Abstract
The Romanian cohort can provide valuable information about the effect of chronic HIV-infection and exposure to combined antiretroviral therapy (cART) on the developing brain, based on its unique characteristics: young adults infected parenterally with HIV clade F in the late 1980s and exposed to cART for a decade. We conducted a prospective study using a neuropsychological test battery validated in other international HIV cohorts, in order to evaluate the rate and severity of neurocognitive impairment in a group of young Romanian adults. The 49 HIV-infected (HIV+) participants and the 20 HIV negative (HIV−) controls were similar for age and gender, although the HIV− group tended to be more educated. We found higher cognitive impairment prevalence in the HIV+ group (59.1 %) versus the HIV− group (10 %), and the impairment rate remained significantly higher even when the groups were matched based on the educational level (38.7 % for the HIV+ group vs. 10.0 % for the HIV− controls; p = 0.025). The nadir CD4 count was <200 in 71.4 % of patients, but at the time of neurocognitive assessment, 89.5 % of patients had normal immunological status and 81.8 % undetectable HIV load. Among the HIV-impaired group, 26 % of the participants had syndromic impairment while the other 74 % had asymptomatic neurocognitive impairment. We found a high prevalence of neurocognitive dysfunction in the Romanian young adults growing-up with HIV. The greatest HIV-related cognitive deficits were in the domains of executive and motor functioning, consistent with a frontosubcortical pattern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human immunodeficiency virus (HIV) infection has increasingly become a chronic illness worldwide. According to the World Health Organization in 2013 approximately 3.2 million individuals under the age of 15 are living with HIV. Because of advances in treatment, more children with HIV have survived into late adolescence and early adulthood; however, little is known about the long-term effects of HIV infection and antiretroviral therapy on brain development.
Children infected with HIV at birth who are not receiving antiretroviral treatment (ART) are at high risk for development of progressive encephalopathy, especially if they have a rapid decline of CD4 count (Nozyce et al. 1994; Smith et al. 2006). However, encephalopathy occurring in older vertically infected children seems to have a different pathophysiologic mechanism than that from infants, similar to that observed in adults (Tardieu et al. 2000; Mitchell 2001).
Studies have found that HIV-infected children showed impairments in language, motor, executive function, and information processing speed, and that the severity and progression of these impairments were correlated with the stage of the HIV infection, CD4 count at initiation of antiretroviral therapy, and duration of treatment (Nozyce et al. 1994; Koekkoek et al. 2008). Nevertheless, psychosocial functioning also may be influenced by living environments and background (Coscia et al. 2001; Steele et al. 2007; Burns et al. 2008). Evaluating the impact of HIV damage on CNS during childhood is complicated in part by the lack of case definitions especially for older children. A recent review examining neurodevelopment in HIV-infected children noted that a “striking finding is the lack of published data specific to the adolescent age group” (Laughton et al. 2013).
Romania had a major HIV epidemic in children between 1987 and 1990, which may represent the world’s largest iatrogenic transmission of blood-borne pathogens in children (Hersh et al. 1991). The majority of HIV-infected children acquired the infection via transfusions of unscreened blood or therapeutic injections through the improper reuse of nonsterile needles and syringes at a time when poverty, malnutrition, and epidemics of diseases were at their zenith (Hersh et al. 1993). Previous studies indicate that the HIV F1 clade was overwhelmingly the predominant (93 % of cases, with 6 % uninterpretable) clade linked to the nosocomial transmission in children (Apetrei et al. 1998). The use of combined antiretroviral therapy (cART) became standard in Romania starting in 1999. Currently, the children infected during the Romanian HIV epidemic have reached adulthood, and have over 20 years of chronic HIV infection and over a decade of exposure to antiretroviral treatment.
The present study was designed to evaluate the prevalence and severity of neurocognitive impairment in a homogenous group of young Romanian adults with the same subtype F who were primarily infected in their first years of life during this era.
Methods
Study population
The study was approved by the institutional review board (IRB) of “Dr. Victor Babes” Hospital for Infectious and Tropical Diseases (VBH) Bucharest, Romania and the University of California San Diego IRB. All participants provided written informed consent to participate in the study.
The HIV-infected participants (HIV+) were recruited from patients seen at the VBH outpatient department. VBH is one of the reference centers for HIV in Romania, and has followed over 1,600 HIV-infected patients since the beginning of HIV epidemic.
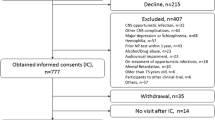
All 49 HIV+ participants were born between 1988 and 1991, had documented HIV infection and parenteral non-IDU transmission risk factors, and were followed at VBH since their HIV diagnosis. Epidemiological data supports infection in their first year of life, during the Romanian pediatric HIV epidemic. Ten of the 49 HIV participants had previous HIV clade evaluation (due to virological failure); all had F1 HIV subtype. Furthermore, previous molecular demographic and phylogeographic analyses (Mehta et al. 2011; Mbisa et al. 2012) demonstrated that F subtype was responsible for the Romanian nosocomial pediatric HIV epidemic. Participants were excluded if they were unable to provide informed consent, had a neurocognitive morbidity unrelated to HIV condition (e.g., recent and/or significant traumatic brain injury, color blindness, hearing deficit that appears to affect auditory comprehension, neurosyphilis, stroke, etc.), or significant CNS opportunistic infection with fixed neurologic damage that may interfere with the neurocognitive assessment (e.g., PML, brain toxoplasmosis), severe psychiatric disorder, current or past psychotic disorder (e.g., schizophrenia, bipolar, or unipolar disorder with psychosis), or other major mental disorder likely to affect participation in the study or confound interpretation of neurocognitive evaluation, use of alcohol or recreational drugs meeting abuse or dependence criteria, or other severe medical confounds (e.g., severe pulmonary or renal disease, significant cardiac disease, and malignancy). We excluded participants with less than 8 years of education or who had been institutionalized during childhood.
Age-matched HIV− participants (n = 20) with similar socioeconomic status were recruited mainly from Bucharest and from surrounding regions. Most of the controls were the siblings of HIV+ patients or their schoolmates.
Neurocognitive assessment
All 69 participants underwent standardized neurocognitive, psychiatric, and medical evaluations by the Romanian investigators (LE, RB, AB, AL), following training and certification at the HIV Neurobehavioral Research Center (HNRC) in San Diego.
The study protocol included a comprehensive neuropsychological (NP) test battery that has been validated for detecting and characterizing neurobehavioral effects of HIV-1 infection in the US (Heaton et al. 2010) and other international settings (Heaton et al. 2008). The battery assessed seven neurocognitive domains (Table 1). The instruments were adapted for linguistic and cultural appropriateness and translated into Romanian using standard back-translation methods.
In order to explore the real-world consequences of HIV-associated neurocognitive impairments, questionnaires were administered in order to assess (1) experiences of cognitive difficulties in the participants’ everyday lives using the Patient’s Assessment of Own Functioning Inventory (PAOFI) (Chelune et al. 1986) as well as (2) the independence with which they perform instrumental activities of daily living, using the Independent Activities of Daily Living Scale (ADL) questionnaire (Heaton et al. 2004).
Substance use and psychiatric disorders
In order to address the question whether neurocognitive impairment is due to HIV or other condition, the psychiatric evaluations focused on depression and substance use. Depression was evaluated with Beck Depression Inventory II (a 21-item self-report scale that measures depressive symptoms). A score of 0–13 represents minimal symptoms, 14–19 indicates mild depression, 20–28 is moderate, and 29–63 represents severe depressive (Beck and Steer 1993). Current and past alcohol and substance use was determined using a substance use history questionnaire.
Estimate of premorbid cognitive ability
In this HIV+ cohort, fewer years of schooling was impacted by noncognitive factors (e.g., not attending school due to stigma), but also possibly due to HIV-related cognitive deficits. Since, in general, children’s academic and occupational success is correlated with their parents’ attainment in these areas, in order to estimate whether the HIV+ and HIV− participants had similar background characteristics and likely premorbid potential, we calculated the Hollingshead four-factor index (Hollingshead 2011). This index, ranging from 1 to 5, is estimated by combining information on the parents’ sex, marital status, education, and occupation. We adapted the occupation of the Romanian group to fit into the nine groups defined in Hollingshead classification. Hollingshead data collection was initiated following the start of the study, so data was available for 40 of 49 HIV+ and 12 of 20 HIV– participants.
Neuromedical evaluation
The neuromedical (NM) examination included a systematic review of past medical and neurological histories, review of history of any current or past antiretroviral medications, and their side effects as well as a brief medical and neurological exam (includes testing for peripheral neuropathy). All NM instruments were adapted from ongoing HNRC studies. The medical records of HIV-infected patients were reviewed in order to record medical history, including nadir CD4 count. Current CD4 and HIV RNA were measured at the moment of neurocognitive evaluation. The HIV RNA detection limit was 400 copies/ml.
All the control participants had a negative HIV serology. For all participants, standard laboratory testing was performed in order to assess other co-infections (HBV, HCV, and syphilis), renal, liver insufficiency, severe anemia, and thrombocytopenia. Data derived from these evaluations were used, in conjunction with laboratory results, to stage HIV+ participants and identify potential confounds.
Statistical analysis
Analyses for individual test raw scores were conducted using independent samples t test. In order to examine performance within and across cognitive domains, each of the tests was transformed into Z scores based on the mean and standard deviation of the HIV– group. The domain-specific Z scores were then averaged and independent samples t test was used to examine if mean group differences exist. In order to estimate impairment rates, we utilized a global deficit-type approach (Carey et al. 2004) by assigning a score from 0 to 5 based on number of Z score standard deviations from normal. Worse cognitive performance results in a higher Z score deficit score (ZDS). A Z score of >0.50 was the cutpoint for classification as neuropsychologically impaired. The effect size (Cohen’s d) was used to demonstrate the magnitude of the differences in NP scores between the HIV+ and HIV– groups.
Results
Demographic characteristics
As shown in Table 2, the HIV+ and HIV− groups were similar with respect to age and gender. The HIV+ group had significantly less education than the control group, although the two groups had similar parental Hollingshead index scores. The average age at HIV diagnosis was 8.82 years (IQR = 7.1 to 10.4).
There were few confounding conditions: one HIV-infected participant was infected with HCV, and only two participants from the HIV+ group admitted significant previous drug abuse (marijuana and cocaine). None of the participants had chronic alcohol use or alcohol abuse during the last 12 months. Eighteen HIV+ and 2 HIV− participants had positive HBs antigen. None of the participants had ALT levels elevated more than two times normal and none of them had signs of liver insufficiency.
Neuropsychological performance
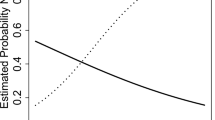
As seen in Table 3, the HIV+ group had lower raw NP scores on all tests, with the following reaching statistical significance: Letter Fluency and Action Fluency, Digit Symbol, Symbol Search, Trails A, Stroop Word and Color, PASAT-50, Category Test, WCST-64, Stroop Incongruent, BVMT Learning, and Grooved Pegboard Dominant and Non-Dominant Hand. Effect sizes across the NP tests ranged from 0.22 to 1.13, with most exhibiting at least a medium (>0.5) effect size. As seen in Table 3 and Fig. 1, the greatest effect sizes were seen in the motor and executive functioning domains in the overall HIV+ group (solid line). Using a ZDS cutpoint of 0.5, 59.1 % of the HIV+ group was identified as cognitively impaired, versus 10.0 % of the HIV− group.
Since the educational differences between the HIV+ and HIV− groups may present an additional confound (higher levels of education are typically associated with better neuropsychological test performance), we also took a more conservative approach and sought to match the HIV− and HIV+ groups with at least 10 years of education attained (biasing our analyses to include the best functioning HIV+ individuals). This resulted in a group of 31 HIV+ individuals (mean [SD] years of education = 10.9 [0.81]) and the same 20 HIV− controls (p = 0.10). As seen in the far right column of Table 3 and Fig. 1, despite this conservative bias, we still find low-to-moderate effect sizes across most cognitive domains, with the strongest differences being found in Fluency (letter fluency), Processing speed (digit symbol), Executive functioning (WCST-64), and Motor functioning (grooved pegboard). In the matched group, the impairment rate was 38.7 % for the HIV+ group versus 10.0 % for the HIV− controls (p = 0.025). The matched group also had the largest effect size for Motor and Executive functioning, with the pattern being similar to that seen with the total HIV+ group (Fig. 1). More than half of the HIV+ participants had impairment in the motor, speed of information processing, executive functioning, and attention/working memory domains.
Relationship between medical variables and cognition
Forty-two participants (85.7 %) had a current CD4 count greater than 200. Current CD4 levels were not correlated with education (p > 0.99). There was a trend for those with a nadir CD4 < 200 (n = 34) to have a higher ZDS than those who never dropped below 200 (n = 13) (ZDS = 1.21 (1.04) vs. 0.75 (0.82), respectively; p = 0.17; effect size = 0.46). Eighteen patients had AIDS-defining diseases, with a mean ZDS of 1.72 (1.14) versus 0.72 (0.69) for those without AIDS events (p < 0.001).
All except one HIV patient had been exposed to antiretroviral treatment. Four patients stopped cART for more than 6 months before testing (nonadherence). Thirty-six (81.8 %) of the 44 HIV patients on cART had undetectable HIV load at testing. The mean time of exposure to ART was 5.8 ± 3.2 (0.46–15.4) years in our group of HIV-infected participants. The median number of ART regimens was 2 (range 1–6). Duration of exposure to antiretroviral therapy was not associated with the ZDS (rho = −0.2, p = 0.16), and there was no difference in neurocognitive impairment rates between participants that spent longer time on an antiretroviral regimen with a total CNS penetration rank above 7 (Letendre et al. 2010) compared to those with an antiretroviral regimen rank below 7.
There was a trend for the HIV+ participants to be more likely to report cognitive problems on the PAOFI (58.5 % with at least one complaint vs. 31.6 % of the HIV− group; p = 0.052). Within the HIV+ group, a higher ZDS score was associated with more cognitive complaints (r = 0.33, p = 0.04), and there was a trend for a higher number of complaints in the HIV-impaired group (mean 2.6 vs. 0.83 in the nonimpaired group, p = 0.058). There were no clear differences in difficulties completing everyday activities (ADL questionnaire), but this was confounded by the fact that the HIV+ group was more likely to report that they were “able” to do an activity, but chose not to or that someone else does it for them (e.g., 5 % of controls vs. 26.5 % of HIV+ reporting being able to handle their own finances, but someone else does it for them), and that the HIV− group may have had more social supports (e.g., the HIV− group was more likely to eat meals prepared by others vs. the HIV+ group which was more likely to prepare their own meals). Thus, we did not utilize the IADL questionnaire in determining syndromic status. However, HIV-infected participants reported problems in initiating and participating to social activities, using transportation, reading, and understanding written materials. The HIV+ NP-impaired group had a higher mean number of reported difficulties in IADLs domains compared to HIV+ NP unimpaired participants (3.4 ± 2.2 vs. 2.2 ± 1.6, p = 0.04).
Using the previously established PAOFI cutpoint of three or more complaints as indicative of complaints with everyday functioning (Blackstone et al. 2012), approximately 26 % of the HIV+ impaired individuals would be classified as having syndromic impairment (Antinori et al. 2007), with the remaining 74 % classified as having asymptomatic neurocognitive impairment.
The overall depression rates were low for the HIV+ group (BDI median of 7.0 [IQR 3–15] vs. 4.5 [IQR 2.5–9.0] for the control group; p = 0.07). No participant had severe depression. However, 8.1 % of HIV participants and 5 % of controls had moderate depression. Depressive symptoms were not significantly associated with the number of cognitive complaints (r = 0.19; p = 0.25).
Discussion
Using a neuropsychological test battery validated in other international HIV cohorts, and a comparison group of HIV seronegative young adults of similar age and socioeconomic background, we found high prevalence of neurocognitive dysfunction among HIV+ young adults infected with HIV since early childhood. Over half of the HIV-infected young adults exhibited neurocognitive impairment. This rate is notable given that most of the participants were on cART, had undetectable HIV plasma load, and good immunological status; there was a low rate of confounding conditions, and that they were part of a “survivor” cohort. To our knowledge, this is one of the largest studies to examine HIV-associated neurocognitive disorders in a homogenous group of long-term surviving adolescents and young adults who grew up with HIV.
Most cognitive studies of individuals infected since early childhood focused on children infected perinatally, and have reported on data collected through early adolescence. Comparisons between studies are complicated by many methodological differences, such as age ranges of the participants, sample sizes, ARV treatment status, comprehensiveness of the neuropsychological test battery, availability of appropriate norms, etc. Nonetheless, findings regarding global cognitive functioning have been mixed, with some studies finding lower functioning in HIV+ children and adolescents (Koekkoek et al. 2008; Paramesparan et al. 2010; Hoare et al. 2012; Ruel et al. 2012), while others did not (Bagenda et al. 2006). The overall impairment rate in the present study was 59 %, which is slightly higher than described in the US CHARTER adult population (45 % for those without severe confounds) (Heaton et al. 2010) and similar to those reported in the French Aquitaine Cohort between 2007 and 2009 (Bonnet et al. 2012), although it should be noted that the current cohort had a history of more advanced HIV disease. Of note, these findings of higher impairment in the HIV+ generally held even when more closely matching the HIV+ and HIV− groups on educational attainment, a conservative bias. Reasons for the lower education in the HIV+ sample were fear of being stigmatized, the parents’ decision to hold them out of school, poor general health status, or limited access to high school or university (Buzducea et al. 2010).
In the present study, the greatest HIV-related decrements in cognitive functioning were in the domains of executive and motor functioning, although more than half of the HIV+ participants had impairment in four of the seven assessed cognitive domains (including speed of information processing and attention/working memory) consistent with a frontosubcortical pattern. Motor impairment is often reported in children with HIV encephalopathy. The young adults with HIV in the present study did not have abnormalities on the neurological exam, but had significantly worse scores at the Grooved Pegboard Test, indicating a deficit of fine motor skills. HIV-associated impaired myelination processes during childhood and adolescence might be responsible for impaired fine motor skills in our group, as reported previously by other pediatric studies (Blanchette et al. 2001; Abubakar et al. 2008). Moreover, demyelination processes were linked to poor executive functioning and attention in a group of HIV-infected children, asymptomatic with slow HIV progression (Hoare et al. 2012).
There are several candidate explanations for the present findings. It is possible that irreversible brain damage occurred prior to initiating cART, which is corroborated by the strong relationship we found between worse cognitive functioning and a history of an AIDS-defining illness, as well as lower nadir CD4 cell counts. All the HIV+ participants had at least 8–10 years of chronic HIV without cART and most had a nadir CD4 count below 200. In children, similar to adult populations, persistent and uncontrolled HIV replication in the brain may lead to cognitive deficits (Pratt et al. 1996; Reger et al. 2002), and nadir CD4+ lymphocyte count has been associated with greater risk of neurocognitive impairment in many reports in adults (Heaton et al. 2010; Ellis et al. 2011) and children (Foster et al. 2006). We postulate a possible neurotropic effect of HIV clade F which could explain the prevalence of NCI in this group of young participants with controlled HIV infection. HIV-associated neurocognitive impairment appears prevalent regardless of the HIV clade (Joseph et al. 2013), although a study of children in Uganda found that HIV-subtype A was associated with poorer neuropsychological performance compared with subtype D, suggesting that subtype-specific neurocognitive deficits may possibly reflect age-related differences in the neuropathogenesis of HIV (Boivin et al. 2010).
Although less studied, the potential neurotoxicity of antiretroviral therapy on the developing brain may also contribute to neurocognitive impairment (Robertson et al. 2012). This is of particular interest in the light of exposure of our study population during childhood to nucleoside reverse-transcriptase inhibitors that have been associated with possible mitochondrial dysfunction in children with perinatal HIV infection (Crain et al. 2010).
The impaired HIV+ participants had a significantly higher number of cognitively complaints than cognitively normal participants, consistent with prior studies demonstrating the relationship between cognitive functioning and real-world difficulties (Heaton et al. 2004; Blackstone et al. 2012). Using cognitive complaints to determine syndromic status, 26 % of the impaired participants met the criteria for mild neurocognitive disorder, similar to the rate recently reported in the CHARTER multisite study of adults in the US (Blackstone et al. 2012). Unfortunately, the use of a questionnaire designed to assess independence in everyday functioning in participants infected in adulthood was problematic for our specific group, due to different living conditions, access to parents and other social support systems, etc. In addition, its utility was limited since both groups could not reference an earlier time of independent functioning. Previous studies using the Adaptive Behavior Assessment System (Harrison and Oakland 2003), which has been proposed as one option for measuring adolescent functioning, have had conflicting results (Gosling et al. 2004; Smith et al. 2012). Almost half of the current HIV+ participants reported problems in initiating or participating in social activities. Although not significantly different from controls, this may be related to the self-stigmatization often observed in our patients.
We found relatively low depression rates in the HIV+ group. This could be the result of good coping mechanisms, given the long course of their HIV-infection, or possibly an underreporting of depressive symptoms. Low prevalence of depression has also been reported among youths in studies from US (Mellins et al. 2009; Gadow et al. 2010) and Thailand (Lee et al. 2011).
Our group of HIV positive participants had HIV negative parents while most studies evaluate perinatally HIV-infected children that are orphans or have HIV-infected parents. The support of the parents and caregivers is associated with better mental health in youth (Elkington et al. 2011). The present study did not comprehensively evaluate for psychiatric disorders, emotional/behavioral problems, or psychosocial problems related to prolonged hospitalization, missed school and social opportunities, and stigma, which could have led to incomplete education, unemployment, or impaired independent functionality.
Our study had several limitations, including the limited HIV+ and control sample sizes, as well as a lack of large-scale normative data. This was somewhat mitigated by the narrow age and education range of the current cohort, but it does limit the overall interpretability of our findings.
Future studies should incorporate a longitudinal design to determine whether cognitive trajectories differ during this critical developmental period, and the maturation that typically occurs during the young adulthood years. In addition, such studies might assess for key behaviors (e.g., risk-taking and decision-making) and neuroanatomic changes (e.g., via imaging) in long-term surviving children/adults to better understand the conditions likely to be encountered as the large international cohort of individuals infected since childhood ages. This in turn might inform the field regarding targets for cognitive and behavioral interventions.
References
Abubakar A, Van Baar A, Van de Vijver FJ, Holding P, Newton CR (2008) Paediatric HIV and neurodevelopment in sub-Saharan Africa: a systematic review. Trop Med Int Health 13:880–887
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799
Apetrei C, Necula A, Holm-Hansen C, Loussert-Ajaka I, Pandrea I, Cozmei C, Streinu-Cercel A, Pascu FR, Negut E, Molnar G, Duca M, Pecec M, Brun-Vezinet F, Simon F (1998) HIV-1 diversity in Romania. AIDS 12:1079–1085
Bagenda D, Nassali A, Kalyesubula I, Sherman B, Drotar D, Boivin MJ, Olness K (2006) Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics 117:729–740
Beck AT, Steer RA (1993) Beck depression inventory manual. Psychological Corporation, San Antonio
Benedict RH (1997) Brief visuospatial memory test-revised. Odessa
Blackstone K, Moore DJ, Heaton RK, Franklin DR Jr, Woods SP, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, Rivera-Mindt M, Deutsch R, Ellis RJ, Hampton Atkinson J, Grant I (2012) Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc 18:79–88
Blanchette N, Smith ML, Fernandes-Penney A, King S, Read S (2001) Cognitive and motor development in children with vertically transmitted HIV infection. Brain Cogn 46:50–53
Boivin MJ, Ruel TD, Boal HE, Bangirana P, Cao H, Eller LA, Charlebois E, Havlir DV, Kamya MR, Achan J, Akello C, Wong JK (2010) HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. AIDS 24:1163–1170
Bonnet F, Amieva H, Marquant F, Bernard C, Bruyand M, Dauchy FA, Mercie P, Greib C, Richert L, Neau D, Catheline G, Dehail P, Dabis F, Morlat P, Dartigues JF, Chene G (2013) Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS 27:391–400
Bowie CR, Harvey PD (2006) Administration and interpretation of the Trail Making Test. Nat Protoc 1:2277–2281
Brandt J, Benedict RH (2001) Hopkins verbal learning test-revised. Professional manual, Lutz
Brucki SM, Malheiros SM, Okamoto IH, Bertolucci PH (1997) Normative data on the verbal fluency test in the animal category in our milieu. Arq Neuropsiquiatr 55:56–61
Burns S, Hernandez-Reif M, Jessee P (2008) A review of pediatric HIV effects on neurocognitive development. Issues Compr Pediatr Nurs 31:107–121
Buzducea D, Lazar F, Mardare EI (2010) The situation of Romanian HIV-positive adolescents: results from the first national representative survey. AIDS Care 22:562–569
Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK (2004a) Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26:307–319
Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, Grant I, Heaton RK (2004b) Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol 18:234–248
Chelune G, Heaton R, Lehman R (1986) Neuropsychological and personality correlates of patient’s complaints of disability. New York Plenum Press, New York
Christensen BK, Girard TA, Bagby RM (2007) Wechsler adult intelligence scale-third edition short form for index and IQ scores in a psychiatric population. Psychol Assess 19:236–240
Coscia JM, Christensen BK, Henry RR, Wallston K, Radcliffe J, Rutstein R (2001) Effects of home environment, socioeconomic status, and health status on cognitive functioning in children with HIV-1 infection. J Pediatr Psychol 26:321–329
Crain MJ, Chernoff MC, Oleske JM, Brogly SB, Malee KM, Borum PR, Meyer WA 3rd, Mitchell WG, Moye JH, Ford-Chatterton HM, Van Dyke RB, Seage Iii GR (2010) Possible mitochondrial dysfunction and its association with antiretroviral therapy use in children perinatally infected with HIV. J Infect Dis 202:291–301
Diehr MC, Heaton RK, Miller W, Grant I (1998) The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment 5:375–387
Diehr MC, Cherner M, Wolfson TJ, Miller SW, Grant I, Heaton RK, Group HIVNRC (2003) The 50 and 100-item short forms of the Paced Auditory Serial Addition Task (PASAT): demographically corrected norms and comparisons with the full PASAT in normal and clinical samples. J Clin Exp Neuropsychol 25:571–585
Elkington KS, Robbins RN, Bauermeister JA, Abrams EJ, McKay M, Mellins CA (2011) Mental health in youth infected with and affected by HIV: the role of caregiver HIV. J Pediatr Psychol 36:360–373
Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I (2011) CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 25:1747–1751
Foster CJ, Biggs RL, Melvin D, Walters MD, Tudor-Williams G, Lyall EG (2006) Neurodevelopmental outcomes in children with HIV infection under 3 years of age. Dev Med Child Neurol 48:677–682
Gadow KD, Chernoff M, Williams PL, Brouwers P, Morse E, Heston J, Hodge J, Di Poalo V, Deygoo NS, Nachman S (2010) Co-occuring psychiatric symptoms in children perinatally infected with HIV and peer comparison sample. J Dev Behav Pediatr 31:116–128
Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK (1999) Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment 6:147–178
Golden CJ (1975) A group version of the stroop color and word test. J Pers Assess 39:386–388
Gosling A, Burns J, Hirst F (2004) Children with HIV in the UK: a longitudinal study of adaptive and cognitive functioning. Clin Child Psychol Psychiatry 9:25–37
Harrison PL, Oakland T (eds) (2003) Adaptive behavior assessment system. The Psychological Corporation, San Antonio
Heaton RK, Grant I, Matthews CG. (1991) Comprehensive norms for an expanded Halstead-Reitan battery: Demographic corrections research findings, and clinical applications. Odessa
Heaton R, Taylor M, Manly J (2003) Demographic effects and use of demographically corrected norms with the WAI-III and WMS-III. Academic Press, San Diego
Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I (2004) The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 10:317–331
Heaton RK, Cysique LA, Jin H, Shi C, Yu X, Letendre S, Franklin DR, Ake C, Vigil O, Atkinson JH, Marcotte TD, Grant I, Wu Z (2008) Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol 14:536–549
Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096
Hersh BS, Popovici F, Apetrei RC, Zolotusca L, Beldescu N, Calomfirescu A, Jezek Z, Oxtoby MJ, Gromyko A, Heymann DL (1991) Acquired immunodeficiency syndrome in Romania. Lancet 338:645–649
Hersh BS, Popovici F, Jezek Z, Satten GA, Apetrei RC, Beldescu N, George JR, Shapiro CN, Gayle HD, Heymann DL (1993) Risk factors for HIV infection among abandoned Romanian children. AIDS 7:1617–1624
Hoare J, Fouche JP, Spottiswoode B, Donald K, Philipps N, Bezuidenhout H, Mulligan C, Webster V, Oduro C, Schrieff L, Paul R, Zar H, Thomas K, Stein D (2012) A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naive “slow progressors”. J Neurovirol 18:205–212
Hollingshead AB (2011) Four factor index of social status. Yale J Sociol 8:21–53
Joseph J, Achim CL, Boivin MJ, Brew BJ, Clifford DB, Colosi DA, Ellis RJ, Heaton RK, Gallo-Diop A, Grant I, Kanmogne GD, Kumar M, Letendre S, Marcotte TD, Nath A, Pardo CA, Paul RH, Pulliam L, Robertson K, Royal W 3rd, Sacktor N, Sithinamsuwan P, Smith DM, Valcour V, Wigdahl B, Wood C (2013) Global NeuroAIDS roundtable. J Neurovirol 19:1–9
Klove H (1963) Clinical neuropsychology. Saunders, New York
Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP (2008) Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol 12:290–297
Kongs S, Thompson LL, Iverson GL, Heaton RK (2000) WCST-64: Wisconsin card sorting test-64 card version professional manual. Odessa
Laughton B, Cornell M, Boivin M, Van Rie A (2013) Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc 16:18603
Lee B, Chhabra M, Oberdorfer P (2011) Depression among vertically HIV-infected adolescents in Northern Thailand. J Int Assoc Physicians AIDS Care (Chic) 10:89–96
Letendre SL, Ellis RJ, Ances BM, McCutchan JA (2010) Neurologic complications of HIV disease and their treatment. Top HIV Med Publ Int AIDS Soc USA 18:45–55
Mbisa JL, Hue S, Buckton AJ, Myers RE, Duiculescu D, Ene L, Oprea C, Tardei G, Rugina S, Mardarescu M, Floch C, Notheis G, Zohrer B, Cane PA, Pillay D (2012) Phylodynamic and phylogeographic patterns of the HIV type 1 subtype F1 parenteral epidemic in Romania. AIDS Res Hum Retroviruses 28:1161–1166
Mehta SR, Wertheim JO, Delport W, Ene L, Tardei G, Duiculescu D, Pond SL, Smith DM (2011) Using phylogeography to characterize the origins of the HIV-1 subtype F epidemic in Romania. Infect Genet Evol 11:975–979
Mellins CA, Brackis-Cott E, Leu CS, Elkington KS, Dolezal C, Wiznia A, McKay M, Bamji M, Abrams EJ (2009) Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry 50:1131–1138
Mitchell W (2001) Neurological and developmental effects of HIV and AIDS in children and adolescents. Ment Retard Dev Disabil Res Rev 7:211–216
Nozyce M, Hittelman J, Muenz L, Durako SJ, Fischer ML, Willoughby A (1994) Effect of perinatally acquired human immunodeficiency virus infection on neurodevelopment in children during the first two years of life. Pediatrics 94:883–891
Orsini A, Trojano L, Chiacchio L, Grossi D (1988) Immediate memory spans in dementia. Percept Mot Skills 67:267–272
Paramesparan Y, Garvey LJ, Ashby J, Foster CJ, Fidler S, Winston A (2010) High rates of asymptomatic neurocognitive impairment in vertically acquired HIV-1-infected adolescents surviving to adulthood. J Acquir Immune Defic Syndr 55:134–136
Pendleton MG, Heaton RK (1982) A comparison of the Wisconsin card sorting test and the category test. J Clin Psychol 38:392–396
Piatt AL, Fields JA, Paolo AM, Troster AI (1999) Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia 37:1499–1503
Pratt RD, Nichols S, McKinney N, Kwok S, Dankner WM, Spector SA (1996) Virologic markers of human immunodeficiency virus type 1 in cerebrospinal fluid of infected children. J Infect Dis 174:288–293
Reger M, Welsh R, Razani J, Martin DJ, Boone KB (2002) A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 8:410–424
Robertson K, Liner J, Meeker RB (2012) Antiretroviral neurotoxicity. J Neurovirol 18:388–399
Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, Rosenthal PJ, Dorsey G, Achan J, Akello C, Kamya MR, Wong JK (2012) Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis Off Publ Infect Dis Soc Am 54:1001–1009
Selnes OA, Jacobson L, Machado AM, Becker JT, Wesch J, Miller EN, Visscher B, McArthur JC (1991) Normative data for a brief neuropsychological screening battery. Multicenter AIDS Cohort Study. Percept Mot Skills 73:539–550
Smith R, Malee K, Leighty R, Brouwers P, Mellins C, Hittelman J, Chase C, Blasini I (2006) Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics 117:851–862
Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, Wilkins M, Nichols S, Mellins C, Usitalo A, Garvie P, Rutstein R (2012) Impact of human immunodeficiency virus severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J
Steele RG, Nelson TD, Cole BP (2007) Psychosocial functioning of children with AIDS and HIV infection: review of the literature from a socioecological framework. J Dev Behav Pediatr 28:58–69
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Tardieu M, Le Chenadec J, Persoz A, Meyer L, Blanche S, Mayaux MJ (2000) HIV-1-related encephalopathy in infants compared with children and adults. French Pediatric HIV Infection Study and the SEROCO Group. Neurology 54:1089–1095
Tombaugh TN, Kozak J, Rees L (1999) Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol 14:167–177
WHO (2014, July 21, 2014) Global summary of AIDS epidemic 2013. http://www.who.int/hiv/data/epi_core_dec2014.png?ua=1. Retrieved 8 Aug 2014
Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Troster AI, Group HIVNRC (2005) Action (verb) fluency: test-retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc 11:408–415
Acknowledgments
The authors would like to thank all participants in the study; Gratiela Tardei, MD for the laboratory assessments for the study participants, and Maiko Sakamoto, PhD for background literature review and editorial comments on the manuscript. This work was supported by an R21 MH077487-01 NIMH grant and the HIV Neurobehavioral Research Center at UCSD (P30MH062512).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ene, L., Franklin, D.R., Burlacu, R. et al. Neurocognitive functioning in a Romanian cohort of young adults with parenterally-acquired HIV-infection during childhood. J. Neurovirol. 20, 496–504 (2014). https://doi.org/10.1007/s13365-014-0275-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-014-0275-1