Abstract
Coagulation is a water and wastewater treatment technology that has been widely used due to its high efficiency and effectiveness. Various inorganic salts are commonly used as coagulant; however, there are some negative impacts of utilization of these chemical coagulants, such as high costs, large sludge volume, and also potential negative impact to human health. To overcome these problems, natural-based coagulants have been explored to reduce and substitute chemical coagulants. In this study we investigated utilization of Leucaena leucocephala seed protein, extracted using 0.5 M MgCl2 solution as natural coagulant to treat synthetic Congo red wastewater. From the protein solubility profile at various pH, it could be observed that leucaena protein’s isoelectric point was around pH 4. The optimization of coagulation condition was done by using Response Surface Method (RSM)—Central Composite Design (CCD) with variations of pH (2.93–4.12), coagulant dose (183.52–496.58 mg eq BSA/L), and dye concentration (44.1–56 mg/L). This experimental design was used to obtain the optimum condition that gave maximum dye removal percentage and minimum sludge volume. It was found that pH, coagulant dose, and dye concentration were significant for dye removal and sludge volume. Furthermore, it became evident that charge neutralization was the coagulation mechanism in this study. Based on the obtained model, 88.61% dye removal with 16.93 mL/L sludge volume was obtained at the optimum condition. The predicted optimum condition was in a good agreement with experimental data with relatively low error value. The obtained result implies good potency of leucaena as natural coagulant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Textile industry is classified as one of the largest and rapidly growing industries in many countries. It is known that the textile industry consumed a large number of water for its production thus generating a large volume of wastewater. Textile wastewater is high in biological oxygen demand (BOD), chemical oxygen demand (COD), total suspended solid (TSS), and other hazardous contents, namely heavy metal substances, halogenated hydrocarbons, pigments, dyes, and organic solvents [1]. According to Verma et al. (2012), typical textile wastewater had a wide range of BOD and COD value, namely 110–5500 mg/L and 50–18,000 mg/L, respectively, depending on the materials used during textile processing. It is also known that textile dyes have high-molecular weight and complex structure that making it non-biodegradable [2]. According to the regulations in Indonesia, the maximum BOD and COD in the discharge water for textile industry are 35–60 mg/L and 115–150 mg/L, depending on the wastewater debit, while the maximum organic content in drinking water is 10 mg/L. Direct discharging of textile wastewater into the river body should be avoided, as it could damage the aquatic ecosystem, as well as cause various diseases in human due to its mutagenic and carcinogenic nature.
Several methods to treat textile wastewater have been extensively studied, such as adsorption [3, 4], membrane filtration [5], electrocoagulation-flocculation [6] and advanced oxidation processes (AOPs) such as Fenton oxidation [7] and photo catalytic oxidation [8]. These previously mentioned technologies have their own advantages and drawbacks in wastewater treatment. However the coagulation—flocculation methods for treating wastewater are still widely used as primary chemical treatment due to its high efficiency and low cost [9]. Chemical coagulants such as alum (AlCl3), ferric chloride (FeCl3), and polyaluminum chloride (PAC) are commonly used [1]. While the effectiveness of these chemical coagulants is well-recognized, there are, nonetheless, disadvantages associated with usage of these coagulants such as: ineffectiveness in low-temperature water, relatively high-procurement costs, detrimental effects on human health (Alzheimer’s, dementia), production of large less-degradable sludge and alteration of the water’s pH [10]. It is therefore desirable to reduce and replace the chemical coagulants with natural-based coagulants to counteract the aforementioned drawbacks.
Natural coagulants have been used for more than 2000 years for traditional water treatment in India, Africa, and China [11]. Natural coagulants can be produced from plants (seeds, leaves, and roots), animals, and microorganisms. There are some advantages of natural coagulant such as non-toxic and non-corrosive nature, and also less volume sludge generation [10]. Based on these facts, utilization of natural coagulant may become an alternative for sustainable water and wastewater treatment, especially in the aspect of waste minimization and prevention, less hazardous chemicals, and its renewability [12]. Some plant-based natural coagulants such as Moringa oleifeira [13], Vicia faba [14], Carica papaya [15], and Plantago ovata [16, 17] have been investigated to treat various wastewaters.
In this study, Leucaena leucocephala, also known as white popinac or petai cina in Indonesian, was investigated its performance as natural coagulant. Leucaena is a native tree that can be easily found in tropical countries [18]. Leucaena is a species of legume that has high content of protein, namely 57–64%w of dry basis [19], making it a potential candidate as natural coagulant [20]. Furthermore, according to Sethi and Kulkarni (1993) 43.5% of total leucaena protein is globulin [21], a protein fraction that is soluble in salt solutions. Direct utilization of leucaena seed in powder form as natural coagulant is possible [22]. However, the active coagulating agent is commonly used in extract form to minimize the existence of organic substances mixed with active coagulant substances that could cause increase COD (Chemical Oxygen Demand) and DOC (Dissolved Organic Carbon) in the treated water [23, 24]. Solution of MgCl2 was used to prepare the crude extract, to investigate utilization of divalent salt that has not been widely used, in comparison with previous studies where NaCl solution was used as solvent [25, 26]. Although the coagulation using leucaena has been previously investigated, it is crucial to investigate the effect of various variables, namely coagulant dose, pH, and dye concentration to the coagulation performance. Furthermore, maximum color removal and minimum sludge volume produced should be optimized for an efficient coagulation process using response surface methodology (RSM). RSM with central composite design (CCD) are commonly used to investigate statistically relation between input variables and response with minimum number of experimental points [27, 28].
2 Materials and Methods
2.1 Extraction of Protein from Leucaena Seed
Leucaena seed was procured from local market in Probolinggo, Indonesia. The seed was de-husked and the kernel was then oven dried (Memmert Un110) at 65 °C for 4 h to obtained kernel with water content 7–10%w. The dried kernel was then pulverized to a fine powder using a commercial food processor (Panasonic MX GX1462). The fine powder was then sieved through a standard 80 mesh and stored in an airtight container for further use. The extraction was carried out at various pH to determine the effect of pH extraction to the protein extractability, as it is known that protein extracted is usually following a “U” shaped curve with minimum value at its isoelectric point, and higher solubility at both acid and basic condition [29]. Five grams of dried leucaena powder was dispersed in 100 mL of 0.5 M MgCl2 (Merck p.a.) with pH adjustments over the range of 2–9 by adding 0.1 M NaOH or HCl. The suspension was rigorously mixed for 60 min and separated by mean of centrifugation at 5000 rpm for 5 min. The protein concentration in the extract was determined using Bradford method [30], in which Bovine serum albumin (BSA, Merck p.a) was used as standard in this analysis method. The protein concentration was then stated as mg equivalent BSA per mL (mg eq BSA/mL).
2.2 Coagulation Study
Congo red (Sigma-Aldrich) was used as a model substance of textile wastewater. A stock solution of 1 g/L was prepared by dissolving Congo red powder in distilled water. This stock solution was diluted as needed to obtain desired concentration. The coagulation study was done by using jar test apparatus. Congo red solution with concentration of 44.1–56 mg/L was adjusted to the desired pH value (2.93–4.12) using either 0.1 M HCl or NaOH. The leucaena crude extract was then added varying from 183.52 to 496.58 mg eq BSA/L according to the experimental design. The volume of extract that was added into the coagulation experiment was calculated using Eq. 1, where M1 is the initial protein concentration, V1 is the volume of extract added, M2 final concentration in the coagulation system, and V2 is the volume of wastewater treated.
The mixture of Congo red solution and leucaena crude extract was rapidly mixed at 200 rpm for 2 min followed by slow mixing at 30 rpm for 30 min. The mixture was then allowed to settle for 1 h. The sludge volume was measured by using 1 L Imhoff cone after 1 h settling, calculated using Eq. 2.
The initial (Co) and final concentration (Ci) of Congo red was measured using a spectrophotometer at its maximum wavelength, and the dye removal was calculated according to Eq. 3.
2.3 Design of Experiments
A response surface methodology (RSM) based on central composite design (CCD) was employed in order to study the optimum condition of coagulation using Leucaena crude extract. RSM is a significant, fast and economical statistical technique for the determination of the interactive effects of parameters on experimental data [31]. Meanwhile, CCD is used for modeling the optimization process so that to obtain maximum dye removal with minimum sludge volume. The range and level of each variable is presented in Table 1. The experimental design in coded value with the responses is shown in Table 2.
The regression and graphical analysis with statistical significance were done using Design-Expert software (ver 7.0.0, Stat-Ease, Inc). In order to visualize the relationship between the experimental variables and responses, the response surface and contour plots were generated from the models. The validity of predicted optimum condition was confirmed by conducting triplicate experiments at the optimum values of pH, dose, and Congo red concentration. Furthermore, the morphology of sludge flocs at the optimum condition was observed using scanning electron microscope (SEM; HITACHI SU3500).
3 Results and Discussions
3.1 Effect of pH on Leucaena Seed Extraction
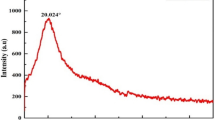
This research was conducted to determine the best pH to extract protein from Leucaena seed. Based on Fig. 1, it could be observed that at acidic pH (pH < 4), the extracted protein concentration increases along with decreasing pH. It was possible due to the presence more positively charged protein molecules at low pH, making electrostatic repulsion between protein molecules were dominant. With the presence of dissociated salts in water, namely Mg2+ and Cl−, positively charged protein molecules become more charged, resulting in more protein molecules repelling each other and subsequently bound to the salt ions, thus increasing the extracted protein [32, 33].
Proteins reached its minimum solubility at pH 4. At this condition, the charges of protein molecules were neutral, which is known as isoelectric point [34]. In this condition, the intermolecular forces between protein molecules would be dominant and the proteins would form peptide bonds, subsequently precipitate due to their increased molecular weight [35]. Above the protein isoelectric point, protein concentration in the solution tends to increase. This phenomenon could happen as the surface of the protein molecules would be negatively charged, making electrostatic repulsion between the protein molecules was dominant and with the presence of salt ions, the proteins’ charge would increase, thus increasing the solubility of the protein in the salt solution [32, 33].
The obtained isolectric point for leucaena protein was important information to determine the possible interaction between colloids and protein, which act as natural coagulant. The obtained isoelectric point was similar with other legumes, such as common beans (Phaseolus vulgaris) and fava beans (Vicia faba) with isoelectric point around 4–5 [36, 37], and soybeans (Glycine max) and winged beans (Psophocarpus tetragonolobus) with isoelectric point around 3.5–5 [38].
3.2 Congo Red Coagulation
3.2.1 Analysis of Variance (ANOVA) and Model Fitting
The results of the ANOVA in Tables 3 and 4 show that the quadratic model is significant with 95% confidence level (α < 0.05). Moreover, the pH (A), coagulant dose (B), and dye concentration (C) were all significant. The effect of those variables and their interactions will create quadratic model equations, respectively, presented in Eqs. (4) and (5).
Lack of fit and R2 describe the suitability of the model with the response data [39]. High p value (> 0.05) of the lack of fit was obtained for both of responses, indicating insignificant lack of fit and significant model correlation between variables (pH, coagulant dose, and dye concentration) and responses (dye removal and sludge volume). While for R2, the values were close to 1, indicating the model and data were well fitted. Furthermore, the difference between adjusted and predicted R2 was less than 0.2, indicating both models were valid and could be used for the optimization process [39]. The suitability between data and model could also be seen in the predicted versus actual graphs, presented in Fig. 2.
3.2.2 Effect of Coagulation Operating Parameters
The profile of the interaction between pH and coagulant dose to dye removal and sludge volume is presented in Fig. 3. Based on Fig. 3, it could be observed that with the increase of the coagulant dose accompanied by decreasing pH value could increase the dye removal and sludge volume. Based on Fig. 1, it could be seen that the extracted leucaena protein had isoelectric point around pH 4, where it exhibited the lowest solubility. On the other hand, Congo red’s isoelectric point was known to be 3 [40], therefore the molecules would be negatively charged at pH > 3, and vice versa. Thus, at pH below 4 the protein became positively charged and was readily available to neutralize anionic Congo red colloids. It is commonly observed that more destabilized colloids would significantly increase dye removal and sludge volume [25]. On the other hand, with increase of pH to 4.12, the dye removal and sludge volume were also decreased. It was possible as at the pH above protein isoelectric point, the protein molecules would be negatively charged, thus hindering the interaction between protein and Congo red molecules [41].
It could also be observed at pH 2.93; coagulant dose of 183.52 mg/L gave 23.25 mL/L sludge volume, while increasing the dose to 496.58 mg/L gave sludge volume increase to 43.75 mL/L with similar removal around 90%. There are generally three possible conditions of dosing in coagulation, namely low, optimum, and overdosing [42]. At low coagulant dose, there was not enough coagulant to neutralize the Congo red molecules, thus resulting in low removal and low sludge formation. Optimum condition could happen when the right amount of coagulant was added, and over addition of coagulant could give similar, or slightly lower removal but with higher sludge volume. This phenomenon could happen due to the presence of excessive coagulant; the colloid molecules became re-charged and re-stabilized. At this condition, the growth of flocs became hindered thus making the sludge became porous and with higher volume [25].
The profile of the interaction between pH and dye concentration to dye removal and sludge volume is presented in Fig. 4. It could be observed that with increase of dye concentration from 44.1 to 56 mg/L, the dye removal and sludge formation were also increased. This trend was possible as with the increase of dye concentration, more colloidal particles was contained in the solution, causing more colloids adsorbed to the protein [43]. On the other hand, the lower coagulation pH, the higher dye removal and sludge formation was observed. The observed trend confirmed the effect of pH on coagulation process in this study as previously explained.
The profile of the interaction between coagulant dose and dye concentration to dye removal and sludge volume is presented in Fig. 5. It could be observed that with the increase of coagulant dose and dye concentration, the dye removal (Fig. 5a–c) and sludge volume (Fig. 5d–f) were also increased. With the higher dye concentration; the more colloidal particles could be adsorbed to the protein. When the dye concentration was too low, less colloidal particles could be destabilized by cationic proteins, making the excessive presence of protein could cause charge re-stabilization. As the result, colloidal particles became repulsive and inhibited coagulation [44]. Similar result was also reported by previous researchers when using protein extracted from Moringa oleifera [45], Cocos nucifera [46], and pine cone [47] as natural coagulant. Furthermore, it could be observed in Fig. 5 that at every level of pH, the addition of coagulant dose and dye concentration had not reached optimum conditions, thus further study, i.e., expanding the range of coagulant dose as it showed a tendency toward the optimum point, is needed.
Based on these results, it could be concluded that the coagulation is influenced by pH (A), coagulant dose (B), and dye concentration (C). Furthermore, based on the ANOVA, parameters A, C, AB, A2 and A2B for the dye removal; B, C, B2 for sludge volume formation had high significance, and B, AC, BC, ABC, AB2 for the dye removal; A, AB, AC, BC, A2, C2 for sludge volume had moderate significance.
3.2.3 Optimum Condition of Coagulation
Optimization was done in objective to obtain operating conditions with maximum dye removal and minimum sludge volume, while other variables (pH, coagulant dose, dye concentration) was set in range and importance for all variables and responses was set equal. According to the desirability value for each parameter, presented in Fig. 6a, it could be seen that all parameters, namely pH, coagulant dose, and dye concentration had desirability value 1.0, while dye removal was 0.89 and sludge volume was 0.76, resulted on the overall desirability of 0.82 (Fig. 6b). The desirability value should lie between 0 and 1, where higher desirability value; closer to 1, corresponds to more desirable response [48]. Based on the models, the optimum condition was found at pH 3.22, coagulant dose 183.53 mg eq BSA/L, and dye concentration 44.1 mg/L. Confirmation of the optimum condition result was done in triplicate, presented in Table 5. Based on this result, it could be observed that the model was valid and could predict optimum condition with low error, calculated using Eq. 6.
The sludge volume produced from leucaena extract coagulant was much lower than alum (40 mL/L) with similar coagulation condition [25]. This was possible due to bigger sludge size compared to alum making the sludge more compact thus occupying smaller volume [13]. The sludge observation is presented in Fig. 7. It could be observed that the flocs structure was dispersed with crack openings. Similar structure was also observed by previous study using protein as active coagulating agent [49]. According to [49], a compact-porous structure as observed in Fig. 6 was desirable due to the presence of large surface area of coagulant could facilitate coagulation of particles.
Based on the findings obtained in this study, it could be seen that further studies of utilization of leucaena seed’s protein as natural coagulant is needed. According to Yin (2010), purification of crude extract using various technologies could improve the coagulant activity and efficiency. Various technologies that could be used such as ion exchange [50], dialysis [51], ultrafiltration [52], spray drying [53], and lyophilization [54]. Furthermore, a combination between natural coagulants with other water-wastewater treatment technologies, such as electrochemical techniques [55], membrane filtration [56], and adsorption [57], could also be explored.
4 Conclusion
In this study, we have investigated the utilization of MgCl2 extracted leucaena protein as natural coagulant to treat synthetic Congo red wastewater. Furthermore, the effect various parameters, namely pH, coagulant dose, and dye concentration, to the dye removal and sludge volume formation was studied using response surface method (RSM)—central composite design (CCD). Based on the experiments, it was found that all parameters are significant to the dye removal and sludge formation. Moreover, pH was found to be highly significant in dye removal as both of the protein and Congo red molecules’ charges were pH sensitive. This phenomenon was in relation with the charge neutralization mechanism that is commonly found in protein-based natural coagulant. It also became evident that coagulant dose and dye concentration was correlated to each other. With the increase of coagulant dose at the same dye concentration, both dye removal and sludge volume also increased until optimal dye removal. Over addition of coagulant resulted on the increase of sludge volume, possibly due to colloid re-stabilization, with similar or slightly lower removal. The model obtained in this study was well fitted, with the optimum condition was achieved at pH 3.22, coagulant dose 183.52 mg eq BSA/L, and dye concentration 44.1 mg/L resulting in dye removal of 88.61% and sludge volume of 16.93 mL/L. In the light of the results obtained in this study, leucaena seed protein showed a great potential to be used as natural coagulant in water—wastewater treatment. Further study such as active coagulating agent purification and its combination with other technologies are needed for effective and efficient implementation as commercial natural coagulant.
References
Verma, A.K.; Dash, R.R.; Bhunia, P.: A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage 93, 154–168 (2012)
Ulucan-Altuntas, K.; Ilhan, F.: Enhancing biodegradability of textile wastewater by ozonation processes: optimization with response surface methodology. Ozone Sci. Eng. 40, 465–472 (2018)
Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M.: Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid Interface Sci. 209, 172–184 (2014)
Kausar, A.; Sher, F.; Hazafa, A.; Javed, A.; Sillanpää, M.; Iqbal, M.: Biocomposite of sodium-alginate with acidified clay for wastewater treatment: kinetic, equilibrium and thermodynamic studies. Int. J. Biol. Macromol. 161, 1272–1285 (2020)
Dasgupta, J.; Sikder, J.; Chakraborty, S.; Curcio, S.; Drioli, E.: Remediation of textile effluents by membrane based treatment techniques: a state of the art review. J. Environ. Manage 147, 55–72 (2015)
Sher, F.; Hanif, K.; Iqbal, S.Z.; Imran, M.: Implications of advanced wastewater treatment: electrocoagulation and electroflocculation of effluent discharged from a wastewater treatment plant. J. Water Process Eng. 33, 101101 (2020)
Rashid, T.; Iqbal, D.; Hazafa, A.; Hussain, S.; Sher, F.; Sher, F.: Formulation of zeolite supported nano-metallic catalyst and applications in textile effluent treatment. J. Environ. Chem. Eng. 8, 104023 (2020)
Sehar, S.; Sher, F.; Zhang, S.; Khalid, U.; Sulejmanović, J.; Limaf, E.C.: Thermodynamic and kinetic study of synthesised graphene oxide–CuO nanocomposites: a way forward to fuel additive andphotocatalytic potentials. J. Mol. Liq. 313, 113494 (2020)
Sher, F.; Malik, A.; Liu, H.: Industrial polymer effluent treatment by chemical coagulation and flocculation. J. Environ. Chem. Eng. 1, 684–689 (2013)
Yin, C.-Y.: Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 45, 1437–1444 (2010). https://doi.org/10.1016/j.procbio.2010.05.030
Asrafuzzaman, M.; Fakhruddin, A.N.; Hossain, M.A.: Reduction of turbidity of water using locally available natural coagulants. ISRN Microbiol. 2011, 632189 (2011). https://doi.org/10.5402/2011/632189
Cheng, S.Y.; Show, P.-L.; Juan, J.C.; Ling, T.C.; Lau, B.F.; Lai, S.H.; Nge, E.P.: Sustainable landfill leachate treatment: optimize use of guar gum as natural coagulant and floc characterization. Environ. Res. 188, 109737 (2020)
Dalvand, A.; et al.: Comparison of Moringa stenopetala seed extract as a clean coagulant with Alum and Moringa stenopetala-Alum hybrid coagulant to remove direct dye from Textile Wastewater. Environ. Sci. Pollut. Res. 23, 16396–16405 (2016)
Kukic, D.V.; Sciban, M.B.; Prodanovic, J.M.; Tepic, A.N.; Vasic, M.A.: Extracts of fava bean (Vicia faba L.) seeds as natural coagulants. Ecol. Eng. 84, 229–232 (2015)
Kristianto, H.; Kurniawan, M.A.; Soetedjo, J.N.M.: Utilization of papaya seeds as natural coagulant for synthetic textile coloring agent wastewater treatment. Int. J. Adv. Sci. Eng. Inf. Technol. 8, 2071–2077 (2018)
Chaibakhsh, N.; Ahmadi, N.; Zanjanchi, M.A.: Use of Plantago major L. as a natural coagulant for optimized decolorization of dye-containing wastewater. Ind. Crops Prod. 61, 169–175 (2014)
Ramavandi, B.: Treatment of water turbidity and bacteria by using a coagulant extracted from Plantago ovata. Water Res. Ind. 6, 36–50 (2014)
Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S.: Agroforestree Database: a tree reference and selection guide version 4.0. http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp (2009)
Sethi, P.; Kulkarni, P.R.: Chemical composition of Leucaena leucocephala seeds. Int. J. Food Sci. Nutr. 45, 5–13 (1994)
Kristianto, H.: The potency of indonesia native plants as natural coagulant: a mini review. Water Conserv. Sci. Eng. 2, 51–60 (2017)
Sethi, P.; Kulkarni, P.R.: Fractionation of Leucaena seed-kernel proteins based on their solubility characteristics. Food Chem. 48, 173–177 (1993)
Kristianto, H.; Paulina, S.; Soetedjo, J.N.M.: Exploration of various indonesian indigenous plants as natural coagulant for synthetic turbid water. IJTech 9, 464–471 (2018)
Baptista, A.T.A.; Silva, M.O.; Gomes, R.G.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S.: Protein fractionation of seeds of Moringa oleifera lam and its application in superficial water treatment. Sep. Purif. Technol. 180, 114–124 (2017). https://doi.org/10.1016/j.seppur.2017.02.040
Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M.: Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 35, 405–410 (2001). https://doi.org/10.1016/S0043-1354(00)00290-6
Kristianto, H.; Rahman, H.; Prasetyo, S.; Sugih, A.K.: Removal of Congo red aqueous solution using Leucaena leucocephala seed’s extract as natural coagulant. Appl. Water Sci. 9, 88 (2019)
Kristianto, H.; Tanuarto, M.Y.; Prasetyo, S.; Sugih, A.K.: Magnetically assisted coagulation using iron oxide nanoparticles-Leucaena leucocephala seeds’ extract to treat synthetic Congo red wastewater. Int. J. Environ. Sci. Technol. 17, 3561–3570 (2020)
Sultana, N.; Hossain, S.M.Z.; Alam, M.S.; Hashish, M.M.A.; Islam, M.S.: An experimental investigation and modeling approach of response surface methodology coupled with crow search algorithm for optimizing the properties of jute fiber reinforced concrete. Constr. Build. Mater. 243, 118216 (2020)
Hossain, S.M.Z.; Taher, S.; Khan, A.; Sultana, N.; Irfan, M.F.; Haq, B.; Razzak, S.A.: Experimental study and modeling approach of response surface methodology coupled with crow search algorithm for optimizing the extraction conditions of papaya seed waste oil. Arab. J. Sci. Eng. (2020). https://doi.org/10.1007/s13369-020-04551-1
Swamylingappa, B.; Srinivas, H.: Preparation and properties of protein isolate from hexane-acetic acid treated commercial soybean meal. J. Agric. Food Chem. 42, 2907–2911 (1994)
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). https://doi.org/10.1016/0003-2697(76)90527-3
Tawakkoly, B.; Alizadehdakhel, A.; Dorosti, F.: Evaluation of COD and turbidity removal from compost leachate wastewater using Salvia hispanica as a natural coagulant. Ind. Crops Prod. 137, 323–331 (2019). https://doi.org/10.1016/j.indcrop.2019.05.038
Albarracín, W.; Sánchez, I.C.; Grau, R.; Barat, J.M.: Salt in food processing; usage and reduction: a review. Int. J. Food Sci. Technol. 46, 1329–1336 (2011). https://doi.org/10.1111/j.1365-2621.2010.02492.x
Curtis, R.A.; Lue, L.: A molecular approach to bioseparations: protein–protein and protein–salt interactions. Chem. Eng. Sci. 61, 907–923 (2006). https://doi.org/10.1016/j.ces.2005.04.007
Voet, D.; Voet, J.G.: Biochemistry, 4th edn. Wiley, USA (2011)
Machado, F.F.; Coimbra, J.S.R.; Rojas, E.E.G.; Minim, L.A.; Oliveira, F.C.; de Sousa, R.C.: Solubility and density of egg white proteins: effect of pH and saline concentration. Food Sci. Technol. (2007). https://doi.org/10.1016/j.lwt.2006.08.020
Antov, M.G.; Šciban, M.B.; Petrovic, N.J.: Proteins from common bean (Phaseolus vulgaris) seed as a natural coagulant for potential application in water turbidity removal. Bioresour. Technol. 101, 2167–2172 (2010)
Fernandez-Quintela, A.; Macarulla, M.T.; Barrio, A.S.D.; Martinez, J.A.: Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum. Nutr. 51, 331–342 (1997)
Makeri, M.U.; Mohamed, S.A.; Karim, R.; Ramakrishnan, Y.; Muhammad, K.: Fractionation, physicochemical, and structural characterization of winged bean seed protein fractions with reference to soybean. Int. J. Food Prop. 20, S2220–S2236 (2017)
Ghafari, S.; Aziz, H.A.; Isa, M.H.; Zinatizadeh, A.A.: Application of response surface methodology (RSM) to optimize coagulation-flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J. Hazard. Mater. 163, 650–656 (2009). https://doi.org/10.1016/j.jhazmat.2008.07.090
Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J.: Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 9, 16530 (2019)
Chethana, M.; Sorokhaibam, L.G.; Bhandari, V.M.; Raja, S.; Ranade, V.V.: Green approach to dye wastewater treatment using biocoagulants. ACS Sustain. Chem. Eng. 4, 2495–2507 (2016)
Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Ramanan, R.N.: A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 12, 367–390 (2015)
Namasivayam, S.N.; et al. (2018) Removal of dye in wastewater by adsorption-coagulation combined system with hibiscus sabdariffa as the coagulant. In: MATEC Web of Conferences, vol. 152, p. 01008. https://doi.org/10.1051/matecconf/201815201008
Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Ramanan, R.N.: A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 12, 367–390 (2013). https://doi.org/10.1007/s13762-013-0446-2
Pritchard, M.; Craven, T.; Mkandawire, T.; Edmondson, A.S.; O’Neill, J.G.: A study of the parameters affecting the effectiveness of Moringa oleifera in drinking water purification. Phys. Chem. Earth 35, 791–797 (2010)
Fatombi, J.K.; Lartiges, B.; Aminou, T.; Barres, O.; Caillet, C.: A natural coagulant protein from copra (Cocos nucifera): isolation, characterization, and potential for water purification. Sep. Purif. Technol. 116, 35–40 (2013)
Hussain, S.; Ghouri, A.S.; Ahmad, A.: Pine cone extract as natural coagulant for purification of turbid water. Heliyon 5, 01420 (2019)
Bobadilla, M.C.; Lorza, R.L.; García, R.E.; Gómez, F.S.; González, E.P.V.: An improvement in biodiesel production from waste cooking oil by applying thought multi-response surface methodology using desirability functions. Energies 10, 130 (2017)
Menkiti, M.C.; Okoani, A.O.; Ejimofor, M.I.: Adsorptive study of coagulation treatment of paint wastewater using novel Brachystegia eurycoma extract. Appl. Water Sci. 8, 189 (2018)
Ghebremichael, K.A.; Gunaratna, K.R.; Dalhammar, G.: Single-step ion exchange purification of the coagulant protein from Moringa oleifera seed. Appl. Microbiol. Biotechnol. 70, 526–532 (2006)
Dezfooli, S.M.; Uversky, V.N.; Saleem, M.; Baharudin, F.S.; Hitam, S.M.S.; Bachmann, T.R.: A simplified method for the purification of an intrinsically disordered coagulant protein from defatted Moringa oleifera seeds. Process Biochem. 51, 1085–1091 (2016)
Prodanović, J.M.; Antov, M.G.; Šćiban, M.B.; Ikonić, B.B.; Kukić, D.V.; Vasić, V.M.; Ivetić, D.Ž.: The fractionation of natural coagulant extracted from common bean by use of ultrafiltration membranes. Desalin. Water Treat. 51, 442–447 (2013)
Mohammad, T.A.; Mohamed, E.H.; Noor, M.J.M.M.; Ghazali, A.H.: Coagulation activity of spray dried salt extracted Moringa oleifera. Desalin. Water Treat. 51, 1941–1946 (2013)
Mohamed, E.H.; Mohammad, T.A.; Noor, M.J.M.M.; Ghazali, A.H.: Influence of extraction and freeze-drying durations on the effectiveness of Moringa oleifera seeds powder as a natural coagulant. Desalin. Water Treat. 55, 3628–3634 (2015)
Barbosa, A.D.; Silva, LFd; Paula, HMd; Romualdo, L.L.; Sadoyama, G.; Andrade, L.S.: Combined use of coagulation (M. oleifera) and electrochemical techniques in the treatment of industrial paint wastewater for reuse and/or disposal. Water Res. 145, 153–161 (2018)
Mateus, G.A.P.; Formentini-Schmitt, D.M.; Nishi, L.; Fagundes-Klen, M.R.; Gomes, R.G.; Bergamasco, R.: Coagulation/flocculation with Moringa oleifera and membrane filtration for dairy wastewater treatment. Water Air Soil Pollut. 228, 1–13 (2017)
Hoong, H.N.J.; Ismail, N.: Removal of Dye in Wastewater by AdsorptionCoagulation Combined System with Hibiscus sabdariffa as the Coagulant. In: MATEC Web Conference, vol. 152, p. 01008 (2018)
Acknowledgement
This research was funded by Parahyangan Catholic University Centre of Research and Community Service (No. III/LPPM/2018-01/11-P). The authors are thankful for the funding support. The authors are also grateful for anonymous reviewers for their suggestions to improve the quality of the manuscript.
Funding
This study was funded by Parahyangan Catholic University Centre of Research and Community Service (No. III/LPPM/2018-01/11-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kristanda, J., Sintiago, K.S., Kristianto, H. et al. Optimization Study of Leucaena leucocephala Seed Extract as Natural Coagulant on Decolorization of Aqueous Congo Red Solutions. Arab J Sci Eng 46, 6275–6286 (2021). https://doi.org/10.1007/s13369-020-05008-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05008-1