Abstract
Peripheral neuropathy (PN) is a common neurological complication of HIV infection that has debilitating effects on quality of life. While there has been a comprehensive evaluation of the prevalence of neuropathic signs/symptoms and risk factors (RFs) for PN or symptomatic PN (SPN) with initiation of combination antiretroviral therapy (cART) in ART-naïve patients, similar evaluation in ART-experienced patients is limited. This study investigated the prevalence and RFs for PN/SPN in ART-experienced patients enrolled in clinical salvage therapy studies. Between February 2000 and June 2007, 522 ART-experienced participants who experienced virologic failure with a prior regimen and started new regimens were followed longitudinally and annually screened for signs and symptoms of PN. Rates of PN/SPN at 3 years since parent study entry were 52.8 and 24.0 %, respectively. Aging, taller height, protease inhibitor use, and female sex were significant RFs for PN/SPN. The use of statin drugs was significantly associated with lower odds of SPN, and it may prevent progression from no SPN to SPN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the declines in the incidences of HIV-associated dementia and central nervous system (CNS) opportunistic infections in the combination antiretroviral therapy (cART) era, sensory neuropathies (SNs) are still a frequent neurological disorder associated with HIV infection and its treatment with ART (Evans et al. 2011; Evans et al. 2012; Keswani et al. 2002; Bacellar et al. 1994; McArthur et al. 2005; Ellis et al. 2010).
There are two major types of HIV-associated distal sensory peripheral neuropathies: primary HIV-associated distal sensory polyneuropathy (HIV-DSP) and ART toxic neuropathy (ATN), which when combined affect approximately 30–67 % of patients with advanced HIV disease (Wulff et al. 2000; Cornblath and McArthur 1988). The signs/symptoms of HIV-DSP and ATN resemble each other as well as common neuropathies encountered in clinical practice including diabetic and alcohol-associated neuropathy. Symptoms include distal numbness, paresthesia, burning sensation, and stabbing pain. Common signs include reduced or absent ankle reflexes relative to patellar reflexes, reduced or absent vibration sensation in the toes, and decreased pin and temperature sensation in a stocking/glove distribution. There are no FDA-approved therapies for HIV-associated SNs, and treatment is limited to symptomatic measures (Keswani et al. 2002).
Risk factors for PN/SPN with initiation of cART in ART-naïve patients have been investigated (Evans et al. 2012), but similar evaluation in ART-experienced patients is lacking. Compared to ART-naïve individuals, treatment-experienced patients may be at higher risk for neurological disorders due to having longer duration of HIV infection, and a greater number of interventions and complications. We evaluated potential risk factors for PN/SPN using data from 522 ART-experienced patients who had virologic failure on prior regimens and started new regimens in ten randomized AIDS Clinical Trials Group (ACTG) trials.
Methods
Participants were selected from the ACTG Longitudinal Linked Randomized Trials (ALLRT), a meta-study of participants prospectively enrolled into randomized clinical trials of cART (defined as three or more antiretroviral agents) regimens. Study participants were followed long term for the purpose of evaluating clinical, virologic, immunologic, and neurologic outcomes associated with treatment of HIV with cART. They can enroll to ALLRT during parent clinical trials or within 8 weeks of completion of parent clinical trials. Therefore, study participants had variable length of follow-up and variable timing (relative to start of new regimens) for their first evaluations. All available data for ART-experienced participants for which neuropathy-related data had been collected were included. A total of 522 participants were enrolled from ten randomized trials, the largest of which were ACTG 398 (n = 213; Hammer et al. 2002), A5146 (n = 115; Demeter et al. 2009), A5211 (n = 70; Wilkin et al. 2007; Gulick et al. 2007), A5076 (n = 39), and A5143 (n = 33; Collier et al. 2008), that each contributing more than 30 participants. Others were ACTG 388 (n = 20; Fischl et al. 2003), ACTG 400 (n = 14), ACTG 347 (n = 11; Murphy et al. 1999), A5126 (n = 4; Eron et al. 2009), and A5135 (n = 3).
Peripheral neuropathy data
The Brief Peripheral Neuropathy Screen (BPNS) was administered in ALLRT every 48 weeks by trained non-neurologist site personnel. The BPNS assesses signs (vibration sensation at the feet and ankle reflexes) and symptoms (pain, parasthesia (“pins and needles” sensation), and numbness). The performance characteristics of the BPNS have been reported (Simpson et al. 2006; Ellis et al. 2005).
The participants had 2,024 patient visits with the median (Q1, Q3) number of neuropathy evaluations per patient 3 (2, 6), the median (Q1, Q3) time to the first neuropathy evaluation from parent study entry 48 (48, 96) weeks, and the median (Q1, Q3) follow-up time per patient 144 (96, 384) weeks.
PN was defined as at least mild loss of vibration sensation in both great toes or absent or hypoactive ankle reflexes bilaterally relative to knees. Asymptomatic PN (APN) was defined as PN without bilateral symptoms. SPN was defined as PN with any bilateral symptoms. Use of nART (i.e., d4T, ddI, or ddC) or protease inhibitors (PIs) was defined as at least 4 weeks of use in the 6 months prior to the evaluation.
Objectives
The objectives of the current analyses were to estimate the prevalence of neuropathic signs and symptoms over time in ART-experienced patients, to investigate risk factors for PN and SPN, and to explore risk factors for progression from no SPN to SPN.
Statistical methods
Descriptive statistics were used to describe the study sample. Longitudinal plots displayed the prevalence of PN and SPN over time. Multivariable logistic regression models (logistic GEE) were used to estimate the association [i.e., odds ratios (ORs) and associated confidence intervals (CIs)] between potential risk factors with PN, SPN, and progression from no SPN to SPN. Multivariable models included all of the risk variables described below since there was interest in estimating the association between each of these variables with PN/SPN/progression to SPN. Model fit was also assessed using methods described in Evans and Li (2005). Forest plots summarize these odds ratio estimates and associated confidence intervals.
Reported model results are from “full” models including all covariates, and thus, OR estimates were adjusted for other variables. Variables included in the models were demographics, HIV disease characteristics, ART use at the time of the evaluation, concomitant therapy use, and other patient characteristics.
Demographic variables included age at the time of evaluation (scaled such that ORs were interpreted for a 10-year increment), race [white (reference), black, Hispanic, other], sex (reference = male), and height at parent study entry (scaled such that odds ratios were interpreted for a 5-cm increment). HIV disease characteristics variables included log10(HIV-1 RNA copies/mL) at parent study entry, CD4 at parent study entry [categories: ≤200, 201–350, 351–500, ≥501 cells/μL (reference)], HIV-1 RNA at the time of evaluation [categories: ≤400 (reference), >400 copies/mL], and CD4 at the time of evaluation [categories: ≤ 200, 201–350, 351–500, ≥501 cells/μL (reference)]. ART use at the time of the evaluation variables included nART use and PI use. Concomitant therapy use variables were the use of the following medications within the 21 days prior to the evaluation: statins, non-statin lipid lowering drugs, insulin, and other glucose-lowering drugs (non-insulin). Other patient characteristics included reported history of diabetes, HCV seropositivity, and history of IV drug use.
Results
Demographics and baseline characteristics
Five hundred twenty-two ART-experienced participants (88 % male, 52 % white, 23 % black, median age = 43 years, median time since first ART use = 6.0 years, 48 % initiating EFV and PI-based ART, 44 % starting ritonavir-boosted PI-based ART (five different regimens were used: boosted Saquinavir, Atazanavir, Indinavir, Tipranavir, and Kaletra), median log10 HIV-1 RNA = 4.5 copies/mL, and median CD4 count = 204 cells/μL at parent study entry) were analyzed (Table 1).
Prevalence
Prior to the initiation of new regimens, the prevalence estimates (95 % CI) of PN and SPN were 57.6 % (46.9 %, 67.9 %) and 33.7 % (24.2 %, 44.3 %), respectively.
The treated cohort displayed viral control (i.e., HIV-1 RNA ≤400 copies/mL) in 57.8 % (1,116 of 1,932) of patient visits. The prevalence estimates (95 % CI) of PN and SPN at 3 years since parent study entry were 52.8 % (46.6 %, 58.9 %) and 24.0 % (19.0 %, 29.6 %), respectively. The prevalence of PN and SPN over time appeared relatively stable despite viral control and improved immune function after new regimen initiation at parent study entry (Fig. 1; Table 2). Consistent with this finding, a sensitivity analysis of the prevalence of neuropathy over time restricting to patients who had neuro tests at parent study entry suggested a similar trend (n = 92; figure not shown here). A univariate logistic GEE regression with time as the predictor does not suggest PN changes significantly with time (P = 0.65).
The majority of PN signs occurred without symptoms. Of 1,018 patient visits with PN, 716 (70.3 %) reported a pain level of zero, 720 (70.7 %) reported absence of paresthesia, and 645 (63.4 %) reported absence of numbness.
Associations with PN and SPN
Evaluation of the associations with PN and SPN was based on appropriate data from 485 patients (88 % male, 53 % white, 23 % black, 49 % were 40–49 years of age, and 27 % were 30–39 years of age, median time since first ART use = 6.1 years, 49 % initiating EFV and PI-based ART, 44 % starting boosted PI-based ART, median log10HIV-1 RNA = 4.6 copies/mL, and median CD4 count = 205 cells/μL at new ART regimen initiation) (model 1, Table 1). The participants had 1,739 patient visits, with the median (Q1, Q3) number of neuropathy evaluations per patient 3 (2, 5).
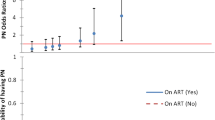
The following variables were associated with a higher odds of PN in a multivariate logistic GEE model simultaneously evaluating all factors: older age [OR = 1.71 per 10-year increment, 95 % CI = (1.41, 2.07), P < 0.001], female sex [OR = 2.45, 95 % CI = (1.40, 4.28), P = 0.001], taller height [OR = 1.24 per 5-cm increment, 95 % CI = (1.12, 1.37), P < 0.001], current PI use [OR = 1.67, 95 % CI = (1.16, 2.41), P = 0.006], and a history of IV drug use [OR = 1.60, 95 % CI = (1.04, 2.46), P = 0.036]. Neither viral suppression (HIV-1 RNA ≤400 copies/mL) (P = 0.78) nor baseline CD4 (P = 0.88) was associated with PN (Fig. 2a).
In a multivariate logistic GEE model, notable factors associated with higher odds of SPN were older age [OR = 1.56 per 10-year increment, 95 % CI = (1.24, 1.95), P < 0.001], female sex [OR = 2.00, 95 % CI = (1.04, 3.85), P = 0.058], taller height [OR = 1.24 per 5-cm increment, 95 % CI = (1.09, 1.40), P = 0.001], and current PI use [OR = 1.44, 95 % CI = (1.00, 2.07), P = 0.039]. The use of statin drugs in the 21 days preceding the neuro-visit was associated with lower odds of SPN [OR = 0.66, 95 % CI = (0.45, 0.98), P = 0.028]. Neither current CD4 (P = 0.27) nor baseline CD4 (P = 0.21) was associated with SPN (Fig. 2b).
Associations with progression from no SPN to SPN
To evaluate risk factors for progression from no SPN to SPN, we restricted analyses to those participants without SPN at their first neurologic visit after starting a new ART regimen. We then used data from subsequent visits to evaluate associations with SPN. Three hundred seven ART-experienced participants (88 % male, 55 % white, 19 % black, 48 % were 40–49 years of age, and 30 % were 30–39 years of age, median time since first ART use = 5.8 years, 53 % initiating EFV and PI-based ART, 38 % starting boosted PI-based ART, median log10HIV-1 RNA = 4.5 copies/mL, and median CD4 count = 226 cells/μL at new ART regimen initiation) were analyzed (model 2, Table 1). These participants had 990 patient visits of which 116 (11.7 %) displayed SPN. For 307 patients analyzed, the median (Q1, Q3) number of evaluations per patient was 3 (1, 5).
In a multivariable logistic GEE model, the following variables were associated with higher odds of progression from no SPN to SPN: older age [OR = 1.56 per 10-year increment, 95 % CI = (1.14, 2.14), P = 0.008], female [OR = 4.54 compared to male, 95 % CI = (1.95, 10.56), P = 0.003], taller height [OR = 1.34 per 5-cm increment, 95 % CI = (1.13, 1.59), P = 0.002], and IV drug use [OR = 3.53, 95 % CI = (1.56, 8.01), P = 0.018]. The use of statin drugs in the 21 days preceding the neuro-visit was marginally significant as a protector against progression from no SPN to SPN [OR = 0.50, 95 % CI = (0.23, 1.12), P = 0.063]. Having APN at the first visit (vs. not having PN) was not significantly associated with higher odds of future SPN [OR = 0.67, 95 % CI = (0.39, 1.18), P = 0.17] (Fig. 2c).
Discussion
Peripheral neuropathy in ART-naïve patients persists despite improved immunologic function and virologic control associated with cART (Evans et al. 2011). ART-experienced patients initiating new regimens due to virologic failure may have increased risk of PN due to longer duration of infection, uncontrolled viremia, and exposure to older ARV including d4T, ddI, and ddC. Our analyses from a diverse group of ART-experienced patients initiating new regimens suggest a relatively stable level of PN prevalence over time, but higher prevalence than for ART-naïve individuals after starting ART.
As is the case in ART-naïve HIV patients, PN and SPN in ART-experienced patients are associated with older age. With the rapidly advancing median age of HIV patients, the consistent association of PN with increasing age suggests that this problem should continue to be the focus of studies to reverse or prevent this troubling complication of HIV.
Our study confirms the clinical impression that neuropathy in HIV is height dependent, with a small, but consistently significant contribution to risk for neuropathy in taller persons, which is consistent with findings from previous studies (Evans et al. 2012; Sosenko et al. 1988). However, the impact of height is modest compared to aging. Our analyses support other observations about the lack of association of HCV co-infection with distal sensory neuropathy (Clifford et al. 2009).
This study suggests that PI use is associated with developing distal sensory neuropathy in ART-experienced patients. The concern that PI might be associated with neuropathy has been reported for some but not all PI drugs, postulated to be mediated by loss of macrophage-derived trophic factors (Pettersen et al. 2006). Other clinical series have suggested this effect is minimal with the most commonly used current PI (Ellis et al. 2008). Our data also suggest that female sex is a significant risk factor for PN/SPN in ART-experienced patients. This has generally not been seen in other PN studies, and it should be noted that the power of this sample is modest since women were under-represented. Ongoing attention to long-term complications in women deserves careful attention in future studies.
The study shows that the use of statins within the 21 days prior to the evaluation is significantly associated with lower odds of SPN, and it may also have a beneficial effect on preventing progression from no SPN to SPN. Since statins were not randomly assigned to patients in this cohort, the source of the “protective” association between statin use and SPN in this analysis cannot be determined from this observational study. The association may be truly protective, since elevated serum triglycerides have been shown to be a risk factor for SPN both in diabetes mellitus and in HIV (Tesfaye et al. 2005; Banerjee et al. 2011). Statins might, by lowering serum triglyceride levels (Huang et al. 2009), reduce progression to symptomatic SPN. Statins also may lower risk of SPN by reducing macrophage activation (Schönbeck and Libby 2004), which has been implicated in the pathogenesis of HIV-SN (Pardo et al. 2001; Purwata 2011; Hahn et al. 2008). Physicians in practice know that statins can cause myopathy and muscle pain. Knowing this, some providers might withhold statins in patients with SPN or discontinue statins if they develop SPN. This would result in a negative association between statin use and SPN that could be misinterpreted as a “protective” effect. Alternative explanations include confounding by unmeasured variables or unrecognized interactions with antiretroviral or other concomitant medications. Some previous studies have suggested that statins may promote neuropathy (Tierney et al. 2013; Coulson 2011), but others have failed to demonstrate such an effect (Otruba et al. 2011).
A natural question is whether there is undiagnosed diabetes in this cohort. Diabetes is often not diagnosed early and can be related to neuropathy. However, this cohort is composed of participants in clinical trials at academic centers. Thus, the participants are in care and being closely monitored for diabetes and other co-morbid complications. The lack of a history of diabetes and the absence of anti-diabetic drugs for these participants offer reasonable reassurance that diabetes in particular and a large variety of medical confounders are not ignored.
One limitation of this study is that the duration of prior nART treatment was not included as a variable when evaluating risk factors for PN, SPN, and progression from no SPN to SPN because these data were not prospectively collected. Other limitations include its observational nature with the potential for informative drop-out/in, self-selection issues in ART and concomitant medication use, and that the observed association may not be causal. There is a potential for selection bias as the analyses are restricted to clinical trial volunteers that were willing and able to return for follow-up visits. Results could be biased if these patients are very different than patients not included in analyses. Estimated association with variables subject to self-selection should be interpreted with caution. Non-significant P values should not be interpreted as “no association.” Instead the confidence intervals should be used to “rule out” associations with reasonable confidence. Some effect estimates, although not significant, cannot rule out potentially large associations. For instance, the use of a glucose-lowering drug was not significantly associated with higher odds of progression from no SPN to future SPN (P = 0.37), while the OR of 2.72 suggested a large association. Also, the neuropathy assessments were made by non-neurologists who had received training, but for some parts of the exam, such as the distal ankle jerks, reliability is very difficult to establish between examiners and may result in imprecision in some observations.
References
Bacellar H, Munoz A, Miller EN, Cohen BA, Besley D, Selnes OA, Becker JT, McArthur JC (1994) Temporal trends in the incidence of HIV-1-related neurologic diseases: Multicenter AIDS Cohort Study, 1985–1992. Neurology 44:1892–1900
Banerjee S, McCutchan JA, Ances BM, Deutsch R, Riggs PK, Way L, Ellis RJ (2011) Hypertriglyceridemia in combination antiretroviral-treated HIV-positive individuals: potential impact on HIV sensory polyneuropathy. AIDS 25(2):F1–6
Clifford DB, Smurzynski M, Park LS, Yeh TM, Zhao Y, Blair L, Arens M, Evans SR (2009) Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology 73:309–14
Collier AC, Tierney C, Downey GF, Eshleman SH, Kashuba A, Klingman K, Vergis EN, Pakes GE, Rooney JF, Rinehart A, Mellors JW, AIDS Clinical Trials Group Protocol A5143 Team (2008) Randomized study of dual versus single ritonavir-enhanced protease inhibitors for protease inhibitor-experienced patients with HIV. HIV Clin Trials 9(2):91–102
Cornblath DR, McArthur JC (1988) Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology 38:794–796
Coulson WF (2011) Statin neuropathy? J Fam Pract 60(4):182–4
Demeter LM, Jiang H, Mukherjee AL, Morse GD, DiFrancesco R, DiCenzo R, Dykes C, Sista P, Bacheler L, Klingman K, Rinehart A, Albrecht M (2009) A randomized trial of therapeutic drug monitoring of protease inhibitors in antiretroviral-experienced, HIV-1-infected patients. AIDS 23(3):357–68
Ellis R, Evans SR, Clifford D, Moo LR, McArthur JC, Collier AC, Benson C, Bosch R, Simpson D, Yiannoutsos CT, Yang Y, Robertson K, Neurological AIDS Research Consortium; AIDS Clinical Trials Group Study Teams A5001 and A362 (2005) Clinical Validation of the Neuroscreen. J Neurovirol 11(6):503–511
Ellis RJ, Marquie-Beck J, Delaney P, Alexander T, Clifford DB, McArthur JC, Simpson DM, Ake C, Collier AC, Gelman BB, McCutchan JA, Morgello S, Grant I, CHARTER Group (2008) Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. AnnNeurol 64:566–572
Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I, CHARTER Study Group (2010) Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol 67(5):552–8
Eron JJ Jr, Park JG, Haubrich R, Aweeka F, Bastow B, Pakes GE, Yu S, Wu H, Richman DD, ACTG5126 Study Team (2009) Predictive value of pharmacokinetics-adjusted phenotypic susceptibility on response to ritonavir-enhanced protease inhibitors (PIs) in human immunodeficiency virus-infected subjects failing prior PI therapy. Antimicrob Agents Chemother 53(6):2335–41
Evans S, Li L (2005) A comparison of goodness of fit tests for the logistic GEE model. Statis Med 24:1245–1261
Evans SR, Ellis RJ, Chen H, Yeh T, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB (2011) Peripheral neuropathy in HIV: prevalence and risk factors. AIDS 25:919–928
Evans SR, Lee AJ, Ellis RJ, Chen H, Wu K, Bosch RJ, Clifford DB (2012) HIV peripheral neuropathy progression: protection with glucose-lowering drugs? J Neurovirol 18(5):428–433
Fischl MA, Ribaudo HJ, Collier AC, Erice A, Giuliano M, Dehlinger M, Eron JJ Jr, Saag MS, Hammer SM, Vella S, Morse GD, Feinberg JE, Demeter LM, Eshleman SH, Adult AIDS Clinical Trials Group 388 Study Team (2003) A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen, in advanced human immunodeficiency virus disease. J Infect Dis 188(5):625–34, Erratum in: J Infect Dis 188(7):1083
Gulick RM, Su Z, Flexner C, Hughes MD, Skolnik PR, Wilkin TJ, Gross R, Krambrink A, Coakley E, Greaves WL, Zolopa A, Reichman R, Godfrey C, Hirsch M, Kuritzkes DR, AIDS Clinical Trials Group 5211 Team (2007) Phase 2 study of the safety and efficacy of Vicriviroc, a CCR5 inhibitor, in HIV-1-infected, treatment-experienced patients: AIDS Clinical Trials Group 5211. J Infect Dis 196(2):304–312
Hahn K, Robinson B, Anderson C, Li W, Pardo CA, Morgello S, Simpson D, Nath A (2008) Differential effects of HIV infected macrophages on dorsal root ganglia neurons and axons. Exp Neurol 210(1):30–40
Hammer SM, Vaida F, Bennett KK, Holohan MK, Sheiner L, Eron JJ, Wheat LJ, Mitsuyasu RT, Gulick RM, Valentine FT, Aberg JA, Rogers MD, Karol CN, Saah AJ, Lewis RH, Bessen LJ, Brosgart C, DeGruttola V, Mellors JW, AIDS Clinical Trials Group 398 Study Team (2002) Dual vs single protease inhibitor therapy following antiretroviral treatment failure: a randomized trial. JAMA 288(2):169–180
Huang XS, Zhao SP, Bai L, Hu M, Zhao W, Zhang Q (2009) Atorvastatin and fenofibrate increase apolipoprotein AV and decrease triglycerides by up-regulating peroxisome proliferator-activated receptor-alpha. Br J Pharmacol 158(3):706–12
Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC (2002) HIV-associated sensory neuropathies. AIDS 16:2105–2117
McArthur JC, Brew BJ, Nath A (2005) Neurological complications of HIV infection. Lancet Neurol 4:543–555
Murphy RL, Gulick RM, DeGruttola V, D'Aquila RT, Eron JJ, Sommadossi JP, Currier JS, Smeaton L, Frank I, Caliendo AM, Gerber JG, Tung R, Kuritzkes DR (1999) Treatment with amprenavir alone or amprenavir with zidovudine and lamivudine in adults with human immunodeficiency virus infection. AIDS Clinical Trials Group 347 Study Team. J Infect Dis 179(4):808–16
Otruba P, Kanovsky P, Hlustik P (2011) Treatment with statins and peripheral neuropathy: results of 36-months a prospective clinical and neurophysiological follow-up. Neuro Endocrinol Lett 32(5):688–90
Pardo CA, McArthur JC, Griffin JW (2001) HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst 6(1):21–27
Pettersen JA, Jones G, Worthington C, Krentz HB, Keppler OT, Hoke A, Gill MJ, Power C (2006) Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. AnnNeurol 59:816–824
Purwata TE (2011) High TNF-alpha plasma levels and macrophages iNOS and TNF-alpha expression as risk factors for painful diabetic neuropathy. J Pain Res 4:169–175
Schönbeck U, Libby P (2004) Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 109(21 Suppl 1):II18–26
Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, So Y, Marra CM, Diaz-Arrastia R, Shriver S, Millar L, Clifford DB, ACTG A5117 Study Group (2006) HIV Neuropathy Natural History Cohort Study: Assessment Measures and Risk Factors. Neurology 66:1679–1687
Sosenko JM, Boulton AJM, Gadia MT, Ward JD, Skylar JS (1988) The association between symptomatic sensory neuropathy and body stature in diabetic patients. Diabetes Res Clin Pract 4:95–98
Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH, EURODIAB Prospective Complications Study Group (2005) Vascular risk factors and diabetic neuropathy. N Engl J Med 352(4):341–50
Tierney EF, Thurman DJ, Beckles GL, Cadwell BL (2013) Association of statin use with peripheral neuropathy in the US population 40 years of age or older. J Diabetes 5(2):207–15
Wilkin TJ, Su Z, Kuritzkes DR, Hughes M, Flexner C, Gross R, Coakley E, Greaves W, Godfrey C, Skolnik PR, Timpone J, Rodriguez B, Gulick RM (2007) HIV type 1 chemokine coreceptor use among antiretroviral-experienced patients screened for a clinical trial of a CCR5 inhibitor: AIDS Clinical Trials Group A5211. Clin Infect Dis 44(4):591–595
Wulff EA, Wang AK, Simpson DM (2000) HIV-associated peripheral neuropathy: Epidemiology, pathophysiology and treatment. Drugs 59:1251–1260
Acknowledgments
This work was supported by National Institute of Health (NIH) grants including the Neurologic AIDS Research Consortium grant NS32228 from NINDS, the AIDS Clinical Trials Grant AI068636 from NIAID, and the Statistical and Data Management Center of the Adult AIDS Clinical Trials Group grant 1 U01 068634. The authors acknowledge the generous dedication of the many participants volunteering for the ALLRT study, and for the contributions of the contributing AIDS Clinical Trials Units, their investigators and staffs, that collected the samples and clinical data used for this analysis. The authors would like to thank reviewers for their thoughtful comments.
Conflict of Interest
Scott R. Evans has grant funding from NIH. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Clifford, D.B., Deng, L. et al. Peripheral neuropathy in ART-experienced patients: prevalence and risk factors. J. Neurovirol. 19, 557–564 (2013). https://doi.org/10.1007/s13365-013-0216-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-013-0216-4