Abstract
In this study, the performance of using two different adsorbents, nano-zero-valent iron (nZVI) and activated carbon (AC), was examined for the treatment of real textile effluents. The porous structure and chemical composition of the synthesized nZVI were detected via X-ray diffraction, scanning electron microscopy and EDX analysis. Batch adsorption studies were conducted to investigate the optimal operating conditions including pH, adsorbent dose, contact time and stirring rate for the removal of COD, TSS and color from real textile wastewater. At same optimal operating conditions, pH 6, dose 0.8 g/L, contact time 20 min and stirring rate 100 rpm, the experimental results showed distinctive removal efficiency by using AC reached to 78.8% for COD, 76.2% for TSS and 84% for color, while nZVI recorded relatively lower removal efficiency reached to 74.7% for COD, 72.6% for TSS and 80% for color. A comparison study between nZVI and AC was conducted to evaluate the potential of using the two sorbent materials based on technical and sustainable criteria using different multi-criteria decision-making methods: TOPSIS, AHP and SAW. The study concluded that generally AC is better than nZVI based on the established criteria and weights.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the earlier decades, water pollution has increased dramatically owing to continuous and large quantities of textile industry discharge. The textile effluents are considered chemically intensive wastewaters and one of the extreme hazardous effluents that have strong color. In addition, it is deeply polluted by chemical oxygen demand (COD), total suspended solids (TSS) accompanied by elevated pH and temperature. The concentrations of textile wastewater pollutants vary from one factory to another. Table 1 summarizes the characteristics of raw textile wastewater along with pollutants concentration ranges [1]. The existence of TSS in water could seriously affect the marine environment by choking the breathing organ of fish and block the way of natural light to penetrate the water causing eutrophication. On the other side, dyes can damage aquatic life cycle as it bear chemical and organic deviations and devour dissolved oxygen from water. In humans, dyes can cause dermatitis and respiratory diseases and also could be central to circulatory disorders, lung irritation and eye problems. Wide categories of dyes have complex chemical structure and not degradable [1]. Numerous researches have been conducted several biological treatment processes for textile wastewater treatment [2, 3]. All of the predictable biological treatment approaches are not efficient enough to degrade the dyes wholly as well as will not keep up with the massive effluent amounts in a short time [2, 4]. Therefore, dyes exclusion from textile discharges remains a key problem. Lately, diverse efficient treatment techniques are applied for textile effluents treatment, such as coagulation [5], membrane separation [6], ozonation [7] and advanced oxidation processes [8]. Each of these treatment methods has its own flaws, such as massive sludge production, periodic maintenance requirements, or energy consumption, and thus the high cost [1]. In view of that, finding effective, economic and eco-friendly treatment method for textile effluents treatment is of great interest. Adsorption process is one of the supreme widely applied methods for textile wastewater treatment since it is very effective in eliminating toxic dyes and it is low cost mainly when adding low adsorbent dose [9, 10]. Key factors affecting adsorption process are as follows: chemical and textural structure of the adsorbent, pH, adsorbent dosage, contact time and temperature [11]. There are several sorbent materials that could be utilized for dyes and heavy metal adsorption, such as agriculture waste adsorbents [12], industrial waste adsorbents [13], natural materials [14, 15] and biosorbents [16]. Activated carbon (AC) is considered the most commonly utilized adsorbent as it has proved the ability of dyes removal owing to its microporous structure, abundant surface area and great adsorption capability [17]. The AC is traditionally obtained from hard coal; however, it could be extracted in a low-cost way from wastes, such as bamboo dust [18], coconut shell [19], rice husk [20] and date pit [21]. The addition of adsorption process based on AC adsorbent has been evaluated by [22] as a second treatment step for real textile wastewater treatment. The obtained results revealed the enhancement of the COD, turbidity and color removal achieving maximum removal rates of 98.3%, 100% and 98.4%, respectively, at optimum adsorbent dose 0.75 g/L and contact time 120 min. Recently, zero-valent metal nanomaterials, such as zero-valent iron (ZVI), zero-valent copper (ZVC), zero-valent aluminum (ZVAl) and others, have been established as a potential sorbent materials for industrial wastewater treatment [23]. The estimable characteristics of these nanomaterials are their nanometer size and large surface area. Moreover, the exceptional electron conduction could offer sorbent nanomaterials with excellent evaluation in dyes removal from real textile wastewaters. Nano-zero-valent iron (nZVI) was reported to have an excellent degradation capability towards dyes and organic substances [11, 23]. Moreover, the high evaluation on the exclusion of metal ions [24]. In early research, the Disperse Red 1 dye adsorption on nZVI has been evaluated by [25]. About 98% color removal was recorded in fairly short time 10 min. Also, the capability of adsorbing methylene blue (MB) basic dye was studied by [26] at optimum operating conditions of pH 9.5, adsorbent dosage 0.5 g/L and contact time 1 min. The extreme adsorption rate of nZVI towards MB was 208.33 mg/g, demonstrating a promising adsorbent for dying effluents treatment. In another study [11], nZVI was examined for the decolorization of real textile wastewater. The achieved results showed remarkable removal ability of color at pH 5, contact time 50 min and stirring rate 150 rpm, reached 71% and 99% for 350 and 50 mg/L Pt/Co initial color concentrations, respectively. On the other side, multi-criteria decision making (MCDM) is an important branch of the decision-making process that deals with decisional problems including various, usually conflicting, criteria. Several methods can be used to solve the multi-attribute decision making problems. Each method has its own characteristics, utilization complexity and the required supplementary information such as the weights of criteria [27]. MADM methodology aims to improve the decision quality by helping the decision maker to evaluate and select the alternatives that have various and conflict technical and sustainable criteria [28]. In the field of water treatment, various MCDM methods were used by previous researchers. For example, fuzzy Delphi method was used to detect the significance of sustainability criteria for the conventional and novel technologies for the treatment of industrial effluents [29]. Analytical hierarchy process (AHP) with fuzzy was used to introduce an environmental risk assessment methodology for engineered nanoparticles [30]. In addition, AHP was utilized to indicate the best removal method of adsorption processes for textile wastewater treatment [31]. Moreover, AHP and grey relation analysis (GRA) were incorporated to select the optimum full-scale tannery effluent treatment plants [32]. Generally, it was concluded that MCDM techniques are useful tools to select the chemicals for the physical-technical treatment [33], and AHP can be considered as an effective tool that can be used for implementation of appropriate wastewater treatment technology [34].
Life cycle assessment (LCA) is a methodology that values environmental impacts caused by a product or service through its life cycle, from extraction of raw materials to final stage when the product becomes a waste. The LCA technique was used in different studies to assess the environmental influences of nZVI and AC. For example, LCA was applied for three methods of nZVI synthesis; milling, chemical reduction with sodium borohydride and chemical reduction with hydrogen gas. Simapro tool, Ecoinvent database and the Impact 2002 + method were used to analyze the environmental impacts of each nZVI method used in the remediation of contaminated sites [35]. Moreover, LCA was utilized to advance the life cycle impact assessment profile of biochar-based carbon activation comparing with commercial coal-based carbon activation in terms of cumulative energy demand and environmental impacts [36]. In addition, LCA was used as an eco-design tool to assess the environmental performance of nZVI synthesis process by traditional and green methods [37]. From previous researches, it was concluded that LCA is a deep-rooted and internationally accepted method for categorizing GHG emissions and other impacts from industrial processes. LCA is a powerful environmental tool dealing with the complicated interaction between the environment and a product by quantifying the consumed resources and energy through whole life cycle of the products or systems [36, 38]. Limited data exist concerning the nZVI life cycle inventory (LCI) and environmental impact assessment. This study compiled available information concerning the environmental assessment of the nZVI particles compared with AC containing the data that should be considered when choosing the best adsorbent for textile effluent treatment and other remediation processes. The key purpose of this research is to investigate the feasibility of using AC and nZVI adsorbents for COD, TSS and color removal from real textile wastewater under diverse operating conditions. Moreover, evaluating the utilization of ZVI and AC is based on technical and sustainable criteria to determine the better adsorbent material for textile wastewater treatment. As a step towards sustainable development, use of AC or nZVI as sorbent materials for the treatment of real textile effluents requires the integration of a multiple criteria, including those related to technical performance, potential impacts to the environment and socioeconomic aspects. Technique for Order Preference by Similarity to the Ideal Solution (TOPSIS), analytical hierarchy process (AHP) and simple additive weighting (SAW) are three MCDM methods that used in this study to assess and select the better material. In addition, LCA technique is used to evaluate the environmental impacts and human health criteria.
2 Materials and Method
2.1 Chemicals
The used chemicals in this study were ferric chloride (FeCl3.6H2O, 98.5% pure, Aldrich Co), ethyl alcohol (C2H6O, 95% pure, World Co.), sodium boron hydride (NaBH4, 99% pure, Win Lab.), sulfuric acid (H2SO4, 97%, Honeywell Co.) and sodium hydroxide (NaOH, 99% pure, Oxford Co.). The used AC was supplied by (Supelco, Co.) in an analytical grade and used without further activation. Table 2 summarizes the basic physical characteristics of the used AC adsorbent. The nZVI sorbent material was prepared at the Housing and Building National Research Center (HBRC), Chemistry Lab.
2.2 nZVI Preparation
The nano-iron particles were prepared by borohydride reduction of ferrous iron (Eq. 1). About 0.5406 gm FeCl3·6H2O was dissolved in a 4/1 (v/v) ethanol/water mixture (24 ml ethanol + 6 ml deionized water) to prepare iron chloride solution. Further, 0.3783 g NaBH4 was dissolved in 100 mL of deionized water to prepare 0.1 molar NaBH4 solution. The NaBH4 solution was added using a burette to the iron chloride solution at a rate of 1 drop per 2 s with vigorous stirring (400 rpm). Black particles of nZVI were formed directly after mixing the two solutions. The wet iron nanoparticles were vacuum filtered twice with Whatman filter papers (Grade 42 circles, diameter 150 mm) to separate the solid particles from the liquid. The separated solid particles were subjected to three times wash with 25 mL portions of absolute ethanol to avoid the rapid oxidation of prepared iron nanoparticles. The iron nanoparticles were dried overnight inside an oven at 80 °C. Finally, a thin layer of ethanol was added to protect the iron nanoparticles from oxidation [39].
2.3 Batch Adsorption Studies
The adsorption of COD, TSS and color onto two different sorbent materials (AC and nZVI) was investigated using batch experiments in 1000-mL Erlenmeyer flasks. A one-factor at-a-time method was used to conduct the effects of operating parameters for instance pH: 2–12, dose: 0.05–1 g, stirring rate: 50–200 rpm and contact time: 5–60 min on the removal efficiencies of the studied parameters, while keeping other parameters constant (pH 6, dose 0.8 g/L, contact time 20 min and stirring rate 100 rpm). Samples treated with nZVI were filtered so that they did not contain high iron content. Therefore, the discharged iron concentrations were negligible and conformed to the Egyptian Standards.
2.3.1 Calculations
The removal efficiency for all studied contaminants was calculated based on Eq. 2.
where C0 is the initial concentration of the contaminants (mg/L) and Ce is the equilibrium concentration after treatment (mg/L).
2.3.2 Samples Collection and Characterization
This study was conducted on the textile wastewater disposed from a textile industry located in El-Sadat City, Menoufia-Egypt. This industry adopts a treatment system consisting of coagulation–flocculation and sedimentation tanks. It receives about 200 m3 of untreated textile wastewater daily. The wastewater received by this plant is normally generated from dyeing of cotton and polyester fabrics (Fig. 1), which is extremely polluted due to the presence of different types of dyes.
Unluckily, the quality of the treated effluents does not meet the Egyptian Standards to be discharged to the sewage networks (Table 3). Composite partially treated textile effluent samples were collected after every two hours during the whole working day (8 h) to provide a comprehensive picture of pollutants for a full working day. Afterwards, the samples were mixed and placed in a container and then conveyed to the HBRC chemistry laboratory for chemical characterization based on the standard methods for the examination of water and wastewater [40]. The collected samples were reddish brown in color and contain high concentrations of TSS, COD, turbidity, TN and color as summarized in Table 3.
2.3.3 Analytical Analysis
The tested samples were analyzed for pH, COD, TSS and color. Moreover, the TN, TDS and turbidity analyses were conducted for the samples collected from the factory. The medium pH and TDS were measured by multi-meter PH/OXI 340 I (JERMANY/ WTW 340i/05,281,018, Germany), while the analysis of COD and TSS was performed using spectrophotometer (HACH DR/2000) set at 620 nm and 810 nm wavelength, respectively. The dichromate-closed reflux method was followed for COD determination. Color was measured by APHA platinum–cobalt method using spectrometer (HACH DR/2000 set at 455 nm wavelength), and distilled water was used as a blank. Turbidity was measured by turbidity meter (COLE PALMER-USA/08,391.45/12,106,698). All measurements were repeated three times and those results in which the standard deviations were found greater than 0.1 mg/L were not accepted.
2.4 Multi-Criteria Decision-Making (MCDM) Methods
2.4.1 Technique for Order Preference by Similarity to the Ideal Solution (TOPSIS)
TOPSIS is one of the popular classical MCDM methods and classified as one of the ideal point methods, which alternatives are ranked according to their separation from an ideal point [41]. TOPSIS determines that the selected solution should have the shortest distance to the positive ideal solution (best measures) and the longest distance from the negative ideal solution (worst measures), and then, the distance Ri+ (similarity or relative closeness the positive-ideal solution) is assigned to each alternative. The final order is obtained sorting the set of alternatives decreasingly in terms of Ri+ [42]. TOPSIS provides a mechanism that is attractive because it follows the principles of automatic machine learning as it can consider several attributes in a systematic manner without hugely subjective human input. Discrete or continuous data are standardized to a range between 0 and 1, and a complete ranking is provided on the relative distance of each alternative to the ideal point [27, 42]. TOPSIS processes can be summarized as follows for an alternative i under evaluation criterion j and its weight wj:
-
1.
Normalization.
$$ r_{ij} = \frac{{x_{ij} }}{{\mathop \sum \nolimits_{i = 1}^{m} x_{ij} }}\quad i = 1, \ldots , m;\;j = 1, \ldots ,n $$(3.1)where xij and rij are evaluation and normalized evaluation matrix R (respectively).
-
2.
Weighting.
$$ v_{ij} = w_{j} \cdot r_{ij} $$(3.2) -
3.
Positive V+ and negative V− ideal solutions.
$$ v_{\max }^{ + } = \max v_{ij} = \left[ {v_{1}^{ + } ,v_{2}^{ + } , \ldots ,v_{j}^{ + } ,v_{n}^{ + } } \right] $$(3.3a)$$ v_{\min }^{ - } = \min v_{ij} = \left[ {v_{1}^{ - } ,v_{2}^{ - } , \ldots ,v_{j}^{ - } ,v_{n}^{ - } } \right] $$(3.3b) -
4.
Separation measure from the positive Si+ and negative Si−
$$ S_{{i}^{ + }} = \sqrt {\mathop \sum \limits_{{j = 1}^{n}} (v_{ij} - v_{{j}^{ + }} )^{2} } $$(3.4a)$$ S_{i}^{ - } = \sqrt {\mathop \sum \limits_{j = 1}^{n} (v_{ij} - v_{j}^{ - } )^{2} } . $$(3.4b) -
5.
Calculate Ri+
$$ R_{{\text{i}}}^{ + } = \frac{{S_{i}^{ - } }}{{S_{i}^{ + } + S_{i}^{ - } }}. $$(3.5)
2.4.2 Analytical Hierarchy Process (AHP)
The AHP is the most popular MCDM methods that uses a hierarchical structure to break the decision problem down into a hierarchy of interrelated decision elements [43]. AHP reduces complex decision by transforming it into a series of simple comparisons and rankings and then synthesizing the outcomes and helps the decision maker to obtain the best decision [44]. AHP is an easy and flexible tool that provides the decision maker by a clear rationale for the choices made. AHP includes three stages [45]:
-
1.
Decomposition: It breaks the complex decision problem into simple decision problems using hierarchy structure.
-
2.
Comparative: It implements pairwise comparisons by developing a comparison matrix at each level of hierarchy, obtaining weights and computation of consistency ratio.
-
3.
Finally, the obtained relative weights should be combined for each level to provide composite weights.
2.4.3 Simple Additive Weighting (SAW)
SAW is the most widely MADM method due to its simplicity. In SAW, the decision maker must assign the relative importance weights. The alternative’s score is estimated by multiplying the assigned weights by the scaled value of the alternative for a criterion. Then, summation of the products for all criteria is computed. Different measurement scales for the criteria scores should be standardized to a known dimensionless unit, taking into consideration which the criterion is beneficial or non-beneficial [27].
2.5 Criterion Weights (Pairwise Comparison Method)
A criterion weight is value given to indicate the importance of each criterion relative to other criteria [27]. Pairwise comparison is the most used method to assign the weight. In this method, a ratio matrix is created using pairwise comparisons as input and produce relative weights as output. After that, the consistency ratio (CR) is estimated to assess the reasonable level of consistency [43].
2.6 Life Cycle Assessment (LCA) Method
A life cycle assessment (LCA) is a methodology that attempts to assess the environmental performance of a product over its total life cycle by considering the flow of raw materials and energy into a system and relating them to the environmental impacts [46]. LCA is currently seen as a key tool to the achievement of sustainable development. The LCA process is a systematic and phased approach. ISO Standard 14040 requires that LCA shall involve the following four phases goal definition and scoping, inventory analysis, impact assessment and interpretation [47]. An inventory analysis gathers system inputs and outputs during its life cycle including the data collection and calculation procedures. The impact assessment phase evaluates the importance of potential environmental impacts based on the life cycle inventory (LCI) results. LCA can be used both as a tool for assessments and a concept in evaluations [48].
3 Experimental Studies
3.1 nZVI Characterization
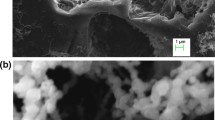
Figure 2a represents the SEM characterization of the prepared nZVI before any treatment processes. Numerous pores were noticed along with heterogeneous and irregular surface structures for the prepared nZVI with an average size of 37.8 nm as shown in Fig. 3a. This permits better mass transfer and diffusion of constituents into the inner iron nanoparticles [49]. Figure 2b displays the XRD for the prepared nZVI. Two reflection peaks were noticed at 2-theta = 44.6° and 65.0° corresponding to 110 and 200 reflections, respectively. The XRD result demonstrated that ZVI was predominant in the prepared sample. Figure 2c displays EDX analysis which performed for detecting the chemical composition of the prepared nZVI. The EDX analysis revealed that the prepared nZVI contains 70.86% iron, which confirmed that the leading product in the prepared sample is iron. The existence of oxygen was observed to be 29.14%. It may be owing to the formation of the iron oxide shells resulting from the iron nanoparticles and water or air reactions throughout observation [50].
3.2 Batch Adsorption Results
3.2.1 Effect of Operating Conditions
3.2.1.1 Effect of pH
Figure 3a displays the effect of pH on the removal of COD, TSS and color by using nZVI and AC. The obtained results suggested that AC and nZVI are effective for COD, TSS and color removal from real textile effluents. However, the removal results for all studied contaminants were observed low at pH < 4. It could be the result of electrostatic attraction subsequent from the excessive presence of H+ ions on the surface of adsorbent particles that compete for the active adsorption sites with the cations in the textile wastewater [11]. A noticeable increase in the removal efficiency was observed with an increase in the value of pH until the maximum reached at optimum pH 6 recording maximum removal efficiency 65.7%, 60.5% and 67.3% by using AC and 64.8%, 60.4% and 65% by using nZVI for COD, TSS and color, respectively. This is could be owing to the reduction in the H+ ions, which improve the electrostatic attraction between the negatively charged surface of the adsorbent and the textile wastewater cations [51]. A dramatic decrease in the removal efficiency for all studied contaminants was observed at pH > 6. This may be caused by the increase in OH− ions, which compete with negative ions on the surface of the adsorbent particles [17]. Similarity, the optimum removal of Cu2+ heavy metal was reported to be (> 98%) using nZVI at acidic medium pH 6 [52]. In addition, the degradation of nitrate based on nZVI has been reported as an acidity-driven process [53]. In another study, the optimum deduction of methylene blue (MB) was recorded at pH 7.2 using commercial AC [54].
3.2.1.2 Effect of Adsorbent Dosage
Figure 3b shows the influence of nZVI and AC dosage on the removal of COD, TSS and color from real textile wastewater. The achieved results showed that the removal efficiency increases by dose increase for AC and nZVI sorbent materials. The obtained results presented that the removal efficiency improved by dose increase and as a result entrapping excessive amounts of molecular contaminants as a result of getting greater vacant site for adsorption [17]. The optimal effective dose by both AC and nZVI was 0.8 g/L achieving removal efficiency of 78.8% for COD, 76.2% for TSS and 84% for color by using AC and 74.7% for COD, 72.6% for TSS and 80% for color by using nZVI. A significant development on the removal rates was observed by using adsorbent dose greater than 0.8 g/L. This could be due to excess of adsorbent particles which could overlap the pore structure of the adsorbent [18].
3.2.1.3 Effect of Contact Time
The impact of contact time was considered for the optimum removal of COD, TSS and color from real textile wastewater as shown in Fig. 3c. The removal efficiency was observed low for all studied contaminants at contact time < 15 min. At contact time 20 min, a significant enhancement in the removal efficiency was recorded to be 65.8%, 59.9% and 66.7% by using AC and 64.3%, 58.6% and 66.3% by using nZVI for COD, TSS and color, respectively. A slight improvement in the removal efficiency was noticed at contact time > 30 min, which could be owing to the adsorbent saturation and equilibrium condition. Consequently, the optimum contact time was detected to be 20 min. In similar, the MB dye removal was evaluated by [55] using AC prepared from bio-waste. About 74% removal efficiency was achieved within 20 min contact time. In another study [56], the optimum uptake of Pb (II) and Hg (II) was achieved at 20 min contact time by using modified nZVI adsorbent.
3.2.1.4 Effect of Stirring Rate
The effect of stirring rate was also studied for the maximum COD, TSS and color removal from real textile wastewater as shown in Fig. 3d. At stirring rate < 100 rpm, the removal efficiency of all studied contaminants was observed low. However, the optimum stirring rate was detected to be 100 rpm. The obtained results of removal at 100 rpm stirring rate for COD, TSS and color were recorded 65.6%, 60% and 66.7% by using AC and 64%, 59.2% and 65.3% by using nZVI, respectively. Stirring rate of 100 rpm enhanced the pollutants molecules interaction with adsorbent particles and thus improved the liquid–solid mass transfer. A slight reduction in the removal efficiency was observed for all studied contaminants after stirring > 100 rpm. This could be caused by the electrostatic forces interruption owing to high stirring rates [17, 57].
4 Evaluation Criteria and Inventory Analysis
The evaluation of the performance on sustainability criteria basis and integrating them into the decision-making process is a primary task to ensure the achievement of long-term benefits [29]. A review has been conducted to investigate the various criteria that used in similar researches to help in selecting the suitable criteria of this study. Table 4 presents samples of the criteria review. Based on that review, the categories and criteria are stated according to the problem nature and the selected alternatives. The selection of optimal alternative involves multiple criteria and hierarchy process as shown in Fig. 4. In this sense, the present MCDM study considered the technical, environmental and socioeconomic aspects as the criteria to evaluate the alternatives (AC and nZVI) including 17 indexes. The data required for these criteria are based on the experiment study executed, life cycle inventory (LCI) datasets available on Ecoinvent, market prices and data available in bibliography. IMPACT 2002 + methodology is used for impact assessment. Environmental and socioeconomic data are determined according to the 1 kg of the material (nZVI or AC) produced.
The life cycle assessment of nZVI includes the activities involved in nZVI manufacturing, from the extraction of raw material to the obtaining of nZVI particles. LCI includes inputs and outputs of the process of chemical reduction with sodium borohydride including the stages of mixtures of reactants, stirring, filtration, washing and drying [35, 37]. Regarding activated carbon (AC), the data collected form Ecoinvent database represent the production of 1 kg of granular activated carbon (GAC) from hard coal. LCA was performed from cradle to gate. LCI includes inputs and outputs of the process including raw materials and energy for activation process as well as emissions and waste.
4.1 Technical Criteria
Treatment efficiency includes the optimum removal percentage of COD, TSS and color to compare technically between alternatives based on their performance. Based on the experimental study, it was 78.8%, 76.2% and 84%, respectively, for AC, and 74.7%, 72.6% and 80%, respectively, for nZVI. Other criterion is the operation stability that includes agglomeration and regeneration and treatment time. The nZVI tends to agglomerate, meaning the separate particles cluster together to form larger particles. This reduces the surface area of the nZVI, which decreases the mobility and reactivity, which in turn exerts negative impact on their application potential [59]. Conversely, AC can be considered less affected by agglomeration than nZVI as AC is a non-magnetic material has large surface area and medium particle size. The regeneration process of nZVI could be considered as a promising process, especially for nZVI used in conventional activated sludge systems [60, 61]. Five-point scale—as presented in Table 5—is used to measure both agglomeration and regeneration indexes. The treatment time using AC or nZVI is the same as indicated in the experimental study (20 min). Data of these criteria for nZVI and AC are based on the experimental work executed in this study as well as data from bibliography [17, 23].
4.2 Environmental Criteria
Resource consumption includes energy consumption and consumed materials. For nZVI, the consumed energy is considered in stirring and filtration processes, while for AC, the consumed energy is mainly in activation process. The consumed materials for nZVI are FeCl3 and NaBH4 in the mixtures of reactants stage, filter papers in the filtration stage and ethanol in the washing stage. Ecosystem quality includes global warming, acidification potential, eutrophication potential and solid waste generation. The impact categories of global warming, human toxicity and acidification potentials play a key role in the overall environmental performance of the production chain [38, 58]. For nZVI, LCI data including quantities are collected from bibliography references [37] as shown in Tables 6 and 7. For AC, data of resource consumption and ecosystem quality are based on Ecoinvent database and IMPACT 2002 + methodology. The higher value of the indicator, the greater the environmental impact. The used environmental and health impact data are presented in Table 7.
4.3 Socioeconomic Criteria
Economic value includes the initial cost and multi-processes usage. The cost is estimated as function commercial price by dividing the alternative market price over the number of reuses that considered “4” for nZVI and “1” for AC [62]. The multi-processes usage means the various processes that the alternative can be used. nZVI can be used as a chemical reduction or degradation or adsorption, while AC is used only as adsorption material. Human health and safety include ozone depletion potential, respiratory effects (inorganics) and photochemical oxidation. Data are collected from LCI datasets available on Ecoinvent (using IMPACT 2002 + methodology for impact assessment), market prices and bibliography [35].
5 Results and Analysis for MCDM Process
Three MCDM methods (TOPSIS, AHP and SAW) are used to evaluate and rank the alternatives. Each method is performed separately, and its results are obtained. According to the experimental work executed in this study, the effective dose of AC and nZVI for best removal efficiency is the same (0.8 g/L); therefore, the functional unit is the same for both alternatives. Due to the big influence of the criteria weight on rank order of the alternatives, the degree of reliability of the results must be known by the decision maker to be able to make the final decision. Therefore, the evaluation study is performed in two scenarios: the first scenario considered equal weights of all criteria; the second scenario considered weights for criteria that calculated by pairwise comparison method. Weights of criteria and indexes which used in the second scenario are presented in Tables 8 and 9.
Tables 10, 11, 12 and 13 present the MCDM matrixes (weighted normalized matrix and weighted sum vector), as well as results (R+, global score and score) for the used methods “TOPSIS, AHP and SAW.” The evaluation results for each criterion: technical, environmental and socioeconomic, in addition to the overall process are compared between AC and nZVI in both scenarios (equal weights for criteria and weighted criteria).
Figures 5, 6 and 7 show the compared the results of different MCDM methods for each criterion as well as to the overall evaluation in the two scenarios. In technical comparison, it is found that there is no significant difference between AC and nZVI either in equal weights or weighted criteria for the three methods. In environmental comparison, there is a difference between the alternatives for TOPSIS and AHP when using equal weights; however, that difference is decreased when using weighted criteria. For SAW method, there is a significant difference between the alternatives when using either equal weights or weighted criteria. In socioeconomic comparison, there is a significant difference between AC and nZVI for TOPSIS and AHP when using either equal weights or weighted criteria especially in TOPSIS. The overall comparison indicates that AC is better than nZVI for all methods in two scenarios.
A sensitivity analysis is conducted to study the effect of the weights of criteria and the type of MCDM method used. The results indicate that when using equal weights of criteria, AC is better than nZVI by 34%, 31% and 87% for TOPSIS, AHP and SAW, respectively. When using weight criteria, AC is better than nZVI by 23%, 27% and 80% for TOPSIS, AHP and SAW, respectively. The variance in results of methods between using equal or weighted criteria is 34%, 13% and 25% for TOPSIS, AHP and SAW, respectively as shown in Fig. 8. It is important to mention that the results of that approach are limited to the MCDM methods used, selected criteria, weights and collected data. Therefore, the generated results may be differed if the decision maker makes changes to these parameters according to the required objectives or directions.
6 Conclusion
This study proved that nZVI and AC are effective sorbent materials for treating textile industry effluents; however, AC showed better removal rates than nZVI for textile effluents decontamination. The optimum operating conditions for the reduction of COD, TSS and color from real textile wastewater was pH 6, contact time = 20 min, dose = 0.8 gm/L, stirring rate = 100 rpm at room temperature, achieving removal efficiency of 78.8%, 76.2% and 84% after using AC and 74.7%, 72.6% and 80% after using nZVI, respectively. Three MCDM methods (TOPSIS, AHP and SAW) are used for evaluation of AC and nZVI for textile wastewater treatment based on technical and sustainable criteria. Two scenarios are prepared: the first for equal weight for all indexes and the second for weighted criteria using a pairwise comparison method. LCA tool is used for environmental impact assessment. According to the results of MCDM study, it is concluded that AC is better than nZVI in technical, environmental and socioeconomic aspects as well as in overall rank based on the selected criteria and weights. The three MCDM methods have similar concluded results taking into consideration the difference in nature and methodology of each method.
References
Ghaly, A.E.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V.V.: Production, characterization and treatment of textile effluents: a critical review. J. Chem. Eng. Process. Technol. 5(1), 1–19 (2014)
Donkadokula, N.Y., Kola, A.K., Naz, I., Saroj, D.: A review on advanced physico-chemical and biological textile dye wastewater treatment techniques. Rev. Environ. Sci. Bio/Technol. 19, 1–18 (2020)
Karam, A.; Mostafa, M.K.; Elawwad, A.; Zaher, K.; Mahmoud, A.S.; Peters, R.W.: Small-Pilot Plant for Tertiary Treatment of Domestic Wastewater Using Algal Photo-Bioreactor, with Artificial Intelligence. In: 2019. AIChE
Elawwad, A., Karam, A., Zaher, K.: Using an algal photo-bioreactor as a polishing step for secondary treated wastewater. Pol. J. Environ. Stud. 26(4), 1493–1500 (2017)
Karam, A., Bakhoum, E.S., Zaher, K.: Coagulation/flocculation process for textile mill effluent treatment: experimental and numerical perspectives. Int. J. Sustain. Eng. 14, 1–13 (2020)
Cinperi, N.C.; Ozturk, E.; Yigit, N.O.; Kitis, M.: Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. J. Clean. Prod. 223, 837–848 (2019)
Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S.: Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chem. Eng. J. 358, 992–1001 (2019)
Sathya, U.; Nithya, M.; Balasubramanian, N.: Evaluation of advanced oxidation processes (AOPs) integrated membrane bioreactor (MBR) for the real textile wastewater treatment. J. Environ. Manag. 246, 768–775 (2019)
Mor, S.; Chhavi, M.K.; Sushil, K.K.; Ravindra, K.: Assessment of hydrothermally modified fly ash for the treatment of methylene blue dye in the textile industry wastewater. Environ. Dev. Sustain. 20(2), 625–639 (2018)
Hynes, N.R.J., Kumar, J.S., Kamyab, H., Sujana, J.A.J., Al-Khashman, O.A., Kuslu, Y., Ene, A., Suresh, B.: Modern enabling techniques and adsorbents based dye removal with sustainability concerns in textile industrial sector-A comprehensive review. J. Clean. Prod. 272, 122636 (2020)
Karam, A.; Zaher, K.; Mahmoud, A.S.: Comparative studies of using nano zerovalent iron, activated carbon, and green synthesized nano zerovalent iron for textile wastewater color removal using artificial intelligence, regression analysis, adsorption isotherm, and kinetic studies. Air Soil Water Res. 13, 1178622120908273 (2020)
Parvaresh, V., Hashemi, H., Khodabakhshi, A., Sedehi, M.: Removal of dye from synthetic textile wastewater using agricultural wastes and determination of adsorption isotherm. Desalin. Water Treat. 111, 345–350 (2018)
Wakkel, M.; Khiari, B.; Zagrouba, F.: Textile wastewater treatment by agro-industrial waste: equilibrium modelling, thermodynamics and mass transfer mechanisms of cationic dyes adsorption onto low-cost lignocellulosic adsorbent. J. Taiwan Inst. Chem. Eng. 96, 439–452 (2019)
Sdiri, A.T.; Higashi, T.; Jamoussi, F.: Adsorption of copper and zinc onto natural clay in single and binary systems. Int. J. Environ. Sci. Technol. 11(4), 1081–1092 (2014)
Gautam, A.K.; Singh, N.B.; Shukla, S.P.; Mohan, D.: Lead removal efficiency of various natural adsorbents (Moringa oleifera, Prosopis juliflora, peanut shell) from textile wastewater. SN Appl. Sci. 2(2), 288 (2020)
da Silva, D.C.C.; de Abreu Pietrobelli, J.M.T.: Residual biomass of chia seeds (Salvia hispanica) oil extraction as low cost and eco-friendly biosorbent for effective reactive yellow B2R textile dye removal: characterization, kinetic, thermodynamic and isotherm studies. J. Environ. Chem. Eng. 7(2), 103008 (2019)
Corda, N.C., Kini, M.S.: A Review on Adsorption of Cationic Dyes using Activated Carbon. In: EDP Sciences, pp. 02022. (2018)
Ahmad, A.A.; Hameed, B.H.: Reduction of COD and color of dyeing effluent from a cotton textile mill by adsorption onto bamboo-based activated carbon. J. Hazard. Mater. 172(2–3), 1538–1543 (2009)
Anirudhan, T.S.; Sreekumari, S.S.: Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J. Environ. Sci. 23(12), 1989–1998 (2011)
Kalderis, D.; Koutoulakis, D.; Paraskeva, P.; Diamadopoulos, E.; Otal, E.; del Valle, J.O.; Fernández-Pereira, C.: Adsorption of polluting substances on activated carbons prepared from rice husk and sugarcane bagasse. Chem. Eng. J. 144(1), 42–50 (2008)
El-Naas, M.H.; Al-Zuhair, S.; Alhaija, M.A.: Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard. Mater. 173(1–3), 750–757 (2010)
Aouni, A.; Lafi, R.; Hafiane, A.: Feasibility evaluation of combined electrocoagulation/adsorption process by optimizing operating parameters removal for textile wastewater treatment. Water Treat. 60, 10890 (2017)
Ruan, W.; Hu, J.; Qi, J.; Hou, Y.; Zhou, C.; Wei, X.: Removal of dyes from wastewater by nanomaterials: a review. Adv. Mater. Lett. 10(1), 09–20 (2019)
Li, S.; Wang, W.; Liang, F.; Zhang, W.-X.: Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 322, 163–171 (2017)
Barreto-Rodrigues, M.; Silveira, J.; Zazo, J.A.; Rodriguez, J.J.: Synthesis, characterization and application of nanoscale zero-valent iron in the degradation of the azo dye Disperse Red 1. J. Environ. Chem. Eng. 5(1), 628–634 (2017)
Arabi, S.; Sohrabi, M.R.: Removal of methylene blue, a basic dye, from aqueous solutions using nano-zerovalent iron. Water Sci. Technol. 70(1), 24–31 (2014)
Yoon, K.P.; Hwang, C.-L.: Multiple Attribute Decision Making: An Introduction, Vol. 104. Sage Publications, New York (1995)
Omann, I.: Multi-criteria decision aid as an approach for sustainable development analysis and implementation. na, (2004)
Kamali, M.; Persson, K.M.; Costa, M.E.; Capela, I.: Sustainability criteria for assessing nanotechnology applicability in industrial wastewater treatment: current status and future outlook. Environ. Int. 125, 261–276 (2019)
Topuz, E.; van Gestel, C.A.M.: An approach for environmental risk assessment of engineered nanomaterials using analytical hierarchy process (AHP) and fuzzy inference rules. Environ. Int. 92, 334–347 (2016)
Ehrampoush, M.H.; Miri, M.; Momtaz, S.M.; Ghaneian, M.T.; Rafati, L.; Karimi, H.; Rahimi, S.: Selecting the optimal process for the removal of reactive red 198 dye from textile wastewater using analytical hierarchy process (AHP). Desalination and Water Treatment 57(56), 27237–27242 (2016)
Dalvand, A.; Ehrampoush, M.H.; Ghaneian, M.T.; Mokhtari, M.; Ebrahimi, A.A.; Malek Ahmadi, R.; Mahvi, A.H.: Application of chemical coagulation process for direct dye removal from textile wastewater. J. Environ. Health Sustain. Dev. 2(3), 333–339 (2017)
Aragonés-Beltrán, P.; Mendoza-Roca, J.A.; Bes-Piá, A.; García-Melón, M.; Parra-Ruiz, E.: Application of multicriteria decision analysis to jar-test results for chemicals selection in the physical–chemical treatment of textile wastewater. J. Hazard. Mater. 164(1), 288–295 (2009)
Pophali, G.R.; Chelani, A.B.; Dhodapkar, R.S.: Optimal selection of full scale tannery effluent treatment alternative using integrated AHP and GRA approach. Expert Syst. Appl. 38(9), 10889–10895 (2011)
Visentin, C.; da Silva Trentin, A.W.; Braun, A.B.; Thomé, A.: Lifecycle assessment of environmental and economic impacts of nano-iron synthesis process for application in contaminated site remediation. J. Clean. Prod. 231, 307–319 (2019)
Kim, M.H.; Jeong, I.T.; Park, S.B.; Kim, J.W.: Analysis of environmental impact of activated carbon production from wood waste. Environ. Eng. Res. 24(1), 117–126 (2018)
Martins, F.; Machado, S.; Albergaria, T.; Delerue-Matos, C.: LCA applied to nano scale zero valent iron synthesis. Int. J. Life Cycle Assess. 22(5), 707–714 (2017)
Gu, H.; Bergman, R.; Anderson, N.; Alanya-Rosenbaum, S.: Life cycle assessment of activated carbon from woody biomass. Wood Fiber Sci. 50(3), 229–243 (2018)
Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N.: Preparation and characterization of zero valent iron nanoparticles. Dig. J. Nanomater. Biostruct. 6(4), 1771–1776 (2011)
American Public Health Association; American Water Works Association; Water Pollution Control Federation; Water Environment Federation: Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, D.C (1920)
Hwang, C.-L., Yoon, K.: Multiple attribute decision making: a state of the art survey. Lect. Notes Econ. Math. Syst. 186(1), 289–289 (1981)
Méndez, M., Galván, B., Salazar, D., Greiner, D.: Multiple-objective genetic algorithm using the multiple criteria decision making method topsis. In: Multiobjective Programming and Goal Programming, Lecture Notes inEconomics and Mathematical Systems, vol. 628, pp. 145–154. Springer, Berlin, Heidelberg (2009)
Saaty, T.L.: The Analytic Hierarchy Process Mcgraw Hill. New York. Agric. Econ. Rev. 70, 97–98 (1980)
Mahmoodzadeh, S.; Shahrabi, J.; Pariazar, M.; Zaeri, M.S.: Project selection by using fuzzy AHP and TOPSIS technique. World Acad. Sci. Eng. Technol. 30, 333–338 (2007)
Zeng, G.; Jiang, R.; Huang, G.; Xu, M.; Li, J.: Optimization of wastewater treatment alternative selection by hierarchy grey relational analysis. J. Environ. Manag. 82(2), 250–259 (2007)
Ljungberg, L.Y.: Materials selection and design for development of sustainable products. Mater. Des. 28(2), 466–479 (2007)
Nebel, B.: White Paper–Life Cycle Assessment and the Building and Construction Industry. Prepared for Beacon Pathway Limited, Auckland (2006)
Mora, E.P.: Life cycle, sustainability and the transcendent quality of building materials. Build. Environ. 42(3), 1329–1334 (2007)
Meda, L.; Marra, G.; Galfetti, L.; Severini, F.; De Luca, L.: Nano-aluminum as energetic material for rocket propellants. Mater. Sci. Eng. C 27(5–8), 1393–1396 (2007)
Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R.: Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci. Total Environ. 466–467, 210–213 (2014). https://doi.org/10.1016/j.scitotenv.2013.07.022
Arabi, S.; Sohrabi, M.R.: Experimental design and response surface modelling for optimization of vat dye from water by nano zero valent iron (NZVI). Acta Chim. Slov. 60(4), 853–860 (2014)
Dada, A.O., Adekola, F.A., Odebunmi, E.O.: Kinetics, isotherms and thermodynamics studies of sorption of Cu2+ onto novel zerovalent iron nanoparticles. Covenant J. Phys. Life Sci. 2(1), 119–133 (2014)
Yang, G.C.C.; Lee, H.-L.: Chemical reduction of nitrate by nanosized iron: kinetics and pathways. Water Res. 39(5), 884–894 (2005)
Kannan, N.; Sundaram, M.M.: Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study. Dyes Pigm. 51(1), 25–40 (2001)
Singh, S.; Sidhu, G.K.; Singh, H.: Removal of methylene blue dye using activated carbon prepared from biowaste precursor. Indian Chem. Eng. 61(1), 28–39 (2019)
Arshadi, M.; Abdolmaleki, M.K.; Mousavinia, F.; Foroughifard, S.; Karimzadeh, A.: Nano modification of NZVI with an aquatic plant Azolla filiculoides to remove Pb (II) and Hg (II) from water: aging time and mechanism study. J. Colloid Interface Sci. 486, 296–308 (2017)
Ahmad, M.A.; Ahmad, N.; Bello, O.S.: Adsorptive removal of malachite green dye using durian seed-based activated carbon. Water Air Soil Pollut. 225(8), 2057 (2014)
Arena, N.; Lee, J.; Clift, R.: Life Cycle Assessment of activated carbon production from coconut shells. J. Clean. Prod. 125, 68–77 (2016)
He, F.; Zhao, D.; Liu, J.; Roberts, C.B.: Stabilization of Fe−Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Ind. Eng. Chem. Res. 46(1), 29–34 (2007)
Ren, J.; Yao, M.; Woo, Y.C.; Tijing, L.D.; Kim, J.-H.; Shon, H.K.: Recyclable nanoscale zerovalent iron (nZVI)-immobilized electrospun nanofiber composites with improved mechanical strength for groundwater remediation. Compos. B Eng. 171, 339–346 (2019)
Khalil, A.M.E. et al.: Treatment and regeneration of nano-Scale zero-Valent iron spent in water remediation. Evergreen: Joint J. Novel Carbon Resour. Sci. Green Asia Strat. 4(1):21–28 (2017)
Pasinszki, T.; Krebsz, M.: Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nanomaterials 10(5), 917 (2020)
Acknowledgements
This research was supported by Nile University (NU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflict of interest with respect to the research, authorship and/or publication of this article.
Rights and permissions
About this article
Cite this article
Badawi, A.K., Bakhoum, E.S. & Zaher, K. Sustainable Evaluation of Using Nano Zero-Valent Iron and Activated Carbon for Real Textile Effluent Remediation. Arab J Sci Eng 46, 10365–10380 (2021). https://doi.org/10.1007/s13369-021-05349-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05349-5