ag–Pd bimetallic nanoparticles were in diameter size of 8.5–16.5 nm with spherical shape. AFM analysis shows the surface roughness of the films decrease as the Pd concentration increase. The sensor performance of PANI–Ag–Pd nanocomposite thin films upon E. coli cells in liquid medium indicates the highest sensitivity is performed by PANI–Ag0.2–Pd0.8. This indicates that the addition of Pd in Ag–Pd bimetallic affects a good reaction between metal nanoparticle and microbe. Thus, this PANI–Ag–Pd nanocomposite thin film has potential application for further research in E. coli detection.
References
Zhang, X.S., Topping, M.E.C., McKendrick, I.J., Savill, N.J., Woolhouse, M.E.J.: Spread of E. coli O157 infection among Scottish cattle farms: stochastic models and model selection. Epidemics. Neth. 2, 11–20 (2010)
Saxena, T., Kaushik, P., Mohan, M.K.: Prevalence of E. coli O157:H7 in water sources: an overview on associated diseases, outbreaks and detection methods. Diagn. Microbiol. Infect. Dis. 82, 249–264 (2015)
Mamani, M., Nobari, N., Alikhani, M.Y., Poorolajal, J.: Antibacterial susceptibility of Escherichia coli among outpatients with community-acquired urinary tract infection in Hamadan. Iran. J. Glob. Antimicrob. Resist. 3, 40–43 (2015)
Fan, N.C., Chen, H.H., Chen, C.L., Ou, L.S., Lin, T.Y., Tsai, M.H., Chiu, C.H.: Rise of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli in children. J. Microbiol. Immunol. Infect. 47, 399–405 (2014)
Ghunaim, H., Abu-Madi, M.A., Kariyawasam, S.: Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Vet. Microbiol. 172, 13–22 (2014)
Arshak, K., Adley, C., Moore, E., Cunniffe, C., Campion, M., Harris, J.: Characterisation of polymer nanocomposite sensors for quantification of bacterial cultures. Sensor. Actuat. B 126, 226–231 (2007)
Arshak, K., Velusamy, V., Korostynska, O., Oliwa-Stasiak, K., Adley, C.: Conducting polymers and their applications to biosensors: emphasizing on foodborne pathogen detection. IEEE Sens. J. 9, 1942–1951 (2009)
Ćirić-Marjanovic, G.: Recent advances in polyaniline composites with metals, metalloids and non-metals. Synth. Met. 170, 31–56 (2013)
Murugavelu, M., Karthikeyan, B.: Study of Ag–Pd bimetallic nanoparticles modified glassy carbon electrode for detection of l-cysteine. Superlattices Microstruct. 75, 916–926 (2014)
Li, J., Feng, H., Li, J., Jiang, J., Feng, Y., He, L., Qian, D.: Bimetallic Ag–Pd nanoparticles-decorated grapheme oxide: a fascinating three-dimensional nanohybrid as an efficient electrochemical sensing platform for vanillin determination. Electrochim. Acta 176, 827–835 (2015)
Mijowska, E., Onyszko, M., Urbas, K., Aleksandrzak, M., Shi, X., Krysztof, M.P., Podolski, J., El Fray, M.: Palladium nanoparticles deposited on graphene and its electrochemical performance for glucose sensing. Appl. Surf. Sci. 355, 587–592 (2015)
Shang, L., Zhao, F., Zeng, B.: Highly dispersive hollow PdAg alloy nanoparticles modified ionic liquid functionalized graphene nanoribbons for electrochemical sensing of nifedipine. Electrochim. Acta 168, 330–336 (2015)
Wang, M., Feng, Y.: Palladium–silver thin film for hydrogen sensing. Sens. Actuat. B 123, 101–106 (2007)
Trung, D.D., Hoa, N.D., Tong, P.V., Duy, N.V., Dao, T.D., Chung, H.V., Nagao, T., Hieu, N.V.: Effective decoration of Pd nanoparticles on the surface of SnO2 nanowires for enhancement of CO gas-sensing performance. J. Hazard. Mater. 265, 124–132 (2014)
Geng, Z.T., He, Q., Jin, C.G.: Study on deposition technique and properties of Pd/Ag alloy film sensor supported on ceramic substrate. IOP Conference Series: Materials Science and Engineering, vol. 137, p. 012009 (2016)
Fernandez, E., Coenen, K., Helmi, A., Melendez, J., Zuniga, J., Pacheco, D.P., Tanaka, D.A.P., van Sint Annaland, M., Gallucci, F.: Preparation and characterization of thin film Pd–Ag supported membranes for high-temperature applications. Int. J. Hydrog. Energy 40, 13463–13478 (2015)
Ali, S.M., Farooq, W.A., Baig, M.R., Shar, M.A., Atif, M., Alghamidi, S.S., Algarawi, M.S., Rehman, N.U., Aziz, M.H.: Structural and optical properties of pure and Ag doped ZnO thin films obtained by sol gel spin coating technique. Mater. Sci. Poland 33, 601–605 (2015)
Chekin, F., Bagheri, S., Abd, S.B.: Hamid, synthesis and spectroscopic characterization of palladium-doped titanium dioxide catalyst. Bull. Mater. Sci. 38, 461–465 (2015)
Abdullah, H., Noor Azmy, N.A., Naim, N.M., Hamid, A.A., Idris, S.: Synthesis and fabrication of ZnO–CuO doped PVA and ZnO–PbO doped PVA nanocomposite films by using γ-radiolysis and it’s microbial sensor application. J. Sol Gel Sci. Technol. 74, 15–23 (2015)
Abdullah, H., Zulfakar, M.S., Jalal, W.N.W., Islam, M.T., Shaari, S.: Synthesis and fabrication of (1 − x)ZnAl2O4–xSiO2 thin films to be applied as patch antennas. J. Sol Gel Sci. Technol. 69, 183–192 (2014)
Kayani, Z.N., Iqbal, M., Riaz, S., Zia, R., Naseem, S.: Fabrication and properties of zinc oxide thin film prepared by sol–gel dip coating method. Mater. Sci. Poland 33, 515–520 (2015)
Foo, K.L., Kashif, M., Hashim, U., Ali, M.E.: Fabrication and characterization of ZnO thin films by sol–gel spin coating method for the determination of phosphate buffer saline concentration. Curr. Nanosci. 9, 288–292 (2013)
Basu, P.K., Indukuri, D., Keshavan, S., Navratna, V., Vanjari, S.R.K., Raghavan, S., Bhat, N.: Graphene based E. coli sensor on flexible acetate sheet. Sensor. Actuat. B 190, 342–347 (2014)
Chowdhury, A.D., De, A., Chaudhuri, C.R., Bandyopadhyay, K., Sen, P.: Label free polyaniline based impedimetric biosensor for detection of E. coli O157:H7 bacteria. Sensor. Actuat. B 171–172, 916–923 (2012)
Ruan, C., Yang, L., Li, Y.: Immunobiosensor chips for detection of Escherichia coli O157:H7 using electrochemical impedance spectroscopy. Anal. Chem. 74, 4814–4820 (2002)
Settu, K., Chen, C.J., Liu, J.T., Chen, C.L., Tsai, J.Z.: Impedimetric method for measuring ultra-low E. coli concentration in human urine. Biosens. Bioelectron. 66, 244–250 (2015)
Prakash, S., Chakrabarty, T., Singh, A.K., Shahi, V.K.: Polymer thin films embedded with metal nanoparticles for electrochemical biosensors applications. Biosens. Bioelectron. 41, 43–53 (2013)
Adams, C.P., Walker, K.A., Obare, S.O., Docherty, K.M.: Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS One 9, e85981 (2014)
Uvarov, V., Popov, I.: Metrological characterization of X-ray diffraction methods at different acquisition geometries for determination of crystallite size in nano-scale materials. Mater. Charact. 85, 111–123 (2013)
Abdullah, H., Noor Azmy, N.A., Naim, N.M., Bolhan, A., Hamid, A.A., Shaari, S.: Synthesis and fabrication of PVA–Ag–Cu and PANI–Ag–Cu nanocomposite thin film sensor for detection of E. coli in water. Adv. Mater. Res. 911, 131–135 (2014)
Reda, S.M., Al-Ghannam, S.M.: Synthesis and electrical properties of polyaniline composite with silver nanoparticles. Adv. Mater. Phys. Chem. 2, 75–81 (2012)
Abdullah, H., Naim, N.M., Bolhan, A., Noor Azmy, N.A., Hamid, A.A.: Morphology, structural and electrical properties of Ag–Cu alloy nanoparticles embedded in PVA matrix and its performance as E. coli monitoring sensor. Arab. J. Sci. Eng. 40, 915–922 (2014)
Prabhu, S., Poulose, E.K.: Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2, 32 (2012)
Díaz-Visurraga, J., Gutiérrez, C., Plessing, C., García, A.: Metal nanostructures as antibacterial agents. In: Mendez-Vilas, A. (ed.) Science Against Microbial Pathogens: Communicating Current Research and Technology Advances, pp. 210–218. Formatex, Badajoz (2011)
Dixit, V., Tewari, J.C., Sharma, B.S.: Detection of E. coli in water using semi-conducting polymeric thin film sensor. Sensor. Actuat. B 120, 96–103 (2006)
Abdullah, H., Naim, N.M., Noor Azmy, N.A., Hamid, A.A.: PANI–Ag–Cu nanocomposite thin films based impedimetric microbial sensor for detection of E. coli bacteria. J. Nanomater. 2015, Article ID 951640 (2015)
Naim, N.M., Abdullah, H., Umar, A.A., Hamid, A.A., Shaari, S.: Thermal annealing effect on structural, morphology and sensor performance of PANI–Ag–Fe based electrochemical E. coli sensor for environmental monitoring. Sci. World. J. 2015, Article ID 696521 (2015)
Carrara, S., Bavastrello, V., Ricci, D., Stura, E., Nicolini, C.: Improved nanocomposite materials for biosensor applications investigated by electrochemical impedance spectroscopy. Sens. Actuat. B 109, 221–226 (2005)
Khanna, P.K., Singh, N., Charan, S., Subbarao, V.V.V.S., Gokhale, R., Mulik, U.P.: Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method. Mater. Chem. Phys. 93, 117–121 (2005)
Acknowledgements
This project was supported by Exploratory Research Grants Scheme (ERGS/1/2012/STG05/UKM/02/5), Islamic Educational, Scientific and Cultural Organization (ISESCO) (KK-2013-006), and Photonic Technology Laboratory, Department of Electrical, Electronic and System Engineering, University Kebangsaan Malaysia, Bangi, Selangor, Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naim, N.M., Abdullah, H. & Hamid, A.A. Influence of Ag and Pd Contents on the Properties of PANI–Ag–Pd Nanocomposite Thin Films and Its Performance as Electrochemical Sensor for E. coli Detection. Electron. Mater. Lett. 15, 70–79 (2019). https://doi.org/10.1007/s13391-018-0087-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-018-0087-1