Abstract
Chronic infection with HIV is associated with neuroinflammation. Prior diffusion tensor imaging (DTI) studies demonstrated increased mean diffusion (MD) and decreased fractional anisotropy (FA) in the white matter (WM) and subcortical brain regions of HIV patients. The current study aims to detect whether there are greater than age-related brain changes in HIV patients after a 1-year follow-up period using DTI. Thirty-nine antiretroviral-stable HIV subjects and 32 HIV-seronegative (SN) controls were evaluated, with neuropsychological tests and DTI, at baseline and after 1 year. MD and FA in the genu and splenium of the corpus callosum and in six other subcortical and white matter regions were evaluated bilaterally. Compared to SN controls, HIV subjects had significantly higher MD in the frontal WM (p = 0.0104) and lower FA in the parietal WM (p = 0.006). After 1 year, HIV subjects showed increase in MD in frontal and parietal WM, putamen, and genu; HIV subjects also showed greater increased genu diffusion than SN controls (p = 0.005). Changes in global cognitive deficit score correlated with changes in MD in the genu and FA in the parietal and frontal WM and putamen (multiple regression, p = 0.0008). Lastly, normal age-dependent changes in frontal WM diffusion and FA in genu and putamen were not observed in HIV subjects. Since increased MD may reflect increased neuroinflammation, our findings suggest greater than normal age-related inflammatory changes in the genu of these HIV patients, which may contribute to the cognitive deficits. Measurements of MD in the genu may be useful for monitoring disease progression in HIV brain infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Human immunodeficiency virus (HIV) infection of the brain is often associated with significant inflammation. This inflammation may be mediated by HIV viral proteins (e.g., tat, gp120, gp41) that upregulate macrophage secretion of proinflammatory cytokines (e.g., tumor necrosis factor, interleukin-1-beta, interlekin-6; Connolly et al. 2005) and chemokines (e.g., macrophage chemotactic protein or MCP-1, RANTES, MIP-1alpha, and MIP-1beta), which in turn would activate microglia and increase astroglial-derived chemokines in the brain (El-Hage et al. 2006; Hauser et al. 2007). These proteins also could lead to elevation of inducible nitric oxide synthetase (Adamson et al. 1996) and other proteins (e.g., matrix metalloproteinases; Conant et al. 2004) that might increase blood–brain barrier permeability and would lead to further ingress of HIV-infected monocytes into the brain and a feed-forward loop of the inflammatory cascade (Banks et al. 2006). In addition to the direct neurotoxic effect of the HIV viral proteins (Nath 2002), the myriad of inflammatory proteins may lead to oxidative stress and ultimately neuronal injury or death, although some of these proteins are activated as a protective response (Steiner et al. 2006).
Several magnetic resonance (MR) techniques have been applied to evaluate brain changes in patients with HIV. MR spectroscopy (MRS) demonstrated elevated myoinositol (Laubenberger et al. 1996), a glial marker that normalizes after effective antiretroviral treatment in HIV patients (Chang et al. 1999b). Therefore, MRS may be able to assess neuroinflammation. MR diffusion tensor imaging (DTI), a technique that evaluates the molecular motion of water molecules, also can detect cerebral white matter and subcortical inflammatory changes associated with HIV-1 infection. Previous cross-sectional studies found increased mean diffusivity (MD) and decreased fractional anisotropy (FA) in multiple brain regions of HIV subjects compared to control subjects (Cloak et al. 2004; Filippi et al. 2001; Pfefferbaum et al. 2007; Pomara et al. 2001; Ragin et al. 2006; Thurnher et al. 2005; Wu et al. 2006), most consistently in the genu of the corpus callosum or frontal white matter. In the current study, DTI changes in the brain were evaluated at baseline and at 1-year follow-up in a group of antiretroviral-stable HIV subjects and in HIV-seronegative controls. We hypothesized that due to the neuroinflammation associated with ongoing HIV infection, HIV subjects would show greater temporal changes on DTI than seronegative controls, specifically greater increases in MD and decreases in FA in the frontal white matter and genu over 1 year.
Methods
Research participants
Participants included 39 HIV-1 positive subjects (HIV+, aged 47.4 ± 1.4, 28–67 years), and 32 HIV-seronegative (SN) controls with similar ranges of age, education and racial distribution (SN, aged 46.7 ± 2.4, 21–71 years). Prior to enrollment in the study, each subject signed a written consent form approved by our institution and was screened and evaluated to ensure the fulfillment of study criteria. Each participant was evaluated clinically and with neuropsychological tests and DTI at baseline and at 1 year follow-up. SN controls were included if they were: (1) age >18 years; (2) seronegative for HIV. HIV subjects were included if they fulfilled the following criteria: (1) age >18 years; (2) HIV positive and either medication naïve or stable on antiretrovirals for 6 months; (3) Memorial Sloan Kettering HIV dementia stage <2 and able to provide informed consent; (4) nadir CD4 < 500/mm3. Exclusion criteria for both subject groups were: (1) chronic medical or neuropsychiatric illnesses that might confound the study; (2) abnormal laboratory studies or electrocardiogram that might confound data; (3) head trauma with loss of consciousness >30 min; (4) history of drug dependence in the past; (5) positive urine toxicology screen for cocaine, methamphetamine, opiates, and benzodiazepines (unless explainable by a known cross-reaction with prescription medication, e.g., efavirenz); or (6) contraindications for MR studies.
Cognitive and mood assessments
The cognitive status of each subject was evaluated by the mini-mental status examination (MMSE; Folstein et al. 1975), the HIV-dementia scale (Power and Johnson 1995), and a well-validated battery of neuropsychological tests known to be sensitive for detecting cognitive deficits in HIV patients and had been applied previously (Chang et al. 2004). Premorbid verbal intelligence was estimated from the National Adult Reading Test-Revised or NART (Blair and Spreen 1989). A global deficit score (GDS) was calculated from the neuropsychological tests and adapted from the established clinical domains and an algorithm reported previously (Woods et al. 2004). Seven cognitive domains were included in the GDS: (1) Speed of Information Processing: Trail Making Part A, Symbol Digit Test, Stroop color and word naming; (2) Executive Functions: Controlled Oral Word Association Test, Ruff Figural Fluency Test, Animal Naming Test, Stroop Color Word Interference Test, Trail Making Part B; (3) Attention/Working Memory: Paced Auditory Serial Addition Test trial 1; WAIS-III Digit Span (backward), WAIS-III Letter-number Sequencing, Arithmetic, CalCAP sequential reaction time (1-back, true positives only, and 2-back, true positives only); (4) Learning: WAIS-III Digit Span (forward), Rey Auditory Verbal Learning Test (AVLT) trials 1 and 5; (5) Memory: Rey Osterrieth Complex Figure test (immediate and delayed), AVLT trial 7; (6) Motor: Grooved Pegboard (dominant and non-dominant hands), Timed Gait; and (7) Reaction Time: CalCAP (simple reaction time, sequential reaction time 1 and 2). Age and education-stratified normative data for computing GDS scores were available from >200 HIV-negative individuals who have been tested in our laboratory, using standardized and scripted instructions for the data collection, same as those used in previous studies (Paul et al. 2007). The normative sample also had similar age, education, and racial distribution as our current subjects in this study (age 41.6 ± 16.3 years, age range 19–92 years, education 15.3 ± 2.4 years, range 9–20 years, 73% whites, 10% Asians, 5% Hispanics, 4% blacks, 1% American Indians, 2% Pacific Islanders, and 8% mixed race). Although subjects with major depression, a potential confound, were excluded from this study, we assessed possible depressive symptoms with the Center for Epidemiologic Studies Depression scale (CES-D; Radloff 1977).
MR imaging protocol
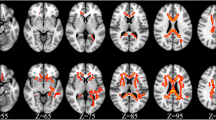
MR scans were performed on a 3 Tesla Siemens Trio scanner. In addition to T1- and T2-weighted structural sequences, a spin-echo DTI series (EPI, TR/TE = 3700/88 ms, b = [0,1000] s/mm2, 12 directions) was acquired. Mean diffusivity and fractional anisotropy (FA) maps were calculated, and regions of interest (ROIs) were manually drawn in a standardized fashion to assess MD and FA using DTI-Studio version 2.03 (Jiang et al. 2006; Fig. 1). In addition to the genu and splenium of the corpus callosum, the following regions were evaluated in both right and left hemispheres: frontal and parietal white matter, thalamus, caudate, putamen, and globus pallidus. The ROIs were placed by two trained investigators (VW, DR) after all the DTI scans from all subjects at both baseline and 1 year were completed. Intraclass correlation coefficients were used to assess intra-rater correlations (FA: r values, 0.56–0.94; MD, 0.5–0.99; using repeat drawings by each rater in ten subjects regardless of subject status or time of scan status, baseline versus 1 year) and inter-rater correlations (FA, 0.4–0.88; MD, 0.65–0.93; using ROI measurements by both raters from scans obtained in 15 subjects, regardless of subject status or scan time status).
Statistical analysis
Statistical analyses were performed in StatView and SAS (SAS Institute, Cary, NC, USA). Repeated-measures analysis of variance (ANOVA) was used to assess group differences between baseline and 1-year follow-up clinical variables and DTI measurements, using HIV status as a between-subject and time of study as a within-subject variable. Post hoc analyses using unpaired or paired t tests were performed to further evaluate group differences in clinical variables (Table 1) and the DTI data (Table 2). For these categorical analyses, the Simes procedure was used to determine which p values remained significant after correcting for multiple comparisons (Simes 1986). Simple or multivariate linear regression analyses were used to explore the relationships among changes in DTI and clinical measures and age, using variables that showed a significant main effect (group or time) or interactions on the repeated measures ANOVA. Exploratory quadratic regression analyses were also performed for the age-dependencies on the DTI measures.
Results
Subject characteristics
HIV+ subjects had similar age and education as SN subjects, but their estimated verbal intelligence quotient was lower (Table 1). The two subject groups also did not differ in racial distribution (p = 0.16); the HIV group comprised 26 (66.7%) whites, two (5.1%) American Indians, two (5.1%) Asians, three (7.7%) blacks, one (2.6%) mixed race subject, and five (12.8%) Pacific Islanders; while the SN group comprised 25 (78.1%) whites, five (15.6%) Asians, and two (6.3%) Pacific Islanders. The HIV subjects showed no significant changes in CD4+ cell counts during the 1-year follow-up period. The plasma viral load was undetectable in 22 (56%) subjects at baseline and 24 (62%) subjects at follow-up, and the average concentration of the plasma HIV RNA, hence the log viral load, was significantly lower at 1-year follow-up. No group difference was observed in any of the clinical variables between subjects with or without detectable viral load. The HIV dementia scale and MMSE were all unchanged after 1 year, whereas the Karnofsky score worsened minimally but significantly. At 1 year, the CES-D scores were significantly higher in the HIV subjects compared to the SN controls (p = 0.001) and to the HIV subjects’ own scores at baseline (p = 0.04).
As expected, the SN subjects had significantly better cognitive performance and lower GDS than the HIV subjects, significant at 1-year follow-up. The SN subjects also showed significant improvement from baseline to 1-year follow-up on this measure (p = 0.04), while the HIV group showed no significant change.
Cross-sectional analyses showed that age-related changes were observed on some of the clinical variables. With advancing age, no changes in CD4 cell count, nadir CD4 cell count, viral load, or duration of HIV diagnosis were observed in HIV subjects. In all subjects, older age was associated with higher education (r = 0.31, p = 0.0078) and hence higher estimated verbal intelligence quotient (r = 0.26, p = 0.0294). The GDS did not correlate with age among these subjects.
Diffusion tensor imaging
Comparison between HIV subjects and seronegative controls
Mean diffusion
Across all subjects, the repeated measures ANOVA revealed a main effect of HIV status on mean diffusivity in the frontal white matter (p = 0.0104). The effects remained unchanged when HIV subjects with or without detectable viral loads were evaluated separately. Post hoc analyses showed that at baseline and at 1 year, the HIV subjects showed significantly higher MD in the frontal white matter compared to the SN controls (Table 2). At 1-year follow-up, the HIV subjects additionally showed higher MD in the genu compared to the SN controls.
Furthermore, the genu showed a significant time-by-HIV-status interaction (p = 0.005), in that the MD increased 3.4% in the HIV+ subjects but was unchanged in the SN controls over the 1-year period (Fig. 2). The putamen showed a trend for time-by-HIV-status interaction (p = 0.134). After 1 year, MD in the putamen was increased in the HIV subjects (+2.2%, p = 0.0152) but not in the SN controls (+0.1%, p = 0.875; Fig. 2). Similarly, only HIV subjects, but not SN subjects, showed significant increases in MD in the frontal white matter and parietal white matter at 1 year compared to baseline (Table 2, Fig. 2). SN and HIV subjects both showed increases in MD in the globus pallidus (SN, +3.3%, p = 0.0276; HIV, +3.9%, p = 0.0671) over the 1-year period.
Mean diffusivity in SN and HIV subjects at baseline and after 1 year (mean ± SE). Compared to SN, HIV subjects showed greater 1-year increases in MD in the genu (top left). Additionally, significant increases in MD were observed in the putamen, frontal, and parietal white matter regions only in the HIV subjects but not in the SN subjects
Fractional anisotropy
The repeated measures ANOVA showed a main effect of HIV status on the FA in the parietal white matter (p = 0.006), where the FA was lower in HIV+ subjects at baseline (−8.5%, p = 0.009) and showed a trend for decrease at 1 year (−6.1%, p = 0.064) compared to SN controls (Table 2). However, no significant interactions between HIV status and time on FA were observed in any brain regions, although there was a slight trend for such an interaction effect in the putamen (p = 0.172). These findings did not change when subjects with or without detectable viral loads were evaluated separately.
Age-related changes in HIV subjects and controls
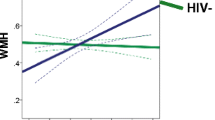
Since there are no significant differences between the slopes for age-related changes at baseline and at 1-year follow-up, the values from the two time points were averaged for analyses. Across all subjects, significant associations between age and MD were found in most brain regions measured (genu, r = 0.47, p < 0.0001; frontal white matter, r = 0.032, p = 0.0058; thalamus, r = 0.25, p = 0.034; caudate, r = 0.26, p = 0.028; and globus pallidus, r = 0.29, p = 0.01), with only a trend in the putamen and no significance in the splenium or the parietal white matter. However, these associations were primarily found in the SN subjects but not in the HIV subjects (Fig. 3, top graphs). Similarly, FA showed a negative association with age in the genu (r = −0.31, p = 0.008) and frontal white matter (r = −0.24, p = 0.045) but a positive association in the putamen (r = 0.38, p = 0.0009). These age-dependent associations were again due primarily to those in the SN but not those in the HIV subjects (Fig. 3, bottom graphs). Exploratory quadratic regressions of diffusion measures on age did not reveal any significant second-order effects.
Age dependence of MD and FA in various brain regions in the two subject groups. Significant age-dependent changes are observed only in the SN subjects but not in the HIV subjects, with trends for significant group difference in the genu FA (p = 0.056), frontal white matter MD (p = 0.18), and the putamen FA (p = 0.11)
Relationship between 1-year changes in DTI, clinical variables, and cognitive function
Multiple regressions were performed between 1-year follow-up variables that showed significant or trends for significant changes in selected DTI, clinical, and cognitive measures. Specifically, MD changes in the genu and putamen, frontal and parietal white matter, thalamus, and globus pallidus and FA changes in the parietal and frontal white matter, putamen, and globus pallidus were independent variables for each multiple regression, and changes in log viral load, Karnofsky score, CES-D, and GDS were dependent variables (each separately). Significant relations were only found for the 1-year change in the GDS (overall p = 0.0008), which was associated with the MD change in the genu (p = 0.0038) and the FA change in both the parietal and frontal white matter (p = 0.049 and 0.0037) and putamen (p = 0.0007); Fig. 4 shows the linear regression data.
Linear regression analyses showing changes in the global deficit scores in relation to changes in MD in genu (top left), FA in the frontal white matter (bottom left), and FA in the putamen (top right) in both subject groups. Multiple regression analyses showed that GDS is associated with change in the genu MD (p = 0.0038), change in parietal white matter FA (p = 0.049), change in frontal white matter FA (p = 0.0037), and change in putamen FA (p = 0.0007)
Discussion
Over 1 year, a group of clinically and cognitively stable HIV+ subjects showed greater increased mean diffusivity in the genu compared to the change observed in SN controls, while all other regions did not show interactions between HIV status and time. Increased water diffusion is often associated with inflammation, and elevation of an inflammatory chemokine, macrophage chemoattractant protein-1 (MCP-1), has been correlated with increased MD in the centrum semiovale, the putamen, and caudate of patients with HIV infection (Ragin et al. 2006). Therefore, our finding suggests that greater than normal age-related inflammatory changes occurred in the genu of the HIV patients, which may be the region most sensitive to white matter changes associated with HIV infection after 1 year. However, since many of the other brain regions evaluated also showed trends for increased diffusion after 1 year, a larger sample size might have illustrated significant effects. The genu as well as the frontal white matter are large and more homogeneous white matter structures; their highly organized and parallel fibers allowed the detection of changes in water diffusion more readily than other regions. The changes in the brain diffusion within the 1-year follow-up period were observed without accompanying significant changes on the global deficit score or on the HIV dementia scale; therefore, DTI appears to be more sensitive than these global cognitive measures. However, significant correlations between changes on the global deficit score and changes on MD in the genu or FA in the putamen also suggest that inflammatory changes may contribute to the cognitive deficits.
One recent cross-sectional study that included a large number of HIV+ subjects (n = 60) also found significantly higher MD and lower FA in the genu relative to control subjects, without significant differences in other brain regions (Thurnher et al. 2005). Another DTI study of HIV patients with alcoholism also found the genu to be more affected than the splenium of the corpus callosum (Pfefferbaum et al. 2007). Compared to SN controls at baseline or at 1 year, our HIV subjects had relatively mild cognitive deficits and showed only increases in MD in the genu and frontal white matter and decreased FA in the parietal white matter. However, due to the smaller variability of intrasubject comparison, small increases in MD in most brain regions in the HIV patients were readily detected after 1 year. These regional increases in MD in the HIV subjects, together with the greater temporal changes in the genu in our HIV subjects compared to SN controls after only 1 year, suggest that DTI may be useful for monitoring disease progression or treatment effects in HIV patients.
Changes in clinical variables, such as improvement in viral loads or Karnofsky scores did not correlate with DTI measurements. However, among all subjects, decline in the global deficit score was associated with increased genu diffusivity, decreased white matter FA, or increased putamen FA. Our findings are similar to those from studies that found correlations between cognitive function and DTI measures in patients with multiple sclerosis (Benedict et al. 2007) or with head trauma (Kraus et al. 2007). Increased inflammation, even from the systemic circulation, may increase the risk or contribute to cognitive decline (Dik et al. 2005; Gemma and Bickford 2007). However, lower FA may reflect greater degradation of the microstructure, such as age-related loss of myelination in the white matter, which might account for the slower performance on many tasks. Conversely, increased FA associated with age in the basal ganglia indicates increased coherence or compactness of these structures with age, which would be consistent with the observation that striatal structures become smaller with age over a 5-year period (Raz et al. 2003). Another explanation for the age-associated increase in FA may be due to the well-documented age-related increase in iron deposition in the deep gray matter structures (Bartzokis et al. 2007; Hallgren and Sourander 1958; Pfefferbaum et al. 2008). However, the exact mechanism for how increased iron would lead to increased FA remains unclear. Additional longitudinal follow-up of these subjects to include techniques that allow iron measurements may further clarify these relationships.
Associations of age with FA and MD on DTI have been well documented in healthy individuals (Sullivan and Pfefferbaum 2003). Our findings of a negative association of age with FA and a positive one with MD in the genu and frontal white matter of SN subjects are consistent with prior findings in healthy subjects of a similar age range (Pfefferbaum et al. 2000). Furthermore, the magnitudes of age-related decrease in FA (−2.7%/decade) and age-dependent increase in MD (+2.0%/decade) in the genu are similar to prior reported increases in tissue diffusion coefficient by 1% per decade after age 40 years (Chen et al. 2001). In contrast, our HIV subjects showed only trends toward age-related changes in the genu and frontal white matter. The altered age-related changes in DTI measures in the HIV subjects may be due to greater inflammatory changes in the younger HIV subjects compared to the older subjects.
We additionally observed age-related changes in MD in the thalamus, caudate and globus pallidus and FA in the putamen across all subjects. Assuming increased MD is related to increased inflammation, aging in both subject groups appears to be associated with increased inflammation. These age-related changes in the basal ganglia might also be related to changes in vasculature since both hemoglobin and hematocrit correlated positively with FA in the putamen, both in our study (data not shown) and as reported previously (Ragin et al. 2005). However, our findings of age-related changes in these brain regions remained significant even after correction for either hemoglobin or hematocrit.
Other neuroimaging markers, such as brain metabolites measured with 1H MRS, also may reflect neuro-inflammation. For instance, myo-inositol is a putative glial marker that may be elevated in disorders that involve glial proliferation (Chang et al. 1999a; Chang et al. 2002). Additionally, since the concentrations of total creatine (CR) and choline compounds (CHO) are higher in glia than in neurons (Brand et al. 1993), these metabolites may be elevated along with myoinositol during glial proliferation. These glial metabolites (myoinositol, CR, CHO) are typically elevated in the brains of HIV patients, especially those with cognitive deficits (Chang et al. 2002). Furthermore, elevated myoinositol, CR and CHO were found to be associated with greater blood oxygenation level dependent signals (Ernst et al. 2003) or increased requirement for brain activation, further suggesting that neuroinflammation may contribute to the neuropathogenesis of HIV dementia.
Limitations and future considerations
Several issues should be considered. First, co-morbid substance abuse, such as alcoholism (Pfefferbaum et al. 2007) or stimulant dependence (Chang et al. 2005; Jernigan et al. 2005), may potentially interact with measures of brain inflammation and degeneration. This study employed rigorous subject selection criteria and excluded subjects with drug dependence, but several subjects had a history of drug abuse or used illicit drugs recreationally. Second, although our sample size is larger than that of most previous studies, an even larger sample size might have demonstrated significant group differences in temporal changes in the remaining brain regions; many showed trends for significant changes on MD or FA after 1 year in these HIV subjects. This consideration is particularly important for designing future medication trials. Third, other brain regions that might show group differences were not evaluated; for example, a trend for lower FA in the hippocampus of HIV patients was observed (Thurnher et al. 2005). Fourth, other image processing approaches, such as a voxel-by-voxel comparison across the entire brain, may be useful in future studies to identify other regional differences. Lastly, future technical advances, such as a higher number of directions for the DTI, may also provide better delineation of changes in MD and FA.
Despite these limitations, changes in mean diffusion at the genu, and possibly the putamen, of HIV patients appear to provide sensitive markers for disease progression. The correlation of temporal changes in diffusion and those in the global cognitive deficit score further suggest that these measurements are sensitive for early detection of HIV-associated brain injury and may serve as objective surrogate makers.
References
Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI et al (1996) Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science 274:1917–1921 doi:10.1126/science.274.5294.1917
Banks WA, Ercal N, Price TO (2006) The blood–brain barrier in neuroAIDS. Curr HIV Res 4:259–266 doi:10.2174/157016206777709447
Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M et al (2007) Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging 28:414–423 doi:10.1016/j.neurobiolaging.2006.02.005
Benedict RH, Bruce J, Dwyer MG, Weinstock-Guttman B, Tjoa C, Tavazzi E et al (2007) Diffusion-weighted imaging predicts cognitive impairment in multiple sclerosis. Mult Scler 13:722–730 doi:10.1177/1352458507075592
Blair J, Spreen O (1989) The new adult reading test—Revised manual. University of Victoria, Vol Victoria, Canada
Brand A, Richter-Landsberg C, Leibfritz D (1993) Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15:289–298 doi:10.1159/000111347
Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E (1999a) Cerebral metabolite abnormalities correlate with clinical severity of HIV-cognitive motor complex. Neurology 52:100–108
Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I et al (1999b) Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology 53:782–789
Chang L, Ernst T, Witt M, Ames N, Jocivich J, Speck O et al (2002) Relationships among cerebral metabolites, cognitive function and viral loads in antiretroviral-naïve HIV patients. Neuroimage 17:1638–1648 doi:10.1006/nimg.2002.1254
Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E et al (2004) Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 56:259–272 doi:10.1002/ana.20190
Chang L, Ernst T, Speck O, Grob C (2005) Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry 162:361–369 doi:10.1176/appi.ajp.162.2.361
Chen ZG, Li TQ, Hindmarsh T (2001) Diffusion tensor trace mapping in normal adult brain using single-shot EPI technique. A methodological study of the aging brain. Acta Radiol 42:447–458 doi:10.1034/j.1600-0455.2001.420504.x
Cloak CC, Chang L, Ernst T (2004) Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol 157:147–152 doi:10.1016/j.jneuroim.2004.08.043
Conant K, St Hillaire C, Anderson C, Galey D, Wang J, Nath A (2004) Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J Neurovirol 10:21–28 doi:10.1080/13550280490261699
Connolly NC, Riddler SA, Rinaldo CR (2005) Proinflammatory cytokines in HIV disease—a review and rationale for new therapeutic approaches. AIDS Rev 7:168–180
Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P (2005) Serum inflammatory proteins and cognitive decline in older persons. Neurology 64:1371–1377
El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL et al (2006) HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia 53:132–146 doi:10.1002/glia.20262
Ernst T, Chang L, Arnold S (2003) Increased glial markers predict increased working memory network activation in HIV patients. Neuroimage 19:1686–1693 doi:10.1016/S1053-8119(03)00232-5
Filippi C, Ulug A, Ryan E, Ferrando S, van Gorp W (2001) Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. Am J Neuroradiol 22:277–283
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198 doi:10.1016/0022-3956(75)90026-6
Gemma C, Bickford PC (2007) Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev Neurosci 18:137–148
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3:41–51 doi:10.1111/j.1471-4159.1958.tb12607.x
Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y et al (2007) HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem 100:567–586 doi:10.1111/j.1471-4159.2006.04227.x
Jernigan T, Gamst A, Archibald S, Fennema-Notestine C, Mindt M, Marcotte T et al (2005) Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry 162:1461–1472 doi:10.1176/appi.ajp.162.8.1461
Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81:106–116 doi:10.1016/j.cmpb.2005.08.004
Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM (2007) White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 130:2508–2519 doi:10.1093/brain/awm216
Laubenberger J, Haussinger D, Bayer S, Thielemann S, Schneider B, Mundinger A et al (1996) HIV-related metabolic abnormalities in the brain: Depiction with proton MR spectroscopy with short echo times. Radiology 199:805–810
Nath A (2002) Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis 186(Suppl 2):S193–S198 doi:10.1086/344528
Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G et al (2007) Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. J Neuropsychiatry Clin Neurosci 19(3):283–292 doi:10.1176/appi.neuropsych.19.3.283
Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV (2008) Diffusion tensor imaging of deep gray matter brain structures: Effects of age and iron concentration. Neurobiol Aging (May 28, Epub ahead of print)
Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M (2000) Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med 44:259–268 doi:10.1002/1522-2594(200008)44:2<259::AID-MRM13>3.0.CO;2-6
Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV (2007) Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain 130:48–64 doi:10.1093/brain/awl242
Pomara N, Crandall D, Choi S, Johnson G, Lim K (2001) White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res 106:15–24 doi:10.1016/S0925-4927(00)00082-2
Power C, Johnson RT (1995) HIV-1 associated dementia: clinical features and pathogenesis. Can J Neurol Sci 22:92–100
Radloff LL (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401 doi:10.1177/014662167700100306
Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG (2005) Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol 11:292–298 doi:10.1080/13550280590953799
Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG (2006) Monocyte chemoattractant protein-1 correlates with subcortical brain injury in HIV infection. Neurology 66:1255–1257 doi:10.1212/01.wnl.0000208433.34723.65
Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD (2003) Differential aging of the human striatum: longitudinal evidence. Am J Neuroradiol 24:1849–1856
Simes RJ (1986) An improved Bonferroni procedure for multiple tests of significance. Biometrika 73:751–754 doi:10.1093/biomet/73.3.751
Steiner J, Haughey N, Li W, Venkatesan A, Anderson C, Reid R et al (2006) Oxidative stress and therapeutic approaches in HIV dementia. Antioxid Redox Signal 8:2089–2100 doi:10.1089/ars.2006.8.2089
Sullivan E, Pfefferbaum A (2003) Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol 45:244–255 doi:10.1016/S0720-048X(02)00313-3
Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC (2005) Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. Am J Neuroradiol 26:2275–2281
Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM et al (2004) Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 26:759–778
Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB (2006) Diffusion alterations in corpus callosum of patients with HIV. Am J Neuroradiol 27:656–660
Acknowledgements
We thank our research participants and support by the NIH (2R01MH61427; K24-DA16170; K02-DA16991; 5P20-RR11091; G12-RR003061) and the Office of National Drug Control Policy (ONDCP). We also thank Kenneth Yue, PhD; Daniel Alicata, MD, PhD; Renat Yakupov, MS; Grace Crocket, BA; and Caroline Jiang, MS, for their assistance in data collection or analyses.
Disclosure
Dr. Miller is the author and distributor of the CalCAP program and has a financial interest in this software.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, L., Wong, V., Nakama, H. et al. Greater Than Age-Related Changes in Brain Diffusion of HIV Patients After 1 Year. J Neuroimmune Pharmacol 3, 265–274 (2008). https://doi.org/10.1007/s11481-008-9120-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-008-9120-8