Abstract
In this work, we reported synthesis of palladium (Pd)-doped titanium dioxide (TiO2) (Pd-TiO2) nanoparticles by the sol–gel-assisted method. The synthesized Pd-doped TiO2 nanoparticles were characterized using X-ray diffraction, transmission electronic microscopy, energy-dispersive spectroscopy, Fourier transform infrared (FT-IR) spectroscopy and voltammetry techniques. The analysis showed that particles are spherical in shape and pure anatase form with average size about 10 nm. To investigate the catalytic efficiency of Pd-TiO2 nanoparticles, the hydrogen evolution reaction using the deposited film of Pd-TiO2 nanoparticles on glassy carbon electrode (Pd-TiO2/GCE) was studied in 0.1 M H2SO4 solution using linear scanning voltammetry. This study demonstrates the feasibility of using gelatin for the synthesis of Pd-TiO2 catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium dioxide (TiO2) thin films have applications in optics and electronics because of their excellent properties, e.g. chemical and physical stability, high refractive index, high dielectric constants, high electrical resistance and interesting catalytic properties. The catalytic activity of TiO 2 is dependent on its crystal structure, crystal size distribution, surface roughness, surface hydroxyl group density, and so on.[1]
Highly dispersed nanoparticles of noble metals, such as Pt, Pd, Rh, Ru and Au in mesoporous supports as titania, alumina, silica are widely used as catalysts in organic synthesis, petrochemistry, etc. In a study concerning the photocatalytic activity of noble-metal-loaded TiO 2,[2–8]an ohmic contact is formed between the metal and semiconductor. Hence electrons can easily flow to the metal sites on TiO 2 under irradiation and the role of the metal is to act as an electron sink and thus to enhance the activity. However, it is difficult to introduce metal nanoparticles into mesopores by traditional impregnation methods, because they tend to deposit richly on outer surface of mesoporous materials and moreover it is difficult to control the loading amount by impregnation.[9]
Various deposition techniques such as electron beam evapouration,[10]metal organic chemical vapour deposition (MOCVD),[11] pulsed laser deposition,[12] reactive sputtering technique,[13] spray pyrolysis,[14] hydrothermal process[15] and sol–gel methods[16] have been used for production of TiO 2 thin films. Besides the above methods, sol–gel technique can be considered as the most influential technique for preparation of the mesoporous thin layers. It can provide good uniform composition and large specific areas via mild processing conditions.[17,18]High-purity and homogeneous oxide materials can be prepared by the sol–gel method, based on hydrolysis and polycondensation of metal-organic precursors, that allows an excellent compositional control. Sol–gel method easily allows the preparation of nanocomposite materials such as inorganic matrices in which a metal phase could be highly dispersed. Many studies deal with Pt dopant.[19–21]Concerning Pd doping, generally, its dispersion by the sol–gel method was made in SiO 2,[22,23]Al 2 O 3[24,25]and in vitreous matrices.[4–8,26]There are few references so far regarding the use of Pd 2+ as a dopant in the case of a sol–gel prepared TiO 2 matrix.
The hydrogen evolution reaction (HER) is an electrochemical process that has received wide attention because of its importance in both fundamental and technological electrochemistry such as fuel cell technology.[27,28]The search for new and less expensive alternative materials for HER has been a topic of current interest.[29–31]Deposition of Pt or Pd particles on the less expensive materials such as carbon supports reduced the cost of anode materials in the industrial applications.
In this paper, we report on construction of Pt-doped TiO2 nanoparticles (Pd-TiO2) by the green synthesis method. Techniques X-ray diffraction (XRD), transmission electronic microscopy (TEM), energy-dispersive spectrometer (EDS), Fourier transform infrared (FT-IR) spectroscopy and voltammetry were used to characterize the structure and property of the Pd-TiO2. It was found that Pd-TiO2 exhibited good catalytic activity toward the hydrogen evolution reaction.
2 Experimental
2.1 Materials and apparatus
Titanium tetraisopropoxide with purity 98% was obtained from Acros Organics. Palladium nitrate was purchased from Aldrich. Glacial acetic acid (100%) was purchased from Merck. Other reagents were of analytical grade and purchased from Aldrich or Merck and used as received without further purification.
Electrochemical measurements were performed with an Autolab Potentiostat/Galvanostat (Netherlands). The three-electrode system consists of a Pd-TiO 2/GCE as working electrode, Ag—AgCl—KCl 3M as the reference electrode and a platinum wire as an auxiliary electrode (Metrohm) were used in all voltammetric experiments. Bruker-D8 powder XRD was used for determination of crystal phase identification and estimation of the crystallite size. LEO-Libra 120 microscope was employed for Pd-TiO 2 nanoparticles TEM images. FT-IR spectroscopy studies were carried out with BRUKER FT-IR spectrometer. OXFORD (INCA Energy 400) EDS was used for the elemental analysis and chemical characterization of the sample. Electrochemical measurements were performed with an Autolab Potentiostat/Galvanostat (Netherlands).
2.2 Synthesis of Pd-doped TiO2 nanoparticles
The precursor solution was prepared by dissolving titanium tetraisopropoxide (TTIP) in the glacial acetic acid (AA), followed by the addition of H 2O. The mol ratio of TTIP:AA:H 2O must be kept at 1:10:200. Gelatin solution was made by dissolving 3.0 g of gelatin in 100 ml of deionized water and stirred for 30 min at 60 ∘C to achieve a clear gelatin solution. Then, the gelatin solution was added into the precursor solution. Then, 10 ml of 1 M Pd solution was added drop wise into the precursor solution for a few minutes. The solution was dried at the temperature of 80 ∘C overnight. The dried gel was grinded and calcined in a muffle furnace at 500 ∘C for 5 h.
2.3 Fabrication of Pd-TiO2/GCE
Prior to modification, the bare GCE was polished on chamois leather with 0.05 μm alumina powder. Then it was thoroughly sonicated in deionized water and absolute ethanol, respectively. One milligram of synthesized Pd-TiO 2 was dispersed in 5 ml dimethyl formamide (DMF) with the aid of ultrasonic agitation. The cleaned GCE was coated by casting 5 μl of the Pd-TiO 2 solution and dried at 50 ∘C in an oven air to remove the solvent.
3 Results and discussion
3.1 X-ray diffraction and FT-IR spectroscopy
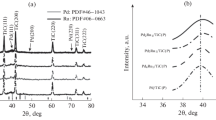
Figure 1 shows XRD patterns of TiO 2 and Pd-TiO 2 powders calcined at 500 ∘C under air for 5 h. From the wide-angle XRD pattern, the titania samples exist only in anatase phase, with their characteristic diffraction peaks of 2 𝜃 values at about 25.4 (101), 37.9 (004), 48.2 (200), 54.0 (105), 55.1 (211) and 62.9 (204), respectively. In consequence, the prepared Pd/TiO 2 (pattern a) and TiO 2 (pattern b) powders are well-crystallized pure anatase form. From the maximum diffraction peak at figure 1 by Scherrer’s formula (D =K λ/ βcos 𝜃, where D is the crystallite size, K the Scherrer constant usually taken as 0.89, λ the wavelength of the X-ray radiation (0.15418 nm for Cu K α), and β the full-width at half-maximum of diffraction peak measured at 2 𝜃, the average particle sizes of pure Pd-TiO 2 and TiO 2 powders for (101) plane, are about 10 and 15 nm, respectively. In comparison with XRD pattern of pure TiO 2, Pd loaded on TiO 2 surface nearly has no influence on crystalline structure. Pd phase has not been detected in the XRD patterns of Pd-TiO 2 powders, possibly because the Pd content on TiO 2 surface is not enough to form clearly crystalline. A comparison of particle size, temperature and synthesis method of Pd-TiO 2 synthesized in this work with those already reported in literatures is shown in table 1. As shown, parameters are comparable with other results.
Figure 2 shows the FT-IR spectra of Pd-TiO 2 (pattern a) and TiO 2 (curve b) nanoparticles in range of 400– 4000 cm −1 which were synthesized via the sol–gel method. In undoped TiO 2, the peaks at 431 and 701 cm −1 are for O–Ti–O bonding. The band centered at 1608 cm −1 is characteristic of δ-H 2O bending. The broad absorption bands between 400 and 800 cm −1 are mainly ascribed to Ti–O and O–Ti–O– flexion vibration. However, the vibration bands between 1300 and 4000 cm −1 are mainly assigned to the chemisorbed and/or physisorbed H 2O and CO 2 molecules on the surface of the compound. When metal ions are doped to the surface of TiO 2, the absorption band transforms and simultaneously new absorption band appears. Upon addition of dopant, a small shift was detected for the stretching vibration of Ti–O.[33]
3.2 Transmission electronic microscopy and energy- dispersive spectroscopy
TEM micrographs of TiO 2 and Pd-TiO 2 are shown in figure 3. Electron microscopy analyses reveal that TiO 2 (image a) and Pd-TiO 2 (image b) samples exhibit similar morphology. As shown in TEM images, particle size of the Pd-TiO 2 is smaller than TiO 2. Energy dispersive X-ray spectroscopy (EDS) of Pd-TiO 2 is shown in figure 4 which confirms the existence of Ti, O and Pd with weight percent.
3.3 Electrochemical behaviour of Pd-TiO 2/GCE
The cyclic voltammetry (CV) curve of Pd-TiO 2/GCE in 0.1 M H 2 SO 4 solution at 50 mV s −1 in a potential window of −0.3 to 1.2 V (figure 5) exhibits a typical H + ion electro-adsorption/desorption region, a double layer charging current region, a Pd pre-oxidation region and a Pd oxy-species reduction region. In the proton adsorption/desorption region, current peaks observed at 0 V and −0.1 V, which corresponds to polycrystalline Pd, confirmed the existence of Pd, as evidenced by EDS, FT-IR and TEM. The surface concentration of the electroactive Pd on Pd-TiO 2/GCE, Γ (in mol cm −2), can be estimated using the equation[34]
where Q is the charge consumed in Coulombs, obtained from integrating the anodic (or cathodic) peak area in cyclic voltammograms under the background correction. The average Γ value of (1.44 ± 0.89) × 10 −10 mol cm −2 was obtained.
3.4 Electrocatalytic behaviour of Pd-TiO 2/GCE
To evaluate the activity of the Pd-TiO 2 catalyst, the electrocatalysis of HER was studied by linear scanning voltammetry (LSV), and results are shown in figure 6. As expected, the electrocatalytic activity improves at surface of Pd-TiO 2/GCE (curve b) as though the onset potential of HER occurs at about −0.4 V vs. Ag—AgCl—KCl 3M and is indicated by an abrupt increase of the cathodic current. Also, the experiment concerning HER on a bare GCE (curve a) was studied as shown in figure 6. As can be seen, at the same current density, the overpotential of HER at Pd-TiO 2/GCE is lower than that at GCE.
4 Conclusion
In summary, we demonstrated that Pd-doped TiO 2 nanoparticles can be easily formed by the sol–gel method. The analysis showed that particles are spherical in shape and pure anatase form with average size about 10 nm. To investigate the catalytic efficiency of Pd-TiO 2, the hydrogen evolution reaction was studied using the deposited film of Pd-TiO 2 on glassy carbon electrode in 0.1 M H 2 SO 4 solution using LSV. The electrocatalytic activity improves at surface of Pd-TiO 2/GCE as though the onset potential of HER occurs at about −0.4 V vs. Ag—AgCl—KCl 3M and is indicated by an abrupt increase of the cathodic current.
References
Sonawane R S, Kale B B and Dongare M K 2004 Mater. Chem. Phys. 85 52
Chen H W, Ku Y and Kuo Y L 2007 Water Res. 41 2069
Huang M, Xu C, Wu Z, Huang Y, Lin J and Wu J 2008 Dyes Pigm. 77 327
Ranjit K T, Varadarajan T K and Viswanathan B 1996 J. Photochem. Photobiol. A: Chem. 96 181
Crisan D, Drăgan N, Crisan M and Răileanu M 2009 J. Phys. Chem. Solids 69 2548
Mardare D, Iftimie N, Crişan M, Răileanu M, Yildiz A, Coman T, Pomoni K and Vomvas A 2011 J. Non-Cryst. Solids 357 1774
Kim S C, Heo M C and Hahn S H 2005 J. Kor. Phys. Soc. 47 700
Yarmand B and Sadrnezhaad S K 2010 Optoelectron. Adv. Mater. Rapid Commun. 4 1572
Yuan S, Sheng Q, Zhang J, Chen F, Anpo M and Dai W 2006 Catal. Lett. 107 19
Bhattacharyya D, Sahoo N K, Thakur S and Das N C 2000 Thin Solid Films 360 96
Zhang X W, Zhou M H and Lei L C 2006 Catal. Commun. 7 427
Gyorgy E, Socol G, Axente E, Mihailescu I N, Ducu C and Ciuca S 2005 Appl. Surf. Sci. 247 429
Mardare D, Nica V, Teodorescu C M and Macovei D 2007 Surf. Sci. 601 4479
Oja I, Mere A, Krunks M, Nisumaa R, Solterbeck C H and Es-Souni M 2006 Thin Solid Films 515 674
Kambe S, Murakoshi K, Kitamura I, Wada Y, Yanagida S, Kominami H and Kera Y 2000 Sol. Energy Mater. Sol. Cells 61 427
Cernigoj U, Stangar U L, Trebxe P, Kraxovec U O and Gross S 2006 Thin Solid Films 495 327
Yun H, Miyazawa K, Honma I, Zhou H and Kuwabara M 2003 Mater. Sci. Eng. C 23 487
Liu K, Zhang M, Shi K and Fu H 2005 Mater. Lett. 59 3308
Facchin G, Carturan G, Campostrini R, Gialanella S, Lutterotti L, Armelao L, Marci G, Palmisano L and Sclafani A 2000 J. Sol–Gel Sci. Technol. 18 29
López T, Gómez R, Pecci G, Reyes P, Bokhimi X and Novaro O 1999 Mater. Lett. 40 59
López T, Gómez R, Romero E and Schifter I 1993 React. Kinet. Catal. Lett. 49 95
López T, Morán M, Navarrete J, Herrera L and Gómez R 1992 J. Non-Cryst Solids 147&148 753
López T, Bosch P, Navarrete J, Asomoza M and Gómez R 1994 J. Sol–Gel Sci. Technol. 1 193
Othman M R and Sahadan I S 2006 Micropor. Mesopor. Mater. 91 145
Noh J, Yang O B, Kim D H and Woo S I 1999 Catal. Today 53 575
Carturan G, Facchin G, Gottardi V, Guglielmi M and Navazio G 1982 J. Non-Cryst. Solids 48 219
Trasatti S 1991 Electrochim. Acta 36 225
Xu Y H, He G R and Wang X L 2003 Int. J. Hydrogen Energy 28 961
Shibli S M A and Dilimon V S 2007 Int. J. Hydrogen Energy 32 1694
Karimi Shervedani R and Madram A R 2007 Electrochim. Acta 53 426
Xu Y, Chen C, Wang X and Wang Q 2007 Int. J. Hydrogen Energy 32 537
Moon J, Park J Ah, Lee Su J, Zyung T and Kim D 2010 Sens. Actuators B 149 301
Zhang X, Zhou G, Xu J, Bai G and Wang L 2010 J. Solid State Chem. 183 1394
Laviron E 1979 J. Electroanal. Chem. 100 263
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
CHEKIN, F., BAGHERI, S. & ABD HAMID, S.B. Synthesis and spectroscopic characterization of palladium-doped titanium dioxide catalyst. Bull Mater Sci 38, 461–465 (2015). https://doi.org/10.1007/s12034-015-0883-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-015-0883-2