Abstract
Species coexistence at a given locality generally implies segregation along one of the three resource dimensions of the ecological niche: spatial, trophic or temporal. Temporal activity patterns of species are ecologically important as they expose how species exploit their environments. Using camera traps, we evaluated the temporal activity patterns and temporal overlap for a mammalian carnivore assemblage from Sierra Nanchititla Natural Park, central Mexico. We characterized and compared temporal activity patterns and temporal overlap between species pairs using circular statistics. Temporal overlap was analyzed using three temporal domains (full diel, diurnal, and nocturnal), and null models were used to contrast the empirical assemblage-wide temporal overlap in relation to randomly generated distributions. We found that pair-wise temporal overlap comparisons among species were quite heterogeneous and dependent on the temporal domain used for the analyses. Two major inferences can be derived from pair-wise analyses and the null model: (a) most of the carnivore assemblage show a nocturnal activity pattern in common and (b) most of the pair-wise comparisons indicate temporal segregation among species. The highest temporal overlap at the assemblage-wide level was found when only the nocturnal domain was evaluated and we found coincident temporal activities at the full domain level, independent of the temporal resolution used. Overall, our results suggest that carnivores with distinct trophic and habitat use like the margay (Leopardus wiedii) and gray fox (Urocyon cinereoargenteus) had temporal overlapping activity patterns to full diel and nocturnal domains, and species with similar trophic and habitat use always presented temporal segregation (gray fox and white-nosed coati, Nasua narica). Finally, species with predator-prey relationships (white-nosed coati and cougar, Puma concolor) showed segregation during the day but overlap at night. Our results indicate that species temporal activity patterns likely change in relation to different interspecific interactions such as predation and competition to allow species coexistence within this carnivore assemblage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An important research focus in ecology has been the mechanisms that allow coexistence of species in local communities. Species segregation along three main resource dimensions of the ecological niche, i.e., the spatial, trophic, and temporal dimensions, is suggested as the main mechanism allowing coexistence (Schoener 1974). Understanding the role of resource partitioning in each one of these dimensions at the levels of communities and assemblages (i.e., group of phylogenetically related species) provides important insight into biodiversity patterns and community dynamics (Pianka 1973; Schoener 1974; Winemiller and Pianka 1990; Linnell and Strand 2000). When limited resources are used by more than one species, coexistence can occur if differences in morphology or behavior lead to variation in the use of these resources (Davies et al. 2007; Di Bitetti et al. 2009).
Niche segregation in the spatial or trophic dimensions has been widely studied given that these axes have been considered the most important for species coexistence, whereas the temporal dimension has received substantially less attention (Schoener 1974, 1983; Castro-Arellano et al. 2010; Oliveira-Santos et al. 2013). Thus, the ecological implications of temporal activity patterns on community dynamics are scarcely known and not fully understood (Kronfeld-Schor and Dayan 2003; Castro-Arellano et al. 2010). Nonetheless, the temporal dimension can be important because changes in temporal activity patterns can alter interactions between species and community composition (Morgan 2004). Moreover, temporal activity patterns of species are ecologically important and indicate how species exploit their environments (Pianka 1973; Kronfeld-Schor and Dayan 2003). Temporal segregation can occur over long (i.e., seasons, years; Tokeshi 1986) or short periods (i.e., daily activity periods; Carothers and Jaksic 1984; Castro-Arellano and Lacher 2009), and presumably each species is able to access space and resources during a given period, allowing coexistence to occur (Schoener 1983).
Top predators and mesopredators are known to have important roles in structuring communities of terrestrial ecosystems; as such, they have been subjects of conservation attention in order to preserve their functional roles in ecosystem processes (Terborgh et al. 2001; Davis et al. 2011). Thus, mammalian carnivore conservation efforts have changed from single species approaches, limited to the largest and most charismatic species, to an approach centered in assemblages (Linnell and Strand 2000; Roemer et al. 2009). Several studies have identified mechanisms of coexistence between large top predators through the analysis of spatial and temporal partitioning, as well as differences in food habits (Taber et al. 1997; Scognamillo et al. 2003; Harmsen et al. 2009). In contrast, there are fewer studies at the whole assemblage level that consider interactions among carnivores in several trophic levels and the implications for coexistence (Di Bitetti et al. 2010; Davis et al. 2011). In our study area, three trophic guilds have been identified: (1) top predators, (2) carnivore mesopredators, and (3) omnivore mesopredators. Top predators prey on both mesopredators, and as consequence, mesopredators may constrain their temporal activity to reduce predation risk (Suselbeek et al. 2014; Gómez-Ortiz et al. 2015).
In the present study, we describe the temporal activity patterns, and test for temporal segregation and overlap, in an assemblage that includes the most frequent and ecologically important carnivores at a mountainous site in central Mexico. These species include two top predators (i.e., the jaguar, Panthera onca Linnaeus and the cougar, Puma concolor Linnaeus), a carnivore mesopredator (the margay, Leopardus wiedii Schinz) and two omnivore mesopredators (the white-nosed coati, Nasua narica, Linnaeus and the gray fox, Urocyon cinereoargenteus, Schreber). We hypothesize that nonrandom temporal segregation exists in this carnivore assemblage as a possible strategy of coexistence.
Material and methods
Study area
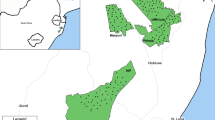
Sampling for our study was done at the Sierra Nanchititla Natural Park (SNNP). This reserve in central Mexico (extreme coordinates: 100° 36′ 49″; 100° 16″ 03” W and 18° 45′ 13″; 19° 04′ 22” N) covers 663.87 km2 and an elevation range of 410–2080 MASL. Vegetation in the park consists of five major types, i.e., oak forest (30%), grasslands (30%), deciduous tropical forest (18%), pine-oak forest (17%), and crops (4%, Monroy-Vilchis et al. 2008). The temperate forest (oak forest and pine-oak forest) and tropical dry forest (deciduous tropical forest) compose the main native vegetation (Fig. 1). Due to the complex topography, there are two main climatic zones, a temperate zone located above 1400 m, and a tropical zone located below 1400 m. In the SNNP, two seasons are recognized—the dry season (June to October) and the wet season (November to May). Mean annual rainfall is 1000–1500 mm and mean annual temperature is 12–16 °C. Mammalian fauna of the reserve consists of 53 species, with the most diverse groups being rodents (17 spp.), chiropterans (15 spp.), and carnivores (14 spp.) (Monroy-Vilchis et al. 2011).
Locations of camera-trap sampling stations within the Sierra Nanchititla Natural Park during the five carnivore species surveys of the study. Tones of gray reflect types of habitat as indicated in the figure inset. Each symbol denotes the geographical location of a camera trap during each survey. This park is located in the southwestern region of the State of Mexico, bordering with the states of Michoacan and Guerrero
Sampling design
Data was collected during five camera-trapping surveys in SNNP from December 2003 to January 2012. These surveys varied in duration and sampling effort. We placed sampling stations (n = 67) in locations where signs of carnivores were previously recorded (footprints, scrapes, scats, etc.) among the two main native habitat types (66% in temperate forest and 34% in tropical dry forest). The effective survey area polygon of sampling stations used covered an area of 443 km2. This polygon included a buffer around each sampling station equal to the mean maximum distance moved of cougar individuals photographed at two or more sites in SNNP (5.5 km; Soria-Díaz et al. 2010).
We used different models of camera traps but all of them were equipped with passive detectors for movement and temperature (MK-8 from Cam Trakker, Watkinsville, GA, USA; RT1004R from Recon Outdoors, Huntsville, AL, USA and ScoutGuard 860c from HCO Outdoor Products, Norcross, GA, USA). We used 13 camera traps for the period of December 2003 to December 2005 (survey 1), 15 camera traps from January 2006 to February 2008 (survey 2), 18 camera traps from October 2008 to September 2009 (survey 3), 7 camera traps from January 2010 to November 2010 (survey 4), and 14 camera traps from January 2011 to January 2012 (survey 5; Fig. 1). Camera traps were affixed to trees 40–60 cm above ground and programmed to operate 24 h per day. Cameras recorded the date and time with each photo and they were programmed to have a minimum interval of 60 s between each photo. Cameras were monitored monthly to change batteries and download images from memory cards.
Temporal activity patterns
Analysis of temporal activity patterns was restricted to the five most abundant carnivore species present at SNNP. These species represented three families (Felidae, Canidae, and Procyonidae) including two top predators (jaguars and cougars) and three mesopredators (margays, gray foxes, and white-nosed coatis). Carnivore species that had less than ten independent events were excluded from the analysis. Photos at a sampling station were all separated by 1 h, except when different individuals were identified through morphological characteristics, or when they occurred in groups. This way we minimized the potential pseudoreplication caused by individuals that triggered camera traps several times within short periods. Temporal activity patterns for each species were assumed to be an overall representation of time use by each species.
Temporal activity patterns for each species were analyzed using circular statistics implemented with the Oriana 2.0 software (Kovach Computing Services, UK). We used circular histograms to classify the species as nocturnal or diurnal focusing on the mean vector length (r) and confidence interval of independent events (95%). Also, we used both the circular variance and the concentration of observations to evaluate of circular dispersion of the independent events. Species with large circular variance values, combined with a low concentration of observations, were characterized by having more uniform temporal activity patterns (Zar 1999; Oliveira-Santos et al. 2012). Additionally, the Rayleigh Uniformity test was used to evaluate whether the independent events of each species were randomly or uniformly distributed. In this uniformity test, Z value is calculated as Z = nr2, where n is the number of observations and r is the length of the mean vector. So, a larger Z value means that there is a greater concentration of observations around the mean, and thus less probality of the data being uniformly distributed (i.e., temporal preferences, temporal activity patterns unidirectional: diurnal, nocturnal, or crepuscular). Conversely, if the independent events from a given species were uniformly distributed, then the species was classified as cathemeral (Zar 1999, Oliveira-Santos et al. 2012).
Temporal overlap patterns
All independent events were classified as occurring in one of four periods: day (0900–1859 h), dusk (1900 h–2059 h), night (2100–06 h59), and dawn (0700–08 h59). Sunrise (monthly mean 0737 h, min 0705 h, max 0817 h; Luvianos, State of Mexico; 28.7 km from study site) and sunset (monthly mean 1943 h, min 1902 h, max 2019 h) varied very little throughout the year, and thus, independent events were classified using the time thresholds we defined above with no adjustments due to seasonal variation. Temporal overlap analyses were done using three temporal domains: full diel domain (i.e., full 24-h diel cycle), a diurnal domain (0700 h–2059 h; day plus dusk and dawn) and a nocturnal domain (1900 h–0859 h; night plus dusk and dawn). We used the Czekanowski index (CI; Feisinger et al. 1981) to determine pair-wise temporal overlap (1 for species that have identical temporal activity patterns and 0 for species that have non-overlapping temporal activity patterns). The proportion of observations within n time intervals for species j and k are pij and pik, respectively. Here, we conduct our analysis using n = 24 (full diel domain) and n = 14 (diurnal and nocturnal domain). The index of Czekanowski niche overlap is calculated as follows:
As a complementary analysis of the Czekanowski index between each species pair, we calculated the W test statistic of the Mardia-Watson-Wheeler test (Zar 1999) using the Oriana 2.0 software. This is a nonparametric test used to determine whether two distributions are identical. Large values of W statistic test increase the probability of rejection of the null hypothesis of identical distributions. In these analyses, jaguar was excluded due to a small sample size and multiple pair-wise tests were conducted among the remaining carnivores. Methods used to maintain Type I error rates at a certain α-level for a group of related pair-wise tests are controversial in ecology (Hulbert and Lombardi 2003). These methods can raise Type II error rates to a point that they could exceed Type I error rates when correction is done for multiple pair-wise tests. In general, corrections for multiple pair-wise tests are not advised for exploratory analyses such as those conducted in our study (Roback and Askins 2005). Finally, a chi-square test was used to check for differences in the number of independent events between the dry and wet seasons. For all analyses, significance was considered at P ≤ 0.05.
Null model simulations in assemblage-wide temporal overlap
We used two temporal resolutions (1 and 2 h) and two temporal domains (full diel and nocturnal domain) to generate species (rows) by time interval (columns) data matrices that were the basis for assemblage-wide temporal niche overlap analyses (diurnal domain was excluded due to the absence of independent events for the margay and jaguar). All matrices were populated with the number of independent events (i.e., measure of temporal activity) for each species and then converted to percentage of total independent events for each species to standardize the species. Due to the potential ecological importance of the jaguar within the assemblage, we compared matrices with and without this species, in spite of its relatively low sample size. Assemblage-wide temporal overlap was generated from the average of all pair-wise overlap values of constituent species calculated using the CI (Feisinger et al. 1981; Gotelli et al. 2015). This average becomes the empirical index that was compared against the null distribution of overlap values of pseudoassemblages generated by the simulation algorithm. An empirical overlap value less than 95% of the overlap values generated from the random pseudo-assemblages (p < 0.05) is an indication of temporal segregation (i.e., less overlap than expected by chance). Opposite of this, an empirical overlap value greater than 95% of the null distribution (p < 0.05) is an indication of temporal coincidence (i.e., more overlap than expected by chance).
For all of our tests, we used 1000 simulations to generate the null distributions that the empirical overlap values (i.e., Czekanowski indices) were compared to. Simulations were implemented by the freely available Time overlap software (available at: http://ivancastroarellano.wp.txstate.edu/analysis_temporal_niche/ and http://hydrodictyon.eeb.uconn.edu/people/willig/Research/activity%20pattern.html). This program uses the Rosario algorithm designed specifically for temporal data (Castro-Arellano et al. 2010). Other models to evaluate niche overlap exist (i.e., RA3 and RA4; Winemiller and Pianka 1990) but those can only be used for nominal unordered resources such as prey or microhabitats, and thus are inadequate for time, because it is an ordered resource. Both RA3 and RA4 eliminate the observed (i.e., real) temporal autocorrelation as these algorithms disrupt the shape of activity patterns when they reshuffle the values of each time interval within each species. On the contrary, Rosario maintains the observed temporal autocorrelation, and thus creates more biologically meaningful null spaces. Moreover, it has a low type-I error rate. Although the Time overlap software implements these null model tests with both Pianka (1973) and Czekanowski (Feisinger et al. 1981) indices to estimate overlap, we elected to report just the latter, because the results with either index were generally congruent (Castro-Arellano et al. 2010). A more detailed description of Rosario algorithm can be found in Castro-Arellano et al. (2010).
Results
Temporal activity patterns
We obtained 791 independent events to describe the temporal activity patterns and temporal overlap. The following species were represented (percentage of events in parentheses): the white-nosed coati (63.59%), cougar (15.93%), gray fox (13.15%), margay (5.18%), and jaguar (2.15%; Table 1).
Most of carnivore species showed a marked temporal activity patterns: the observations of margay, jaguar, gray fox, and cougar were primarily during the nocturnal domain (Fig. 2a, b, c, d). However, the white-nosed coati was more active within the diurnal domain (Fig. 2e). The white-nosed coati and cougar showed the greatest circular dispersion in their temporal activity distributions over the full diel domain, but no species in this carnivore assemblage was classified as cathemeral because none of the distributions were uniformly distributed (p < 0.05; Table 1).
Pair-wise and assemblage-wide temporal overlap
Overall, within the nocturnal domain, there was temporal overlap in the activity patterns of gray fox and margay (CI = 0.79; W = 0.89, p = 0.460); as well as between cougar and white-nosed coati (CI = 0.76; W = 3.96, p = 0.138). In the full diel domain there was also temporal overlap between gray fox and margay (CI = 0.72; W = 0.46, p = 0.462, Table 2a, b). The least temporal overlap occurred between the margay and white-nosed coati (CI = 0.32; W = 66.31, p < 0.001) as well as with the gray fox and white-nosed coati (CI = 0.39; W = 141.55, p < 0.001) within the full diel domain (Table 2a).
Pair-wise comparisons of temporal activity patterns, as indicated by Mardia-Watson-Wheller tests, were highly dependent on the temporal domain used in the analysis (Table 2a, b, c). When the full diel domain was analyzed, most of the pair-wise comparisons indicated differences of temporal activity among species except for between gray fox and margay which showed temporal overlap (Table 2a). The analysis of temporal activities during the nocturnal domain demonstrated temporal segregation in most of the pair-wise comparisons except for between the cougar and white-nosed coati and the gray fox and margay (higher values of temporal overlap; Table 2b). When the comparisons were restricted to the diurnal domain these differences between species pairs were maintained except for between the cougar and gray fox (Table 2c). Finally, we found no differences in the number of independent events between the wet and dry seasons expect for white-nosed coati that had more independent events in the dry season (X2 = 10.68, p < 0.05; all other P values > 0.05).
In most cases (6 of 8), assemblage-wide temporal overlap was indistinguishable from a random expectation, independent of the temporal resolution (1 and 2 h), temporal domain (full diel or nocturnal), and inclusion of jaguar in the analyses (Table 3). However, it is noteworthy that the higher temporal overlap in the assemblage-wide was found in the nocturnal domain. When all species were considered within the nocturnal domain at the 1-h resolution, it approached significance for coincident activities. On the other hand, when the full diel domain and all species were considered, we found significant coincident nonrandom temporal activities, independent of the temporal resolution used (Table 3).
Discussion
Globally, the majority of mammal species are nocturnal and it has been argued that nocturnality is an ancestral trait of the group (Bennie et al. 2014). However, within local mammal assemblages there is great range of variation in the periods of activity. These differences in behavior are functional traits associated with morphological adaptations and have strong links to the ecology of each species (Bennie et al. 2014). Moreover, phenotypic disparity between species can be an important factor for their coexistence given that phenotypes dictate or limit resource use overlap among species that occupy similar niches (Davies et al. 2007; Harmsen et al. 2009). When species are competing for space, segregation in temporal activity or separation in use of key foods can arise in response to interference competition (Carothers and Jaksic 1984; De Almeida et al. 2004).
Temporal overlap among species in the carnivore assemblage at SNNP showed temporal-domain dependence as the results change in relation to the temporal domains used for analyses. In the same manner that results from spatial analyses can provide different outcomes depending on the scale (i.e., domain) and grain used in the analysis, results from temporal analyses have to be interpreted in light of the temporal resolutions and domains used (Loreau 1989). Overall, we detected coincident temporal overlap patterns when only the nocturnal domain was considered, whereas we found temporal coincidence patterns in the full diel domain for both of the temporal resolutions we tested (i.e., 1 and 2 h). The coincident activity patterns for the whole assemblage indicate the shared preference (or constraints) for nocturnal activities among species. Even more, as indicated by the coincident activities result approaching significance within the nocturnal domain, there might be further assemblage-wide constraints for activities within the nocturnal assemblage. Two potential hypotheses for the shared preference for nocturnal activities are that these carnivore species are guided by the activity of prey or a common avoidance of human activity.
Consistent with the incongruent interpretations provided by the null model analyses, the outcomes from the pair-wise contrasts done within these temporal domains are better interpreted in a hierarchical context, first looking at inferences derived from assemblage-wide analyses and how these are related to individual pair-wise analyses. Null model analyses comprising the full diel domain indicated a nonrandom assemblage-wide temporal overlap with species activities more coincident compared to the random expectation, likely as a consequence of most species activities occurring in the nocturnal domain. Although most of carnivore species do trend towards nocturnal activities, pair-wise tests of the full diel domain showed that they exploit this temporal domain in different ways given that only the gray fox and margay species pair had indistinguishable activity patterns. In contrast to the full diel domain results, null model analyses of the nocturnal domain showed that the assemblage-wide temporal overlap is indistinguishable from a random expectation using a 2-h resolution but approached to temporal coincidence with the 1-h resolution. This corresponds well with the outcomes from the pair-wise contrasts done within this temporal domain as this included two species pairs with high temporal overlap (cougar-white-nosed coati and gray fox-margay) and significant differences among the rest of species pairs. Overall, two major inferences can be derived from the pair-wise and null model analyses: (a) the majority of the carnivore assemblage (excluding white-nosed coati) shows a common constraint for nocturnal activities and (b) most species use different strategies for their use of the nocturnal portion of the diel domain. Interestingly, amid the highly heterogeneous pair-wise contrasts, the response of two groups was very consistent: (a) carnivores with very distinct habitat use like margay and gray fox always had overlapping temporal activity patterns, independent of time domain used; (b) carnivores with similar trophic and habitat use always presented segregated temporal activities (gray fox and white-nosed coati).
One consideration for the interpretation of results from pair-wise comparisons with the CI and the Mardia-Watson-Wheeler test is that these analytical procedures provide complementary views of the data. The first one is an index that measures overlap between two resource use curves (Feisinger et al. 1981; Gotelli et al. 2015), whereas the latter assesses if two distribution curves are identical. Graphically, the Czekanowski Index represents the area under the curve where two distributions overlap (Gotelli et al. 2015), whereas the Mardia-Watson-Wheeler test compares the shape of these resource use curves. In general, results we obtained with these tests provide two aspects to contrast how a pair of species are using time as a resource.
Temporal activity patterns from species in this assemblage were mostly similar to previous descriptions from other Neotropical localities (Lucherini et al. 2009; Di Bitetti et al. 2010). Both of the largest predators from SNNP have previously been identified as having a nocturnal activity pattern. The jaguar has a nocturnal activity pattern in both the Venezuelan Llanos and the Western and Central regions of Mexico (Núñez et al. 2000; Scognamillo et al. 2003; Monroy-Vilchis et al. 2009), but the cougar shows a more variable activity pattern, as this species has been reported to have variable temporal activity patterns in different regions (Gómez et al. 2005; Lucherini et al. 2009; Di Bitetti et al. 2010). The margay, which are a smaller and more arboreal felid species than the cougar, has also been reported as nocturnal (Gómez et al. 2005; Di Bitetti et al. 2010; Vanderhoff et al. 2011). The remaining species in our carnivore assemblage, the gray fox and white-nosed coati, have been reported with nocturnal and diurnal activity respectively at the northern part of their geographic distribution (Valenzuela and Ceballos 2000; Farías et al. 2012; Lira-Torres and Briones-Salas 2012).
An interesting contrast of activities is indicated by cougars and white-nosed coatis as these species had different activity patterns during the diel and diurnal domains but showed temporal overlap during the nocturnal domain. In general, our data suggests strong temporal niche segregation between white-nosed coati and the rest of the carnivores, especially during the diurnal domain. Selection of temporal activity pattern (e.g., diurnal, cathemeral) is a trait that distinguishes procyonids, and likely allows them to reduce negative encounters with their potential predators, who usually are terrestrial and nocturnal (Davies et al. 2007). In SNNP, the white-nosed coati is known to be an important part of diet of both top felid predators (Monroy-Vilchis et al. 2009; Gómez-Ortiz et al. 2011; Gómez-Ortiz and Monroy-Vilchis 2013). Additionally, some predators can show temporal activity patterns similar to their prey, particularly when prey are active only in short periods of the day (Ramesh et al. 2012, 2015). The crepuscular activity of the cougar is a key time that suggests a strategy to increase the probability of encounters and facilitate predation of the white-nosed coati. This behavior would support the idea that temporal activity patterns of species can change in relation to both selection forces such as the competition and predation. Thus, prey species (white-nosed coati) might change their activity to minimize overlap not only with top predators (jaguar and cougar) but also to reduce negative interactions with potential intra-guild competitors (gray fox and margay; Linnell and Strand 2000; Di Bitetti et al. 2009; Hayward and Slotow 2009). Interestingly, our data do not support the same response (i.e., mainly diurnal activities) in the gray fox, another terrestrial species of small body size, probably because this species is not a part of the diet of large felids at SNNP (Donadio and Buskirk 2006; Davis et al. 2011; Monroy-Vilchis et al. 2011). This result strengthens the idea that procyonids actively avoid potential predators like canids and felids, through selection of different forest strata or habitat type as well as different temporal activity patterns (Hunter and Caro 2008). Overall, our results not only agree with the idea that smaller carnivores may show temporal segregation from the larger carnivores, to reduce predation risk (Lucherini et al. 2009; Suselbeek et al. 2014), but our study also revealed temporal differences between mesopredators possibly due to competition (white-nosed coati with gray fox and margay; Gómez-Ortiz et al. 2015). Interestingly, mesopredators with very distinct habitat use like the gray fox (scansorial) and margay (arboreal) had strong temporal overlap of activities. Likely, the differential habitat use precludes the need for temporal segregation of activities.
Although diverse environmental factors may influence temporal activity patterns of wildlife, no significant differences between seasons (wet and dry) were identified in the carnivore assemblage except for the white-nosed coati that showed seasonal variation. In other sites, season had strong effects on temporal activity patterns and was related to the high availability of prey items during particular seasons, which in turn implies low search effort and less activity of species (González et al. 1992; Patterson et al. 1999; Suselbeek et al. 2014).
Temporal dimension analysis is clearly a valuable additional approach to ascertain ecological requirements of a species to plan conservation and management strategies. In SNNP, carnivore species have been robustly studied; and after several years, the temporal, trophic, and spatial dimension analyses now offer a baseline for the management of carnivores from this previously little-studied temperate forests in the Neotropics of central Mexico. Evaluation of resource use between sympatric species is a part of the information needed to understand their coexistence and understand their ecological dynamics. The number of species considered in our analyses was determined by abundance and capture success rate of each species. Due to the ecological importance of jaguar in ecosystems, it was included in some analyses although it occurs in low abundance at SNNP (Soria-Díaz et al. 2010). Nonetheless, interpretation should be considered with caution. Our analytical approach in which we included results both with and without jaguar allows the exploration of data for a species with an important role in the community but for which data is inherently scarce. As in most studies of mammalian carnivores, the nature of these elusive mammals and their low densities, present great challenges to obtain large sample sizes needed to make strong inferences.
References
Bennie JJ, Duffy JP, Inger R, Gaston KJ (2014) Biogeography of time partitioning in mammals. Proc Natl Acad Sci U S A 111:13727–13732
Carothers JH, Jaksic FM (1984) Time as a niche difference: the role of interference competition. Oikos 42:403–406
Castro-Arellano I, Lacher TE (2009) Temporal niche segregation in two rodent assemblages of subtropical Mexico. J Trop Ecol 25:593–603
Castro-Arellano I, Lacher TE, Willig MR, Rangel TF (2010) Assessment of assemblage-wide temporal niche segregation using null models. Methods Ecol Evol 1:311–318
Davies TJ, Meiri S, Barraclough TG, Gittleman JL (2007) Species co-existence and character divergence across carnivores. Ecol Lett 10:146–152
Davis ML, Kelly MJ, Stauffer DF (2011) Carnivore co-existence and habitat use in the mountain pine ridge Forest reserve, Belize. Anim Conserv 14:56–65
De Almeida AT, Silveira L, Diniz-Filho JAF (2004) Niche separation between the maned wolf (Chrysocyon brachyurus), the crab-eating fox (Dusicyon thous) and the hoary fox (Dusicyon vetulus) in Central Brazil. J Zool (London) 262:99–106
Di Bitetti MS, Di Blanco YE, Pereira JA, Paviolo A, Jiménez I (2009) Time partitioning favors the coexistence of sympatric crab-eating foxes (Cerdocyon thous) and pampas foxes (Lycalopex gymnocercus). J Mammal 90:479–490
Di Bitetti MS, De Angelo CD, Di Blanco YE, Paviolo A (2010) Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta Oecol 36:403–412
Donadio E, Buskirk SW (2006) Diet, morphology, and interspecific killing in carnivora. Am Nat 167:524–536
Farías V, Fuller TK, Sauvajot RM (2012) Activity and distribution of gray foxes (Urocyon cinereoargenteus) in Southern California. Southwest Nat 57:176–181
Feisinger P, Spears EE, Poole RW (1981) A simple measure of niche breadth. Ecology 62:27–32
Gómez H, Wallace RB, Ayala G, Tejada R (2005) Dry season activity periods of some Amazonian mammals. Stud Neotropical Fauna Environ 40:91–95
Gómez-Ortiz Y, Monroy-Vilchis O (2013) Feeding ecology of puma Puma concolor in Mexican montane forests with comments about jaguar Panthera onca. Wildl Biol 19:179–187
Gómez-Ortiz Y, Monroy-Vilchis O, Fajardo V, Mendoza GD, Urios V (2011) Is food quality important for carnivores? The case of Puma concolor. Anim Biol 61:277–288
Gómez-Ortiz Y, Monroy-Vilchis O, Mendoza GD (2015) Feeding interactions in an assemblage of terrestrial carnivores in Central Mexico. Zool Stud 54:1–8
González G, Sánchez V, Íñiguez L, Santana E, Fuller T (1992) Activity patterns of coyote (Canis latrans), grey fox (Urocyon cinereoargenteus) and opossum (Didelphis virginiana) in Serra of Manantlán, Jalisco. An Inst Biol Univ Nac Auton Mex Ser Zool 63:293–299
Gotelli NJ, Hart EM, Ellison AM (2015) EcoSimR: null model analysis for ecological data. Zenodo. https://doi.org/10.5281/zenodo.16636
Harmsen BJ, Foster RJ, Silver SC, Ostro LET, Doncaster CP (2009) Spatial and temporal interactions of sympatric jaguars (Panthera onca) and pumas (Puma concolor) in a Neotropical forest. J Mammal 90:612–620
Hayward MW, Slotow R (2009) Temporal partitioning of activity in large African carnivores: tests of multiple hypotheses. S Afr J Wildl Res 39:109–125
Hulbert SH, Lombardi CM (2003) Design and analysis: uncertain intent, uncertain result. Ecology 84:810–812
Hunter J, Caro T (2008) Interspecific competition and predation in American carnivore families. Ethol Ecol Evol 20:295–324
Kronfeld-Schor N, Dayan T (2003) Partitioning of time as an ecological resource. Ann Rev Ecol Evol Syst 34:153–181
Linnell JCD, Strand O (2000) Interference interactions, co-existence and conservation of mammalian carnivores. Divers Distrib 6:169–176
Lira-Torres I, Briones-Salas M (2012) Relative abundance and activity patterns of mammals at Chimalapa’s forest, Oaxaca, Mexico. Acta Zool Mex 28:566–585
Loreau M (1989) On testing temporal niche differentiation in carabid beetles. Oecologia 81:89–96
Lucherini M, Reppucci J, Walker RS, Villalba M, Wurstten A, Gallardo G, Iriarte A, Villalobos R, Perovic P (2009) Activity pattern segregation of carnivores in the high Andes. J Mammal 90:1404–1409
Monroy-Vilchis O, Zarco-González M, Rodríguez-Soto C, Suárez P, Urios V (2008) Uso tradicional de vertebrados silvestres en la Sierra Nanchititla, México. Interciencia 33:308–313
Monroy-Vilchis O, Gómez Y, Janczur M, Urios V (2009) Food niche of Puma concolor in Central Mexico. Wildl Biol 15:97–105
Monroy-Vilchis O, Zarco-González M, Rodríguez-Soto C, Soria-Díaz L, Urios V (2011) Mammals’ camera-trapping in Sierra Nanchititla, Mexico: relative abundance and activity patterns. Rev Biol Trop 59:373–383
Morgan E (2004) Ecological significance of biological clocks. Biol Rhythm Res 35:3–12
Núñez R, Miller B, Lindzey F (2000) Food habits of jaguars and pumas in Jalisco, Mexico. J Zool 52:373–379
Oliveira-Santos LG, Graipel ME, Tortato MA, Zucco CA, Cáceres NC, Goulart FV (2012) Abundance changes and activity flexibility of the oncilla, Leopardus tigrinus (Carnivora: Felidae), appear to reflect avoidance of conflict. Zoologia 29:115–120
Oliveira-Santos LGR, Zucco CA, Agostinelli C (2013) Using conditional circular kernel density functions to test hypotheses on animal circadian activity. Anim Behav 85:269–280
Patterson BR, Bondrup-Nielsen S, Messier F (1999) Activity patterns and daily movements of the eastern coyote, Canis latrans, in Nova Scotia. Can Field Nat 113:251–257
Pianka ER (1973) The structure of lizard communities. Annu Rev Ecol Syst 4:53–74
Ramesh T, Kalle R, Sankar K, Qureshi Q (2012) Dietary partitioning in sympatric large carnivores in a tropical forest of Western Ghats, India. Mamm Study 37:313–321
Ramesh T, Kalle R, Sankar K, Qureshi Q (2015) Role of body size in activity budgets of mammals in the Western Ghats of India. J Trop Ecol 31:315–323
Roback PJ, Askins RA (2005) Judicious use of multiple hypothesis tests. Conserv Biol 19:261–267
Roemer GW, Gompper ME, Valkenburgh BV (2009) The ecological role of the mammalian mesocarnivores. BioScience 59:165–173
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Schoener TW (1983) Field experiments on interspecific competition. Am Nat 122:240–285
Scognamillo D, Maxit I, Sunquist M, Polisar J (2003) Coexistence of jaguar (Panthera onca) and puma (Puma concolor) in a mosaic landscape in the Venezuelan llanos. J Zool (London) 259:269–279
Soria-Díaz L, Monroy-Vilchis O, Rodríguez-Soto C, Zarco-González MM, Urios V (2010) Variation of abundance and density of Puma concolor in zones of high and low concentration of camera traps in central Mexico. Anim Biol 60:361–371
Suselbeek L, Emsens WJ, Hirsch BT, Kays R, Rowcliffe JM, Zamora-Gutierrez V, Jansen PA (2014) Food acquisition and predator avoidance in Neotropical rodent. Anim Behav 88:41–48
Taber A, Novaro A, Neris N, Colman F (1997) The food habits of sympatric jaguar and puma in the Paraguayan Chaco. Biotropica 29:204–213
Terborgh J, Lopez L, Nuñez P, Rao M, Shahabuddin G, Orihuela G, Riveros M, Ascanio R, Adler GH, Lambert TD, Balbas L (2001) Ecological meltdown in predator-free forest fragments. Science 294:1923–1926
Tokeshi M (1986) Resource utilization, overlap and temporal community dynamics: a null model analysis of an epiphytic chironomid community. J Anim Ecol 55:491–506
Valenzuela D, Ceballos G (2000) Habitat selection, home range, and activity of the white-nosed coati (Nasua narica) in a Mexican tropical dry forest. J Mammal 81:810–819
Vanderhoff EN, Hodge A, Arbogast BS, Nilsson J, Knowles WT (2011) Abundance and activity patterns of the margay (Leopardus wiedii) at a mid-elevation site in the eastern Andes of Ecuador. Mastozool Neotrop 18:271–279
Winemiller KO, Pianka ER (1990) Organization in natural assemblages of desert lizards and tropical fishes. Ecol Monogr 60:27–55
Zar JH (1999) Bioestatistical analysis. Prentice Hall, New Jersey 662p
Acknowledgements
We thank all students that helped as volunteer field assistants and to the rangers of Sierra Nanchititla Biological Station and CEPANAF for their support in field. We also thank to Dr. Joseph Veech for reviewing the English grammar of the first version of our draft. The comments from one anonymous reviewer and the detailed help of the editor enhanced the clarity of the final version of this manuscript.
Funding
This work was supported by CONACYT (project #105254 and the scholarship #255868 for graduate studies of Gómez-Ortiz Y.) and PROMEP (project #103.5/10/0942).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Quinn Fletcher
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gómez-Ortiz, Y., Monroy-Vilchis, O. & Castro-Arellano, I. Temporal coexistence in a carnivore assemblage from central Mexico: temporal-domain dependence. Mamm Res 64, 333–342 (2019). https://doi.org/10.1007/s13364-019-00415-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-019-00415-8