Abstract
Highly active antiretroviral therapy (HAART) has increased life expectancy among HIV-infected individuals, and by 2015, at least half of all HIV-infected individuals will be over 50 years of age. Neurodegenerative processes associated with aging may be facilitated by HIV-1 infection, resulting in premature brain aging. This review will highlight brain abnormalities in HIV patients in the setting of aging, focusing on recent neuroimaging studies of the structural, physiological, functional and neurochemical changes. Magnetic resonance imaging (MRI) and magnetic resonance spectroscopy studies performed during the pre-HAART era or on antiretroviral-naive subjects suggest an accelerated aging process, while those on HAART-treated subjects suggest premature brain atrophy. Diffusion tensor imaging studies yielded conflicting findings on the relationship between HIV and age in neuroasymptomatic individuals. Functional MRI studies found evidence of premature or accelerated aging processes in the brains of HIV subjects. Lastly, many age-related illnesses such as diabetes, stroke, and depression, as well as comorbid substance abuse, may further exacerbate the aging process in the HIV-infected brain, leading to premature or accelerated age-related brain changes. Given the different pathologic or physiologic changes in the brain assessed by the different neuroimaging techniques, using a multimodal approach in longitudinal follow-up studies is recommended for future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Highly active antiretroviral therapy (HAART) has increased life expectancy among HIV-infected individuals, and hence, by 2015, at least half of all HIV-infected individuals will be over 50 years of age (Smith 2005). However, HIV and HAART combined may exacerbate many comorbidities (e.g., cardiovascular diseases, diabetes, etc.) associated with normal aging (Effros et al. 2008), and HIV patients exceed the expected risk for the development of these complications (Deeks 2011). HIV-1 infection may also facilitate the neurodegenerative processes associated with aging, which ultimately could result in premature aging (Brew et al. 2009) (i.e., due to HIV-associated brain injury, changes occur earlier than, but may be parallel to, the normal aging process) or accelerated aging (i.e., due to interaction between HIV and aging, changes progress at a faster rate than in normal aging). The increased life expectancy and progressive neurodegeneration associated with HIV may contribute to the development of HIV-1-associated neurocognitive disorders (HAND) in as many as 50 % of HIV-1-infected individuals. HAND includes asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and the most severe form, HIV-1-associated dementia (HAD). While the prevalence of HAD has decreased to <7 %, the incidence of both ANI and MND have been on the rise over the past decade (Antinori et al. 2007; Woods et al. 2009). HAND persists among HIV-infected individuals despite the decreased morbidity and mortality with the advent of HAART. The current higher prevalence of the milder form of HAND coincides with the aging population of HIV-infected individuals; therefore, aging may be an important risk factor for HAND.
In seronegative (SN) individuals, aging results in global increases in immune activation, even in the central nervous system (CNS). The number of microglia increases with increasing age, but these “aging” microglia are less functional in terms of neuroprotection (Streit and Xue 2010), resulting in a less-than-optimal inflammatory environment in the brain (Schuitemaker et al. 2012). In patients with HIV-1 infection, HIV-infected monocytes traverse the blood–brain barrier, disseminate the virus, and induce a proinflammatory environment that further propagates HIV-1 infection in the CNS (Haase 1986). HIV-induced glial activation may lead to neuronal dysfunction and greater reactive neuroinflammation, and the dystrophic microglia in the aging brain may not provide the necessary repair or neuroprotection, which ultimately results in premature aging in HIV-1-infected individuals. With recent technical advances, various neuroimaging techniques are available for assessing the effects of HIV-1 on the living, aging brain.
Neuroimaging techniques are widely used in clinical and research settings to aid in the diagnosis and delineation of neurological disorders or to assess neurological sequelae. They can be divided broadly into four categories based on what they characterize, these include: brain structures, neurochemistry, neurophysiology, and brain activation networks. Several different techniques are available in each of these categories. Structural neuroimaging includes X-ray computed tomography (CT), structural magnetic resonance imaging (MRI), and diffusion MRI. Neurochemicals or neurometabolites can be measured by magnetic resonance spectroscopy (MRS) and with positron emission tomography (PET) using radiotracers. Techniques that allow for imaging of physiology, such as blood flow, can be assessed with perfusion (or arterial spin labeling [ASL]) MRI and glucose metabolism with fluorodeoxyglucose PET. Carbon 11-labeled Pittsburgh compound B was also used to assess fibrillary amyloid plaque binding in HIV patients, which might detect preclinical Alzheimer’s disease (Ances et al. 2012a). Lastly, brain activation networks are studied using functional MRI (fMRI) by employing blood oxygen level-dependent (BOLD) imaging and ASL. Each of these techniques has provided insights in the brain changes associated HIV-1 infection, including those that occur during aging.
Many factors contribute to brain changes in aging individuals and may further exacerbate changes in the HIV-1-infected brain, either by the disease or by some HAART regimens. Examples of these comorbid factors include hypertension, diabetes, stroke, depression, and particular host genetic factors. Many of these risk factors are further affected by the administration of HAART, which is known to have toxic side effects at the cellular level (e.g., mitochondrial toxicity), the vasculature (e.g., increasing lipids and atherosclerosis), and ultimately, on the nervous system. This review will highlight brain abnormalities in HIV patients in the setting of aging, focusing on recent neuroimaging studies that contributed to our understanding of the structural, physiological, functional, and chemical alterations in the HIV-infected brain.
Structural magnetic resonance imaging studies in HIV and aging
MRI can easily assess structural changes that occur during normal aging, which include shrinkage of almost all brain regions. For example, with advancing age, the hippocampi, entorhinal cortices, inferior temporal cortices, and prefrontal white matter (WM) show volume loss (Raz et al. 2005). Similarly, the gray matter (GM) shows age-dependent decreases in density (Sowell et al. 2004), while the ventricles increase in size with age (Sowell et al. 2003). Despite the widespread structural changes that occur during aging, many functions are still preserved, in part due to restructuring of the circuitry and reassigning or shared functions between regions (Tyler et al. 2010). Such reallocation of neural resources might lead to limited cognitive reserve capacity in the normal aging brain. However, in the aging HIV-infected brain, both structural and cognitive reserve may be even more limited.
Neuronal and myelin loss in the HIV-infected brain have been well documented on neuropathology (Bell 1998), which may be related to the cortical atrophy seen and measured on MRI (Archibald et al. 2004; Ghafouri et al. 2006). Morphometric analyses of MRI have shown volumetric loss in the caudate, amygdala and hippocampus (Archibald et al. 2004; Chang et al. 2011; Chiang et al. 2007; Harezlak et al. 2011; Lepore et al. 2008), and corpus callosum (Chiang et al. 2007; Dewey et al. 2010) in HIV-infected individuals.
Several studies evaluated gross structural brain volume or GM density changes in the aging, HIV-infected brain (Table 1). Two cross-sectional studies utilizing voxel-based morphometry (VBM), a technique that measures the brain volumes at each voxel independently, reported age-related GM changes that were distinct from HIV infection-related GM atrophy. Towgood et al. (2011) found that despite similarly normal cognitive performance between cognitively asymptomatic HIV subjects and controls, the younger HIV subjects had an area of smaller GM in the medial and superior frontal area and the older subjects had smaller GM in the superior frontal gyrus than their respective age-matched control group. They also found significant aging effects in the frontotemporal GM and WM, independent of the effect of HIV. Becker et al. (2011) reported similar age effects in the frontal and temporal regions, but more extensive atrophy in HIV subjects than SN controls in the frontal GM and inferior and posterior temporal, parietal, and cerebellar regions. Overall, they concluded that, in the post-HAART era, HIV infection is still linked to GM and WM volume loss, independent of age effects. A third cross-sectional study found that the estimated age at the time of HIV infection and the subjects’ age at the time of the scan independently and significantly correlated with smaller anterior cingulate volumes in HIV+ subjects but not in HIV+ subjects with an alcohol abuse or dependence history. Furthermore, a multiple regression revealed that age at HIV infection was a unique predictor, over age, of anterior cingulate volume (Pfefferbaum et al. 2012).
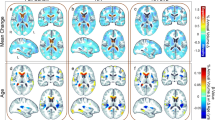
A longitudinal study performed during the pre-HAART era found greater loss of WM and caudate, with greater enlarged cerebrospinal fluid (CSF) space in HIV subjects than SN controls over a 2-year follow-up period, especially in HIV subjects who were medically symptomatic or had lower CD4 counts (Stout et al. 1998). A more recent study of HIV subjects maintained on more potent antiretroviral (ARV) regimens still found that, over a 24-month period, WM loss was greater in HIV subjects than SN controls (Cardenas et al. 2009). Recent morphometry studies, using an automated image segmentation technique, found that both HIV and aging independently contributed to volume reductions in many brain regions (Ances et al. 2012b; Chang et al. 2011). While atrophy in the amygdala was associated with aging, reductions in the corpus callosum were associated with HIV in a small study (Ances et al. 2012b). In addition, HIV subjects with apolipoprotein E (APOE)-ε4 genotype showed premature brain atrophy (in subjects <50 years), especially in the WM and bilateral putamen (Chang et al. 2011) (Fig. 1a).
In summary, HIV infection is associated with greater than age-related brain atrophy, beyond that found with normal aging in the frontal and temporal regions, as well as the basal ganglia (BG), parietal, and cerebellar regions. HIV-infected individuals with genetic risk factors, such as APOEe4, may have even greater risk for premature age-related brain atrophy.
Diffusion tensor imaging studies in HIV and aging
Diffusion tensor imaging (DTI) is another MR technique that measures the molecular motion of water molecules, which reflects changes in the microstructural environment of the brain. Since neuroinflammation is associated with increased brain water, DTI is sensitive to changes in WM and inflammatory changes associated with HIV infection (Chang et al. 2008a). Two common measures are fractional anisotropy (FA), which reflects the organization or integrity of WM fibers and the movement of water molecules along them, and mean diffusion (MD), which reflects the averaged diffusion from both diffusion along the axons (axial diffusion) and diffusion perpendicular to axonal fibers (radial diffusion). WM pallor suggestive of myelin loss is apparent soon after HIV infection (Gray et al. 1996). DTI quantitatively assesses WM integrity (Lim and Helpern 2002) and has detected WM abnormalities in HIV-infected subjects who had normal-appearing MRI images (Pomara et al. 2001). Multiple DTI studies have documented higher diffusion and lower FA in many brain regions in HIV subjects compared to controls; however, only three studies evaluated HIV subjects in relation to age.
In a 1-year follow-up study (Chang et al. 2008a), a group of neuroasymptomatic (NAS) HIV subjects had significantly higher MD in the frontal WM and lower FA in the parietal WM than SN controls at baseline. After 1 year, these HIV subjects showed increases in MD in the frontal and parietal WM, putamen, and genu; HIV subjects also showed greater increased genu diffusion than SN controls. These changes in MD in the genu and FA in the parietal and frontal WM and putamen correlated with changes in global cognitive deficit scores. Therefore, the greater than normal age-related inflammatory changes in the genu of these HIV patients might have contributed to the cognitive deficits. Furthermore, although the age-dependent changes in mean diffusivity and FA were not significantly different between HIV subjects and SN controls, the younger HIV subjects tended to show elevated diffusion and lower FA, suggesting greater neuroinflammation than the younger SN subjects (Chang et al. 2008a) (Fig. 1b).
Similarly, a cross-sectional DTI study also found only normal age-dependent changes in FA and MD in a group of NAS HIV subjects, but no HIV effect or HIV status-by-age interaction on their diffusion measures. The investigators attributed these normal DTI findings to their exceptional cohort of HIV subjects who had relatively high premorbid intelligence quotients, no comorbid disorders or substance abuse, and well-suppressed viral load (Towgood et al. 2011). Another recent study that evaluated various clinical variables on diffusion measures in 85 HIV subjects found that, in addition to the age-dependent decreases in FA in most WM regions, especially the frontal regions, those with a common coinfection, hepatitis C, also had lower frontal WM FA and higher diffusion primarily in the parietal and occipital WM regions (Gongvatana et al. 2011). The authors suggested that since different comorbid conditions may contribute differentially to brain changes, future DTI studies of HIV patients should evaluate the entire brain.
Proton magnetic resonance spectroscopy studies in HIV and aging
HIV infection causes glial activation and neuronal injury (Everall et al. 1993; Navia et al. 1986). Proton MRS is a sensitive method for detecting brain metabolites that reflect glial and neuronal changes. For example, myoinositol (MI) and choline compounds (CHO) are elevated in brain disorders with chronic inflammation and glial activation, while the neuronal marker N-acetylaspartate (NAA) is decreased in later or more severe stages of HIV dementia (Chang et al. 2012). With normal aging, the glial marker MI and, to a lesser extent, total creatine (tCr) and CHO increase linearly with age (Chang et al. 1996). MRS has also proven useful in identifying subtle changes associated with cognitive decline; for example, the MI/tCr ratio has been used to discriminate healthy subjects from those with mild cognitive impairment (Catani et al. 2001). Similarly, in ARV-naïve HIV subjects, elevated levels of MI in the frontal WM was associated with poorer performance on tasks that require executive function (Stroop interference task), as well as lower CD4 count and higher viral load, while elevated CHO in the same brain region correlated with slower performance on a working memory task (Chang et al. 2002). Numerous MRS studies have been applied to evaluate HIV patients with and without HAND; some studies also demonstrated that MRS may be a useful biomarker to monitor the effects of HAART (Chang et al. 2012).
Among the many MRS studies, only four reports specifically evaluated the effects of HIV in relation to age (Table 2). In a group of relatively young (mean age, 36 years) ARV-naïve HIV subjects, accelerated age-associated metabolite changes were observed. Only HIV subjects showed age-related decline in NAA (−3.7 %/decade) and tCr (−4 %/decade) in the BG, suggesting neurodegeneration in this brain region. The HIV subjects also showed greater than age-related increases in MI (+12 % instead of 3 %/decade) and CHO (+10 % instead of 2 %/decade), suggesting greater neuroinflammation in the frontal WM (Ernst and Chang 2004). A multicenter MRS consortium also found evidence of premature age-associated brain metabolite abnormalities in primarily HAART-treated HIV-infected individuals. Those with AIDS dementia complex (ADC) or Memorial Sloan–Kettering (MSK) stage 1 (equivalent to the MND) and MSK stages 2 and 3 (equivalent to HAD) had the highest level of the MI/tCr and CHO/tCr in the WM and BG and higher but parallel elevations of Cho/tCr and MI/tCr in their BG across the age groups compared to the NAS subjects. The BG of the NAS group, even the younger HIV subjects (<40 years of age), showed higher MI/tCr and CHO/tCr than the younger SN subjects, at levels similar to the older SN subjects. In contrast, the NA/tCr levels were lower in the ADC (HAND) group, even in the younger subjects, and decreased further with age in both the frontal WM and the parietal cortex, but the NAS subjects showed only mild decreases of NA/tCr in the WM which did not decline with age. Therefore, the higher CHO/tCr and MI/tCr in the BG and WM of the NAS (no HAND or ANI) and ADC (HAND) subjects and the lower NA/tCr in the WM and parietal cortex in the ADC (HAND) subjects, which were already present in the younger HIV subjects, indicate a premature aging process (Chang et al. 2004a).
A more recent multicenter study of slightly older (median ages, 45–49 years) HAART-treated HIV-infected individuals continued to show elevated MI/tCr and CHO/tCr throughout the brain, with decreased NAA/tCr in the frontal WM only in ADC (MSK stage >0.5 or HAND) subjects and decreased glutamate+ glutamine (Glx)/tCr in NAS (MSK stage 0, no HAND diagnosis or ANI) subjects in the frontal WM (Harezlak et al. 2011). This study also found that, while the glial metabolite ratio MI/tCr increased with age in NAS subjects and those with ADC 0.5 (equivalent to MND), MI/tCr decreased with age in subjects with ADC (HAND). These finding may reflect the age-related increase in microglia cells in the subjects with NAS or ADC 0.5 but a premature age-related microglial senescence process in those with ADC (HAND).
Another study that specifically evaluated brain glutamate (GLU) using a special MRS technique (TE-averaged PRESS) found that HIV subjects with HAND had lower parietal GLU levels, but those without HAND had higher BG GLU. Additionally, although HIV subjects showed similar rates of age-related decline in brain GLU levels, their GLU levels were already lower in the younger subjects, equivalent to those of SN subjects who were approximately 10 years older, again suggesting premature aging (Ernst et al. 2010) (Fig. 1c).
MRS has proven to be a valuable technique for studying age-related brain changes associated with HAND and can measure region-specific changes in brain metabolites that reflect glial activation and neuronal injury or loss. The few studies that evaluated age-related changes suggest that ARV-naïve HIV subjects show accelerated age-dependent changes in glial activation or neuronal injury; however, HAART-treated HIV subjects show only a premature aging process, especially those with HAND. All of these studies were done cross-sectionally, which may suffer biases from intersubject variability; longitudinal studies are needed to validate these cross-sectional findings.
Functional neuroimaging studies in HIV and aging
Although HIV does not infect neurons directly, it is believed that viral proteins released by infected macrophages and microglia leads to neuronal apoptosis and ongoing aberrant neuroinflammation, which contribute to the development of HAND (Kaul et al. 2005). The BOLD contrast on fMRI is an indirect measure of neuronal function (Arthurs and Boniface 2002) and detects brain activity while subjects are resting or while they are engaged in cognitive or motor tasks that do not involve head motion. Several fMRI studies evaluated brain function in HIV-infected individuals. The major findings included decreased activation in the normal attention network (Chang et al. 2004b), but greater activation in adjacent or contralateral brain regions, suggesting greater usage of the reserve brain networks (Chang et al. 2001, 2004b). The need to use the reserve brain network, with greater activation, in order to maintain normal cognitive performance was also demonstrated in NAS HIV patients (Ernst et al. 2002). However, the reserve network has a limited capacity, especially in HIV subjects, which may not provide sufficient reserve for the more difficult tasks or during competing brain activities (e.g., simultaneous visual and auditory functions) that would interfere with the attention network(Tomasi et al. 2006). Furthermore, neuroinflammation, as demonstrated by the elevated glial metabolites (MI, CHO, and tCr) in HIV-infected individuals, may lead to greater BOLD signals, suggesting a greater requirement for usage of the brain reserve (Ernst et al. 2003). Since both HIV and aging may lead to greater neuroinflammation, additive effects on brain activation and the usage of the reserve network would be expected. Lastly, since some ARVs, such as the nucleoside reverse transcriptase inhibitors (NRTIs), might be neurotoxic (Dagan et al. 2002), long-term treatment with HAART in aging HIV patients might also lead to further decreases in the brain network capacity. A study that evaluated the effects of ARVs on brain function found that HIV subjects who were taking ARVs with NRTIs required greater brain activation during a set of parametric attention tasks than those without ARVs (Chang et al. 2008b).
To date, only two fMRI studies evaluated brain activation or perfusion in relation to age. A longitudinal fMRI study of cognitively unimpaired HIV and SN control subjects showed greater BOLD activation at baseline in the occipital, cerebellar, and right prefrontal regions of HIV subjects during performance of the most difficult level of a visual attention task. After 1 year, although there were no changes in task performance for either group, HIV subjects had significantly increased BOLD activation, primarily in the prefrontal and parietal regions, for all three levels of the task. Conversely, SN participants showed only decreased BOLD signals, consistent with practice effects (Ernst et al. 2009) (see Fig. 1). These finding are consistent with a premature aging model, since older healthy individuals showed greater activation on a visual attention task than younger individuals (Madden et al. 2007). Further evidence of premature aging in HIV-infected individuals is shown in a study that utilized ASL, which evaluated resting cerebral blood flow (CBF) as well as CBF changes in response to visual stimulation. Similar to the other MR studies in HAART-treated HIV patients, HIV+ subjects had lower CBF across the age span, equivalent to that in SN subjects who were 15 years older, and also lower brain activation during visual stimulation across the age span, equivalent to that of SN subjects who were 21 years older (Ances et al. 2010) (Fig. 1d).
These fMRI studies suggest that HIV-associated brain injury leads to a less efficient network, with lower resting CBF like older individuals that require greater usage of neural resources to maintain cognition, and the aging HIV infected brain will have an even lower cognitive reserve that may not provide sufficient capacity to maintain cognition. Hence, the prematurely aged brains of HIV patients will likely suffer the development of HAND as they age. Again, longitudinal studies are needed to validate these hypotheses.
Common comorbidities with aging in HIV
Many comorbidities are common in the aging population, which may further affect the aging HIV-infected brain. Aging is associated with increased incidence of cardiovascular diseases, including hypertension and stroke (Gorelick et al. 2011), as well as diabetes (McBean et al. 2004) and depression (Vink et al. 2009). In addition, particular genotypes (e.g., APOEe4 allele) may further prevent the normal repair processes needed for the aging brain and HIV-associated neurodegeneration (Chang et al. 2011). Furthermore, substance abuse, which is prevalent among HIV-infected individuals, may lead to additive effects on brain injury (Chang et al. 2005b, 2006), especially in the aging brains of HIV patients. Other coinfections, such as hepatitis C, can also lead to brain inflammation and injury, which might further impact the aging process. Few studies have evaluated how these comorbid conditions affect the HIV-infected brain, especially in the setting of the aging brain, and even fewer have applied neuroimaging to evaluate comorbid conditions in the HIV aging brain. We will briefly review a few neuroimaging studies of these potential contributing factors to the aging process in HIV-infected individuals.
Diabetic complications in the aging HAART-treated HIV-infected brain
Multiple studies have shown that HAART significantly increases the prevalence of diabetes mellitus in HIV-infected individuals, with 13–14 % having insulin resistance (Brown et al. 2005; Palacios et al. 2006). In particular, protease inhibitors are associated with increased incidence of insulin resistance and new-onset diabetes (Carr et al. 1999; Dever et al. 2000; Palacios et al. 2006; Walli et al. 1998). Neuroimaging studies have shown significant brain pathologies in diabetic patients. Specifically, MRI showed atrophy throughout the brains of diabetic patients, with smaller cortical and subcortical volumes (Tiehuis et al. 2008), as well as smaller hippocampi and amygdalae (den Heijer et al. 2003). Furthermore, patients with type 2 diabetes have increased WM hyperintensities associated with neuroinflammation and more lacunar infarcts from the small vessel disease (Tiehuis et al. 2008). In diabetic patients, increased WM lesions are evident even with CT (Gorelick et al. 2011). In the aging HIV-infected brain, the neuropathology associated with diabetes would likely exacerbate those already found in HIV and aging, leading to additional brain atrophy, WM inflammation, and increased likelihood for cerebral infarcts.
Increased prevalence of strokes in HIV-infected individuals
Approximately one third to half of older people have had infarcts; many of whom do not show cognitive deficits (Schneider et al. 2009), probably due to their cognitive reserve. HIV infection is also associated with an increased risk of stroke (Cole et al. 2004; Qureshi et al. 1997), up by 60 % between 1997 and 2006 in stroke patients with the coexisting diagnosis of HIV (Ovbiagele and Nath 2011). Treatment with protease inhibitors may further increase the incidence of vascular events. Therefore, strokes may occur in younger HIV-infected individuals, as shown in a recent study that the majority of HIV-infected stroke patients were under 46 years of age (Tipping et al. 2007). These younger patients did not have the typical risk factors for strokes, but 20 % had HIV vasculopathy. Therefore, co-occurrence of HIV and strokes will likely decrease the cognitive reserve and lead to greater prevalence of HAND.
Substance abuse and HIV on the aging brain
Substance abuse is another common comorbid condition among HIV-infected individuals, and little or no data exist regarding how drug use might further impact the aging brain. Stimulants, such as methamphetamine, may lead to more severe microglia activation and more pronounced loss of synaptophysin in HIV-associated encephalitis (Langford et al. 2003). Methamphetamine abuse also may lead to greater than age-related cortical volume reductions (Nakama et al. 2011) but larger subcortical volumes (Chang et al. 2005a; Jernigan et al. 2005). Furthermore, additive or interactive effects between HIV and methamphetamine usage were observed on brain morphometry (Jernigan et al. 2005), alterations in WM integrity (Thames et al. 2011), additive effects on neuronal and glial metabolites (Chang et al. 2005b; Taylor et al. 2004), as well as possible additive effect on lower resting CBF and greater CBF changes in response to brain activation (Ances et al. 2011). Similarly, HIV+ cocaine users also had greater reductions in dopamine transporter density and D2 receptors than HIV subjects without drug use (Chang et al. 2008a). Since dopamine receptors decline with age, cocaine abuse in HIV subjects could exacerbate their age-associated dopaminergic receptor loss (Chang et al. 2008a). Furthermore, a growing number of HIV patients are identified to have alcohol use problems (Bonacini 2011), which is well known to contribute to the aging process in the brain (Pfefferbaum et al. 2012). Alcohol abuse appears to contribute to additional brain changes in HIV patients who had greater brain atrophy with larger ventricular volumes (Pfefferbaum et al. 2006), decreased neuronal marker NAA (Pfefferbaum et al. 2005), and greater diffusion abnormalities with lower FA and higher MD (Pfefferbaum et al. 2007). Lastly, despite the high prevalence of marijuana use by the HIV-infected population, for both therapeutic and recreational purposes (Prentiss et al. 2004), virtually no data is available regarding how marijuana might influence brain aging in these individuals. Only one paper evaluated the independent and combined effects of HIV and marijuana use on brain metabolites (Chang et al. 2006). While few studies have examined the combined or interactive effects of HIV and substance abuse on the aging brain, the limited data suggest that comorbid substance abuse may have negative impact in the aging brains of HIV patients.
Summary and conclusion
A variety of neuroimaging techniques have been applied to evaluate possible additive or interactive effects of age and HIV infection on the brain. The neuroimaging studies reviewed evaluated anatomical (MRI) or microstructural changes (DTI), neurochemical alterations (MRS), and brain activation (fMRI) in HIV-infected individuals. Because these techniques evaluated different pathophysiological aspects of HIV-associated brain injury and had differential sensitivities for detecting brain changes, we found differences in the rate of brain degeneration. For example, while the fMRI studies found accelerated aging, the structural MRI studies found premature aging. Similarly, brain activation measured by fMRI (unpublished data) and cognitive performance may be different across subjects with or without HAND despite similar volume loss detected by structural MRI (Chang et al. 2011). Most importantly, the majority of these studies were performed cross-sectionally in a small number of subjects, which may be affected by intersubject variability or biases in subject selections. Therefore, cross-sectional studies are limited in differentiating between premature aging and accelerated aging.
Earlier MRI and MRS studies from the pre-HAART era or in ARV-naive subjects suggest an accelerated aging process, with greater than normal age-dependent brain atrophy and brain metabolite abnormalities. However, more recent studies of HIV subjects maintained on HAART demonstrate premature brain atrophy, with earlier brain changes that may then parallel the aging process. With chronic long-term HIV infection, those with and without HAND may show similar degrees of brain atrophy; however, those with APOE-ε4 allele(s) may have the greatest brain atrophy and early onset brain atrophy. Although HIV subjects with minimal cognitive deficits may show higher than normal levels of MI across the age span, MI appears to decline with age in those with HAND, suggesting a decline in glial function.
DTI studies yielded conflicting findings on the relationship between HIV and age in NAS individuals. One study found similar age-related changes in both SN and HIV subjects (Towgood et al. 2011), while another study found greater than normal age-related changes in diffusion after 1-year, especially in the genu (Chang et al. 2008a). This discrepancy may result from differences in the image-processing techniques, brain regions evaluated, and the research designs (e.g., cross-sectional vs. longitudinal).
fMRI studies found evidence of premature or accelerated aging processes in the brains of HIV subjects. Lesser CBF changes and BOLD activation were found across the age span of HIV subjects during a simple visual stimulation task (Ances et al. 2010). However, increased activation, rather than the normal decreased activation (practice effects), were seen during attention-requiring tasks after 1 year in cognitively normal HIV subjects (Ernst et al. 2009). These studies demonstrate that BOLD fMRI, especially with tasks that require attention (or cognitive “stress”), may be the most sensitive technique for assessing the decline of neural efficiency and the aging effects of the HIV-infected brain.
Lastly, many age-related illnesses such as diabetes, stroke, and depression may further exacerbate the combined or interactive effects of HIV and aging on the brain, leading to premature or accelerated age-related brain changes. However, only a few small neuroimaging studies have evaluated these comorbid issues in HIV patients but did not assess these effects on the aging process. Other concurrent issues, such as the potential direct or indirect neurotoxic effects of particular ARV medications (e.g., NRTIs or protease inhibitors) and the greater prevalence of substance abuse (Ostrow 1994) and nicotine smoking among HIV subjects (Reynolds 2009), could also impact the HIV-infected aging brain. Future studies evaluating the independent and combined effects of these comorbid issues in HIV patients are needed.
Since the different neuroimaging techniques assess different pathologic or physiologic changes in the brain, using a multimodal approach in longitudinal follow-up studies would lead to a better understanding of the relationships between structural, chemical, and functional changes in the aging brains of HIV patients.
References
Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ (2010) HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis 201:336–340
Ances BM, Vaida F, Cherner M, Yeh MJ, Liang CL, Gardner C, Grant I, Ellis RJ, Buxton RB (2011) HIV and chronic methamphetamine dependence affect cerebral blood flow. J Neuroimmune Pharmacol 6:409–419
Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, Aldea P, Fagan AM, Holtzman DM, Morris JC, Clifford DB (2012a) 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol 69:72–77
Ances BM, Ortega M, Vaida F, Heaps J, Paul R (2012b) Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 59:469–477
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799
Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL (2004) Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol 61:369–376
Arthurs OJ, Boniface S (2002) How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 25:27–31
Becker JT, Maruca V, Kingsley LA, Sanders JM, Alger JR, Barker PB, Goodkin K, Martin E, Miller EN, Ragin A, Sacktor N, Selnes O (2011) Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology 54:113–121
Bell J (1998) The neuropathology of adult HIV infection. Rev Neurol 154:816–829
Bonacini M (2011) Alcohol use among patients with HIV infection. Ann Hepatol 10:502–507
Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G (2009) Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol 4:163–174
Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS (2005) Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 165:1179–1184
Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, Neuhaus J, Grant RM, Chao LL, Truran D, Weiner MW (2009) Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol 15:324–333
Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA (1999) Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 353:2093–2099
Catani M, Cherubini A, Howard R, Tarducci R, Pelliccioli GP, Piccirilli M, Gobbi G, Senin U, Mecocci P (2001) (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport 12:2315–2317
Chang L, Ernst T, Poland RE, Jenden DJ (1996) In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci 58:2049–2056
Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T (2001) Neural correlates of attention and working memory deficits in HIV patients. Neurology 57:1001–1007
Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E (2002) Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage 17:1638–1648
Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, Kolson D, Schifitto G, Jarvik JG, Miller EN, Lenkinski R, Gonzalez G, Navia BA (2004a) A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage 23:1336–1347
Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T (2004b) Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 56:259–272
Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T (2005a) Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry 57:967–974
Chang L, Ernst T, Speck O, Grob CS (2005b) Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry 162:361–369
Chang L, Cloak C, Yakupov R, Ernst T (2006) Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol 1:65–76
Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T (2008a) Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol 3:265–274
Chang L, Yakupov R, Nakama H, Stokes B, Ernst T (2008b) Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol 3:95–104
Chang L, Andres M, Sadino J, Jiang CS, Nakama H, Miller E, Ernst T (2011) Impact of apolipoprotein E epsilon4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage 58:1017–1027
Chang L, Feger U, Ernst T (2012) Bioimaging. In: Gendelman H, Grant I, Everall IP, Fox HS, Gelbard HA, Lipton SA, Swindells S (eds) The neurology of AIDS. Oxford University Press, New York, pp 763–797
Chiang MC, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM (2007) 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage 34:44–60
Cole JW, Pinto AN, Hebel JR, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Price TR, Sloan MA, Stern BJ, Wityk RJ, Wozniak MA, Kittner SJ (2004) Acquired immunodeficiency syndrome and the risk of stroke. Stroke 35:51–56
Dagan T, Sable C, Bray J, Gerschenson M (2002) Mitochondrial dysfunction and antiretroviral nucleoside analog toxicities: what is the evidence? Mitochondrion 1:397–412
Deeks SG (2011) HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 62:141–155
den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM (2003) Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 46:1604–1610
Dever LL, Oruwari PA, Figueroa WE, O'Donovan CA, Eng RH (2000) Hyperglycemia associated with protease inhibitors in an urban HIV-infected minority patient population. Ann Pharmacother 34:580–584
Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezlak J, Sem E, Anyanwu JC, Guttmann CR, Navia B, Cohen R, Tate DF (2010) Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage 51:1334–1344
Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, Nayfield SG, Plaeger SF, Schmader KE, Ashworth JR, Campanelli C, Clayton CP, Rada B, Woolard NF, High KP (2008) Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 47:542–553
Ernst T, Chang L (2004) Effect of aging on brain metabolism in antiretroviral-naive HIV patients. AIDS 18(Suppl 1):S61–S67
Ernst T, Chang L, Jovicich J, Ames N, Arnold S (2002) Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59:1343–1349
Ernst T, Chang L, Arnold S (2003) Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage 19:1686–1693
Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L (2009) Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol 65:316–325
Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L (2010) Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging 32:1045–1053
Everall I, Luther P, Lantos P (1993) A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol 52:561–566
Ghafouri M, Amini S, Khalili K, Sawaya BE (2006) HIV-1 associated dementia: symptoms and causes. Retrovirology 3:28
Gongvatana A, Cohen RA, Correia S, Devlin KN, Miles J, Kang H, Ombao H, Navia B, Laidlaw DH, Tashima KT (2011) Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J Neurovirol 17:477–486
Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:2672–2713
Gray F, Scaravilli F, Everall I, Chretien F, An S, Boche D, Adle-Biassette H, Wingertsmann L, Durigon M, Hurtrel B, Chiodi F, Bell J, Lantos P (1996) Neuropathology of early HIV-1 infection. Brain Pathol 6:1–15
Haase AT (1986) Pathogenesis of lentivirus infections. Nature 322:130–136
Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B (2011) Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 25:625–633
Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I (2005) Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry 162:1461–1472
Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA (2005) HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 12(Suppl 1):878–892
Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E (2003) Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr 34:467–474
Lepore N, Brun C, Chou YY, Chiang MC, Dutton RA, Hayashi KM, Luders E, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM (2008) Generalized tensor-based morphometry of HIV/AIDS using multivariate statistics on deformation tensors. IEEE Trans Med Imaging 27:129–141
Lim KO, Helpern JA (2002) Neuropsychiatric applications of DTI—a review. NMR Biomed 15:587–593
Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA (2007) Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging 28:459–476
McBean AM, Li S, Gilbertson DT, Collins AJ (2004) Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: Whites, Blacks, Hispanics, and Asians. Diabetes Care 27:2317–2324
Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T (2011) Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction 106:1474–1483
Navia BA, Cho ES, Petito CK, Price RW (1986) The AIDS dementia complex: II. Neuropathology. Ann Neurol 19:525–535
Ostrow DG (1994) Substance abuse and HIV infection. Psychiatr Clin North Am 17:69–89
Ovbiagele B, Nath A (2011) Increasing incidence of ischemic stroke in patients with HIV infection. Neurology 76:444–450
Palacios R, Merchante N, Macias J, Gonzalez M, Castillo J, Ruiz J, Marquez M, Gomez-Mateos J, Pineda JA, Santos J (2006) Incidence of and risk factors for insulin resistance in treatment-naive HIV-infected patients 48 weeks after starting highly active antiretroviral therapy. Antivir Ther 11:529–535
Pfefferbaum A, Adalsteinsson E, Sullivan EV (2005) Cortical NAA deficits in HIV infection without dementia: influence of alcoholism comorbidity. Neuropsychopharmacology 30:1392–1399
Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV (2006) Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage 33:239–251
Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV (2007) Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain 130:48–64
Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Sullivan EV (2012) Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biol Psychiatry. doi:10.1016/j.biopsych.2012.02.018
Pomara N, Crandall D, Choi S, Johnson G, Lim K (2001) White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res 106:15–24
Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM (2004) Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. J Acquir Immune Defic Syndr 35:38–45
Qureshi AI, Janssen RS, Karon JM, Weissman JP, Akbar MS, Safdar K, Frankel MR (1997) Human immunodeficiency virus infection and stroke in young patients. Arch Neurol 54:1150–1153
Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005) Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15:1676–1689
Reynolds NR (2009) Cigarette smoking and HIV: more evidence for action. AIDS Educ Prev 21:106–121
Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 18:691–701
Schuitemaker A, van der Doef TF, Boellaard R, van der Flier WM, Yaqub M, Windhorst AD, Barkhof F, Jonker C, Kloet RW, Lammertsma AA, Scheltens P, van Berckel BN (2012) Microglial activation in healthy aging. Neurobiol Aging 33:1067–1072
Smith GH (2005) Opening statement of Senator Gordon H. Smith. Aging hearing: HIV over fifty, exploring the new threat. Senate Committee on Aging, Washington, DC, U.S. Government Printing Office
Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003) Mapping cortical change across the human life span. Nat Neurosci 6:309–315
Sowell ER, Thompson PM, Toga AW (2004) Mapping changes in the human cortex throughout the span of life. Neuroscientist 10:372–392
Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I (1998) Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol 55:161–168
Streit WJ, Xue QS (2010) The brain's aging immune system. Aging Dis 1:254–261
Taylor MJ, Letendre SL, Schweinsburg BC, Alhassoon OM, Brown GG, Gongvatana A, Grant I (2004) Hepatitis C virus infection is associated with reduced white matter N-acetylaspartate in abstinent methamphetamine users. J Int Neuropsychol Soc 10:110–113
Thames AD, Foley J, Panos SE, Singer EJ, Patel SM, El-Saden S, Hinkin CH (2011) Past stimulant abuse is associated with reduced basal ganglia and hippocampal integrity in older HIV+ adults: a diffusion tensor imaging study. J AIDS Clinic Res 2:129. doi:10.4172/2155-6113.1000129
Tiehuis AM, van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman AP, Kappelle LJ, Mali WP (2008) Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke 39:1600–1603
Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A (2007) Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry 78:1320–1324
Tomasi D, Ernst T, Caparelli EC, Chang L (2006) Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp 27:694–705
Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Kumar A, Soni S, Sibtain NA, Reed L, Bradbeer C, Barker GJ, Kopelman MD (2011) Mapping the brain in younger and older asymptomatic HIV-1 men: frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex 48:230–241
Tyler LK, Wright P, Randall B, Marslen-Wilson WD, Stamatakis EA (2010) Reorganization of syntactic processing following left-hemisphere brain damage: does right-hemisphere activity preserve function? Brain 133:3396–3408
Vink D, Aartsen MJ, Comijs HC, Heymans MW, Penninx BW, Stek ML, Deeg DJ, Beekman AT (2009) Onset of anxiety and depression in the aging population: comparison of risk factors in a 9-year prospective study. Am J Geriatr Psychiatry 17:642–652
Walli R, Goebel FD, Demant T (1998) Impaired glucose tolerance and protease inhibitors. Ann Intern Med 129:837–838
Woods SP, Moore DJ, Weber E, Grant I (2009) Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 19:152–168
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holt, J.L., Kraft-Terry, S.D. & Chang, L. Neuroimaging studies of the aging HIV-1-infected brain. J. Neurovirol. 18, 291–302 (2012). https://doi.org/10.1007/s13365-012-0114-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-012-0114-1