Abstract
Differences in availability of food resources are often manifested in the differentiation of feeding habits of closely related mammal species. Therefore, we assumed that the diet composition and trophic niche of house (i.e., highly dependent on human households), feral (i.e. independent on human households) domestic cats (Felis silvestris catus) and wildcats (Felis s. silvestris) differs. Based on the literature data from Europe (53 study sites), we compared the diet of these three felids analyzed with use of indirect methods (stomach and scat analyses). In the case of the house cat, we additionally compared consumption data obtained directly from prey brought home. Data were expressed as the relative frequency of occurrence to compare dietary patterns. The main prey of the three cat types were small mammals in different ratios. According to the stomach and scat samples, the diet composition of the cat types showed differences in the consumption of rodents, insectivores, wild ungulates, and household food, supporting the “dietary differences originate from varying resources” hypothesis. More opportunistic house cats had a broader trophic niche than feeding specialist wildcats, while feral cats had an intermediate position. The trophic niche breadth of all three cat types increased along a latitudinal gradient from northern to southern areas of Europe. The predation of the house cat which was examined from prey brought home differed from the data obtained by indirect diet analysis; however, it yielded similar results to the diet of the feral cat and the wildcat. Due to their high numbers and similarity of its diet to the wildcat, house cats are a threat to wild animals; therefore, their predation pressure needs to be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Discovering the ecological role of the mesopredator species, especially recognizing the differences and interactions between foraging habits of these species (MacArthur and Pianka 1966; Colwell and Futuyma 1971; Pianka 1973; Schoener 1974), could promote the management and conservation of predator and prey populations (Courchamp et al. 1999; Crooks and Soulé 1999; Nogales et al. 2004; Matias and Catry 2008; Bonnaud et al. 2010).
Domestic cats (Felis silvestris catus) are popular pet animals, which have coexisted with humans for thousands of years (Fitzgerald 1988; Randi and Ragni 1991; Driscoll et al. 2009). They have populated almost the whole world with human mediation causing serious conservation problems and occasionally the extinctions of species (Fitzgerald 1988; Dickman 1996; Lowe et al. 2000; Medina et al. 2011; Blancher 2013; Doherty et al. 2014). Several domestic cat groups could be defined according to the behavior and closeness of the association with humans (e.g., farm, stray, poaching, or feral; Liberg 1984; Liberg and Sandell 1988; Pearre and Maass 1998), and these groups have different prey sources. Cats living around the house, i.e., house cats, depend largely on the food supplied by the owner (Liberg 1984), but do not rely on hunting for food (Dickman 1996). However, house cats are able to readily revert to the feral or wild state (Fitzgerald 1988; Liberg and Sandell 1988) and maintain their population without human care and food supply (Liberg 1984; Biró et al. 2005; Doherty et al. 2015), as they are excellent hunters (Turner and Meister 1988).
The diet of house cats frequently contains foods originating from human sources (e.g., pet food, kitchen remnant; reviewed: Fitzgerald 1988). Although feeding cats might reduce their motivation to hunt (Turner and Meister 1988), a high level of predation is still demonstrated (e.g., Churcher and Lawton 1987; Woods et al. 2003; Loyd et al. 2013) and it has been suggested that these cats kill prey independently of hunger. Beside the household food, the main diet component contains small mammals (mainly rodents) (Fitzgerald 1988), but the consumption of small birds (Achterberg and Metzger 1980) or insects (Krauze-Gryz et al. 2012) is also frequent.
The dietary pattern of feral cats is well known from reviews or meta-analyses performed on data from islands (Canary Islands: Medina and Nogales 2009, global: Bonnaud et al. 2011; Medina et al. 2011) or Australia (Dickman 1996; Doherty et al. 2015). The main prey are generally small mammals (consisting also pests), but bird (Liberg 1984; Pearre and Maass 1998) or European rabbit (Oryctolagus cuniculus; Corbett 1979; Liberg 1984; Ozella et al. 2016) dominance and frequent reptile predation (Dickman 1996; Doherty et al. 2015; Lanszki et al. 2016) were also demonstrated.
Wildcats (Felis silvestris silvestris) have disjunctive distribution in Europe due to a strong population decrease as a result of habitat loss and hunting pressure (Stahl and Artois 1994; McOrist and Kitchener 1994; Yamaguchi et al. 2015). Recently, the main conservation risk has been hybridization with domestic cats (Pierpaoli et al. 2003; Yamaguchi et al. 2015; Kilshaw et al. 2016). Competition in habitat use (Corbett 1979; Biró et al. 2004) and feeding (Corbett 1979; Biró et al. 2005) could emerge in case of high population density of feral cats (Woods et al. 2003) in areas with a low density of wildcat populations. The dietary pattern of wildcat has been analyzed in several publications; primary foods are small mammals or rabbits (where it is available) (e.g., Moleon and Gil-Sánchez 2003; Malo et al. 2004; Biró et al. 2005; Lozano et al. 2006; Apostolico et al. 2016) and also the role of some prey types (birds, reptiles, and invertebrates) could be considerable (Table S1, Online Resource 1).
Our hypothesis (“dietary differences originate from varying resources” hypothesis), based on the literature data, was that the dietary patterns and trophic niche of the cat types studied vary in Europe due to differences in habitat use (Corbett 1979; Turner and Bateson 1988; Lozano et al. 2003; Biró et al. 2004), associated with varying food resources (Corbett 1979; Liberg 1984; Niewold 1986; Daniels et al. 2001; Moleon and Gil-Sánchez 2003; Malo et al. 2004; Lanszki et al. 2016). On this point, the various hunting techniques existing in the Felis genus and also the dietary opportunism (Corbett 1979; Fitzgerald 1988) have an important role.
We assumed (first prediction) that the diet composition of the feral cat is more similar to the wildcat (both use similar resources) than to the house cat. According to this, we supposed that the niche overlap between the feral cat and the wildcat is high, but moderate between feral and house cat types. Our second prediction was that the trophic niche of the facultative food specialist—the wildcat (Moleon and Gil-Sánchez 2003; Malo et al. 2004; Lozano et al. 2006)—will be narrow, while the trophic niche of house and feral cats will be wider, considering that these are versatile generalist (less specialized) predators, exploiting a wide range of prey (Fitzgerald 1988). We assumed (third prediction) that the tropic niche breadth depends on the geographic latitude (Lozano et al. 2006), with a varying closeness between the cat types. It is demonstrated that house cats in contrast with feral cats predate independently of hunger. The killed prey is consumed only partially, and it is brought home (Churcher and Lawton 1987; Fitzgerald 1988; Woods et al. 2003) or left on the predation site (Loyd et al. 2013). Therefore, we predicted (fourth prediction) that the differences in the food compositions were determined by direct (prey brought home) or indirect (stomach and scat analysis) methods, based on the local experiences of the study by Krauze-Gryz et al. (2012).
The aims of our investigation were (1) to compare the food composition and (2) trophic niche breadth of the three cat types, (3) to determine the differences in the trophic niche breadth in accordance to the geographic latitude based on the literature data of the stomach and scat analyses from European habitats, and (4) to explore the differences in the food composition of the house cat group, using varying methods.
Materials and methods
Literature compilation and variable selection
We collected dietary data across Europe from literature found in electronic databases (Web of Science, Scopus, ScienceDirect) using combinations of the following keywords: Felis catus, Felis silvestris, domestic cat, feral cat, house cat, wildcat, wild cat, food, prey, trophic, niche and Europe, and from additional studies (e.g., PhD theses, archival published materials). Data compilation was conducted up to February 2017. Detailed data and full list of references are given in Table S1 (Online Resource 1).
We separated three cat types on the basis of the information found in the publication: (1) house cats which are highly dependent on human households (named in papers also as stray, pet, or poaching cats), (2) feral cats which are independent of human households (Liberg 1984; Pearre and Maass 1998), and (3) wildcats. We have not taken into account the assessment of the hybrid wildcats (Table S1, Online Resource 1), as well as the ambiguously classified domestic cats.
The sample types used in these studies were scats (n ≥ 75) or stomach (or gut; n ≥ 22) contents (as the indirect diet analysis method group). In the case of house cats, a third sample type, records of “prey brought home” (e.g., Fitzgerald 1988; Woods et al. 2003) were also evaluated (as the direct diet analysis method). When studies used more than one sample type, we analyzed each sample type as a separate study (Fitzgerald et al. 1991; Bonnaud et al. 2011). The sampling period was annual (samples were collected during all seasons) or periodic (covering at least two seasons). If a study contained separate seasonal values but not overall values, we averaged data across all seasons (Doherty et al. 2015). In the case of multiple publications, we considered the most recent one (with larger sample size). We included in the assessment only publications in which all recognizable food items were documented. Results from the same study site were pooled to avoid pseudo-replication (Zhou et al. 2011). Studies where prey types did not clearly fit the proportion of household food consumption (Table S1, Online Resource 1) were excluded from the evaluation.
We only used sources with results expressed as percentage relative frequency of occurrence of food types (RFO, number of occurrences of a certain food type divided by the total number of occurrences of all food types × 100) or those that contained N data (number of food items for each food types) to recalculate the RFO. We discarded studies that only reported data as frequency of occurrence (FO, expressed as the percentage of scats or stomachs containing a certain food type) or biomass estimates (BIO, estimated biomass ratio of main food types consumed). With the known limitations and advantages of occurrence data for diet studies (Reynolds and Aebischer 1991), we used RFO data, because these (and/or N data) were often reported in studies. RFO values are considered to be highly suitable for inter-population comparisons in diet studies (McDonald 2002; Clavero et al. 2003; Lozano et al. 2006; Zhou et al. 2011), although FO data are also widely used (Bonnaud et al. 2011; Doherty et al. 2015; Soe et al. 2017) and biomass estimates are also considered to be useful methods (Reynolds and Aebischer 1991), but the variety of known calculation methods would make the comparison more difficult.
We distinguished 11 main food types (categories), namely, 1, rodents; 2, insectivores (shrews and very rarely bats, moles, and hedgehogs); 3, lagomorphs (and in some cases, other medium-sized herbivorous mammals, i.e., porcupine and marmot); 4, carnivores; 5, wild ungulates (consumption from carrion); 6, household or human-linked food (e.g., domestic animals, pet food); 7, wild birds (and eggs); 8, reptiles; 9, amphibians; 10, fish; and 11, invertebrates (arthropods, molluscs, earthworms). The names of the detected species are shown in Table S2 (Online Resource 2). Plants (as well as matter originating from litter) were also identified from domestic cat and wildcat diets, but because cats are obligate carnivores (Bradshaw et al. 1996), these dietary elements were excluded (e.g., Lozano et al. 2006; Bonnaud et al. 2011; Hervías et al. 2014; Doherty et al. 2015) from the evaluation. The occurrence of their consumption is only indicated in the database (Table S1, Online Resource 1).
Stomach and scat sample numbers (n) in the studies were divided into classes according to quartiles of a comparative analysis: 1, ≤ 75 (22–75); 2, 76-137; 3, 138–214; 4, > 214 (215–561). For each geographical location, we recorded bioclimate, i.e., Mediterranean and temperate (other than Mediterranean), and included latitude (decimal degrees) taken from the information given in the studies or from a geographical database. When samples were collected over a large area, the latitude was estimated for the largest part of the sample (Lozano et al. 2006).
For each study, the trophic niche breadth was calculated by using Levins’ (1968) index: B = 1/Σp i 2, where p i = the relative frequency of occurrence of the ith food taxa; and standardized across 11 main food taxa (Krebs 1989): B A = (B − 1)/(n − 1), rating from zero (dietary specialization) to one (broad diet). The trophic niche overlap was calculated by means of the Renkonen index (Krebs 1989): P jk = Σn(minimum p ij , p ik )]100, where P jk = percentage overlap between cat type (i.e., house, feral, or wildcat) j and cat type k; p ij and p ik = the proportion of resource i represented within the total resources used by cat type j and type k; n = the total number of resource taxa (= 11), rating from 0 (no overlap) to 100% (full overlap). Mean dietary data of three cat types were used.
The names of prey animals used in the analyzed studies were checked (Internet sources: http://www.iucnredlist.org, http://datazone.birdlife.org/userfiles/file/Species/Taxonomy/BirdLife_Checklist_Version_90), and the IUCN Red List categories of species were also added (Table S2, Online Resource 2).
Statistical analyses
The non-normal dependent variables, i.e., percentage of RFO data of main food types were arcsin transformed prior to analysis (e.g., Bonnaud et al. 2011; Díaz-Ruiz et al. 2013). Variances showed homogeneity for the main food types (i.e., rodents, insectivores, lagomorphs, birds, reptiles, and invertebrates).
In order to test for bias caused by the bioclimate (Mediterranean or temperate), sampling period (annual or two seasonal), sample type (stomach or scat), and sample size (one to four category) (i.e., whether these variables have an effect on diet composition; Lozano et al. 2006; Zhou et al. 2011; Díaz-Ruiz et al. 2013; Doherty et al. 2015), the multivariate general linear model (GLM) was applied with the cat type (house cat, feral cat, wildcat) as the fixed factor; bioclimate, sampling period, sample type, and sample size category as covariates; and the arcsin-transformed RFO of each of the 11 food types as response variable (multivariate test: Hotelling’s Trace).
We used the one-way analysis of variance (ANOVA, Tukey post hoc test) to explore differences in diet composition (11 main food types) and standardized trophic niche breadth (B A) data among three cat types. In the case of the three cat types, we separately used Spearman correlation to analyze the statistical relationships among main food types and between consumption ratios and B A data.
We evaluated associations between geographic (latitude) distances and standardized trophic niche breadth (B A) differences (Table S1, Online Resource 1) for dietary data of three cat types with linear regression and the Mantel test. The simple Mantel tests were performed in XLSTAT 2014.5.03 version, with a significance determined via 10,000 permutation tests, using Pearson correlation. Where significant correlation was observed, regression analysis was used to describe the relationship of the latitudinal values versus the B A values, with the significance determined using ANOVA.
We compared consumption data from the direct method (i.e., prey brought home) and indirect diet analysis methods (i.e. stomach and scat analyses) within the house cat type by using independent sample t test (arcsin-transformed RFO, 11 main food types). We applied hierarchical cluster analysis (with Ward as the cluster method and Euclidean distance as the interval of measure, range from 0 to 100; e.g., McDonald 2002) to compare and to visualize the diet composition data recorded for the three cat types (and direct and indirect sampling methods in the case of the house cat) from the literature across Europe on the basis of arcsin-transformed RFO data of 11 main food types (same food types as listed previously).
The SPSS 11.5 for Windows and R statistical package (v. 3.2.3., R Development Core Team, Vienna, Austria) were used for data processing. We accepted a minimum probability level of P < 0.05.
Results
General overview
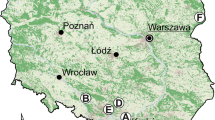
We examined 52 publications (72 sites) in total, from which the data from 39 studies (53 sites; Fig. 1) were used for our comparative analysis. For each cat type, a relatively large number (n > 75) of samples were analyzed in year-round studies (Table 1). The distribution of studies between regions is slightly unbalanced; relatively, few studies have been conducted on house cats in the Mediterranean area, while there are more on feral cats in temperate areas. On the basis of literature reviewed (Table S2, Online Resource 2), in Europe, house cats, feral cats, and wildcats preyed upon the following: 35, 14, and 40 mammals; 71, 42, and 23 birds; 7, 7, and 5 reptiles; 3, 0, and 1 amphibians; 1, 1, and 1 fish; and 26, 6, and 9 invertebrate species, respectively. Among these, according to the IUCN Red List categorization, all three cat types consumed two vulnerable and five near threatened species (Table S2, Online Resource 2).
Study sites across Europe from which data were used in the analysis. White circles, house cat studies; grey circles, feral cat studies; black circles, wildcat studies; white circles with dot, prey brought home studies. Numbers are cross-referenced with the studies reported in Table S1, Online Resource 1
We found no significant differences in the consumption of food types for the three cat types pooled among studies deriving diet composition from different sample types (GLM, F 1,43 = 0.65, P = 0.776) and sample size (F 3,43 = 1.27, P = 0.297), while bioclimate (F 1,43 = 4.51, P = 0.001) and sample period (F 1,43 = 2.89, P = 0.012) had a significant effect in the case of some less important food types (i.e., bioclimate: reptiles and invertebrates; sample period: wild ungulates, fish, and invertebrates). For further analyses, we pooled data with additional explanation for interpretation results. We detected significant differences across cat types (F 2,43 = 5.35, P < 0.001).
Differences in the diet of cat types
The diet composition of the three cat types on the basis of stomach and scat samples differed significantly in four main food types (Table 2). House cats consumed household food more frequently than feral cats and wildcats, while wildcats consumed rodents, insectivores, and ungulates more frequently compared to house cats. Feral cats consumed rodents and household food with similar frequency to wildcats, while they consumed insectivores similarly to house cats.
Compared to temperate areas (T), in the Mediterranean areas (M), reptiles were consumed more frequently (GLM, F = 25.24, P < 0.001) by house cats (M: 2.08% vs. T: 0.78%), feral cats (M: 6.90% vs. T: 0.79%), and wildcats too (M: 4.30% vs. T: 0.03%). Invertebrates (F = 4.78, P = 0.035) were consumed more frequently in Mediterranean areas by feral cats (M: 8.99% vs. T: 2.37%) and wildcats (M: 11.09% vs. T: 0.96%), whereas it was opposite in case of house cats (T: 4.34% vs. M: 3.39%).
Depending on the sampling period, differences were found in the case of occasionally consumed wild ungulates (GLM, F = 5.59, P = 0.024) and fish (F = 16.00, P < 0.001). Compared to annual (A) studies, in the periodic (P) studies, ungulates were consumed more frequently by wildcats (A: 0.5%, P: 3.7%), and fish were consumed more frequently by house cats (A: 0%, P: 9.0%) and feral cats (A: 0.2%, P: 2.0%). In the case of invertebrate consumption of a wildcat, a significant difference was detected depending on the sampling period (F = 4.73, P = 0.036, A: 6.1%, P: 0.3%).
Consumption of rodents (as main prey type) negatively correlated with household foods in the case of house cats (Spearman correlation, r S = − 0.835, P < 0.001), whereas consumption of rodents negatively correlated with lagomorphs in the case of wildcats (r S = − 0.578, P = 0.012).
Factors affecting trophic niche
House cats had a broader trophic niche compared to wildcats (Table 2), while feral cats were closer to wildcats. The trophic niche overlap between house cat and feral cat and between house cat and wildcat was 59.9 and 56.4%, respectively; while between feral cat and wildcat, it was 91.7%.
Standardized trophic niche breadth (B A) negatively correlated with consumption of rodents in the case of feral cats (Spearman correlation, r S = − 0.927, P < 0.001) and wildcats (r S = − 0.957, P < 0.001), while B A positively correlated with the consumption of lagomorphs (r S = 0.491, P = 0.039) and reptiles (r S = 0.546, P = 0.019) in the case of wildcats or with birds in the case of house cats (r S = 0.636, P = 0.011).
Latitude negatively correlated with the consumption of reptiles in the case of house cats (Spearman correlation, r S = − 0.564, P = 0.029) and wildcats (r S = − 0.777, P < 0.001) and with invertebrates (r S = − 0.677, P = 0.032) in the case of feral cats.
Negative linear relationships were found between latitude and B A values in all three cat types (Fig. 2). However, it was statistically significant only in the case of wildcat (simple regression analysis, r = − 0.511, P = 0.030), while in the case of house cat (r = − 0.373, P = 0.170) and wildcat (r = − 0.511, P = 0.131), the relationship was not significant. The evaluation of the same relationship based on permutation test (Mantel test) resulted in similar correlation but different probability, representing a more realistic association. Associations between latitude distances and B A differences were found in all three cat types (negative linear relationship), with only marginally significant P value in the case of the house cat (Mantel test, R 2 = 0.077, P = 0.050), while the association was significant in the case of feral cat (R 2 = 0.192, P = 0.029) and wildcat (R 2 = 0.295, P < 0.001).
Direct versus indirect diet analysis
Comparing dietary data of house cats from the direct method, i.e., prey brought home with data from indirect diet analysis methods (stomach and scat) (Table 2), detection of rodents (independent samples t test, t 23 = 2.89, P = 0.08), insectivores (t 23 = 6.26, P < 0.001), birds (t 23 = 4.61, P < 0.001), and amphibians (t 23 = 2.66, P = 0.014) was more frequent in the case of prey brought home, while household food (t 23 = 5.62, P < 0.001) and invertebrates (t 23 = 2.54, P = 0.018) were less frequently detected. The trophic niche breadth did not differ significantly in the two diet analysis methods (t 23 = 0.870, P = 0.393). Considering the effect of the source of dietary data (i.e., direct and indirect analyses; Fig. 3), the diet of house cats was most different from both the wildcat (E d = 44.1) and the feral cat (E d = 33.0) when analyzed indirectly; however, the difference was little when only the prey brought home was considered (E d = 21.3 and 22.7, respectively).
Similarity dendogram of the Euclidean distances among general diet compositions of house, feral, and wildcat from the reviewed studies across Europe. In the case of the house cat, two diet analysis methods, i.e., stomach (St) and scat (Sc) samples (indirect methods) and prey brought home (PBH—direct method) were taken into account. Arcsin-transformed RFO data; cluster method: Ward; 11 main food types
Discussion
Differences in the diet of cat types
According to our comparative investigation of literature data based on the stomach and scat analyses, we showed the differences in dietary compositions of the three cat types, which confirmed our first prediction. The differences in food resources between settlements (Fitzgerald 1988) and natural habitats (Corbett 1979; Turner and Bateson 1988; Biró et al. 2005) resulted in considerable dietary differences between the house cat and the wildcat. Although, the diet composition of the feral cat showed moderate characteristics from several points of view, it was more similar to the wildcat. These diet composition differences were also manifested in the lower niche overlap (around 60%) between the house cat and the other cat types as well as the higher overlap between the two wild living cat types (over 90%) supporting our first prediction.
The primary prey for all three cat types (in varying degrees) are rodents, principally the available Microtus and Apodemus species with high abundance in European sites and partially rats (Rattus sp.). The secondary item was household food in the diet of house cat type and rabbits in the diet of feral and wildcat types. As the consumption of rodents decreased, the predation of rabbits by wildcats increased, supporting the finding by Lozano et al. (2006) that the wildcat is a facultative specialist predator (Corbett 1979; Sarmento 1996; Gil-Sánchez et al. 1999). In the case of feral cats, in Australia, Doherty et al. (2015) also found that consumption of rabbits is negatively correlated with consumption of small mammals. In the case of house cats, decreasing household food consumption increased the predation of rodents, indicating its opportunistic hunting (foraging) strategy (Turner and Meister 1988). The consumption of other food types showed no significant correlation with small mammal consumption in any of the three cat types. In most cases, we used annual data sets; however, in some cases, differences in consumption of some less important food types were dependent on the sampling period.
Apart from the four food types (rodents, insectivores, wild ungulates, and human-linked food), no significant differences were found due to the high standard deviance (indicating the high variability of the food resources and foraging techniques), although significant biological differences were detected between the cat types (Table S1, Online Resource 1). Basically, all of the cat types frequently consumed birds and insects with similar prevalence. However, for prey types consumed only occasionally, small-sized carnivores were consumed more frequently by the wildcat. Reptiles (mainly lizards) were captured by feral cats, and fish was consumed mainly by house cats (Table S2, Online Resource 2). The difference from the general tendency, e.g., the higher consumption of rabbits (23.3%: Niewold 1986), birds (22.6%: Achterberg and Metzger 1980), fish (18.1%: Lanszki et al. 2016), and invertebrates (32.0%: Krauze-Gryz et al. 2012), was detected in house cats. In the case of feral cats, a higher consumption of rabbits (64.8%: Corbett 1979), birds (22.0%: Ozella et al. 2016), reptiles (20.9%: Lanszki et al. 2016), and insects (32.6%: Millán 2010) was demonstrated, while wildcats had higher rabbit (73.3%: Corbett 1979), carnivore (3.6%: Biró et al. 2005), wild ungulate (6.5%: Kozená 1990), bird (16.4%: Biró et al. 2005), reptile (10.3%: Apostolico et al. 2016), and invertebrate (27.6%: Malo et al. 2004) consumption (Table S1, Online Resource 1).
Trophic niche
We found various prey species in the studies based on scat and stomach samples in all of the three cat types. Prey diversity was high in the wildcat diet based on the reviews of European literature (Apostolico et al. 2016), and similar results were found in the case of feral cats in meta-analyses from Australia (Doherty et al. 2015), several islands of the world (Bonnaud et al. 2011), and at a global range (Fitzgerald 1988). The high diversity of prey species also exists in house cats, especially in accordance with home-brought prey (e.g., Woods et al. 2003; Krauze-Gryz et al. 2012). Several estimations are published on the negative impact of domestic cats on wildlife representing a disadvantageous and invasive species all around the world (e.g., Liberg 1984; Fitzgerald 1988; Dickman 1996; Woods et al. 2003; Medina et al. 2011; Loss et al. 2013). Vulnerable species among the prey of house cats [e.g., south-western water vole (Arvicola sapidus): Woods et al. 2003, red grouse (Lagopus lagopus scoticus): Corbett 1979], in feral cat [e.g., European souslik (Spermophilus citellus): Biró et al. 2005, yelkouan shearwater (Puffinus yelkouan): Tranchant et al. 2003; Bonnaud et al. 2011], and in wildcat [great capricorn beetle (Cerambyx cerdo): Moleon and Gil-Sánchez 2003] were detected in Europe. Although the main prey taxa of house and feral cats in Europe could be pest species, e.g., rats, voles, and mice (e.g., Niewold 1986; Tranchant et al. 2003; Biró et al. 2005), occasionally, their preference for more rare species is also apparent (Lanszki et al. 2016).
Unexpectedly, we showed that all of the three cat types had narrow trophic niche, which indicates that only a narrow range of the available food resources is utilized. Nevertheless, house cats had a broader trophic niche than wildcats, which partially confirms our second prediction. House cats are more opportunistic: they consume easily acquirable household food in the human environment (Fitzgerald 1988), but at the same time, they follow a facultative feeding strategy (Krauze-Gryz et al. 2012), similarly to the wildcat (Moleon and Gil-Sánchez 2003; Malo et al. 2004). In the case of low small mammal resources, or in the nesting period of birds, house cats could switch prey type from small mammals to birds or lizards (Fitzgerald 1988; Peck et al. 2008). Moreover, the predation is not always accompanied by consumption in the case of house cats (Churcher and Lawton 1987; Woods et al. 2003; Loyd et al. 2013), while wildcats are rather food specialists or facultative food specialists (Malo et al. 2004; Biró et al. 2005; Lozano et al. 2006). Feral cats had an intermediate position, since they rarely consumed household food (Table S1, Online Resource 1) whereas they predated for survival like the wildcat. The trophic niche of the more specialized feral cat and wildcat was broadened with the decrease in the consumption of small mammals (the main prey type); additionally, it was also broader in the case of the wildcat, consuming rabbits and reptiles more frequently, as was also found by Lozano et al. (2006). In the case of house cats, the trophic niche was broader with the frequent consumption of birds, presumably due to bird feeding (Lepczyk et al. 2008) and migration (Lanszki et al. 2016), which indicates the importance of this prey type. In the case of the wildcat, a remarkably broad trophic niche was found in Spain (e.g., Malo et al. 2004), while an extremely narrow trophic niche was detected in a study on house cats performed in Switzerland (Weber and Dailly 1998).
Importance of geographical latitude
Our analysis on the literature data suggested that varying trophic niche breadth along a geographical gradient could be verified not only in the case of wildcats (Lozano et al. 2006) but also in the case of feral cats (Fitzgerald 1988) (confirming our third prediction). In the case of house cats, the lower, marginally significant negative correlation could be explained by the consumption of household food in a high ratio independently from the geographic latitude. Our results emphasized that feral cats have feeding habits (foraging) more similar to wildcat than the house cat. However, the similar regression coefficient and constant values indicate that the frequency of consuming reptiles and invertebrates increases toward the southern regions of Europe in the case of all three cat types, but at a different level, following the diversity increase of the potential prey species (Fitzgerald 1988; Malo et al. 2004; Lozano et al. 2006). In the case of house cats, other factors could also have a significant effect, for example, Fitzgerald (1988) found that the decreasing consumption of household food could also be the result of lower human population density. Other factors could be the differences of motivation and hunting methods between the three cat types. The lack of significance in the regressions between B A and latitude values originates rather from the variation of the data than from the sample number, indicating that the house cat studies have more diverse observations. This could be rooted in the more complex phenotypic composition of the house cat group as an outcome of the variability of the different cat breeds as a result of the fast domestication due to artificial selection in the last 200 years (Driscoll et al. 2009; Dickman and Newsome 2015).
Difference between consumption and predation
Our meta-analysis on the literature data confirmed our fourth prediction that the consumption and predation of the house cat group differed depending on direct and indirect food composition analyses, as was expected from the local study published by Krauze-Gryz et al. (2012). Hall and Bradshaw (1998) revealed that in adult house cats, the act of playing has indistinguishable motivation from predation, as both behaviors are similarly affected by hunger and the size of the toy or prey. Prey size depends on the level of hunger, and it decreases with increased hunger. Every object that covers characteristics associated with prey, such as size or texture, could trigger both predation and playing. If stimulus characteristics remain unchanged, rapid habituation occurs and playing behavior ceases (Hall et al. 2002). In the case of live prey, the stimulus is strong and the prey characteristics change and predation occurs, even if there is no need for predation since the level of hunger is low. Young cats learn predation through playing situations. The mother cat introduces the different prey types early on through a series of situations with dead prey and later on by releasing live individuals to initiate their own behavior responses in the young (Turner and Bateson 1988). These habits could result in the separation of predation due to hunger or other motivations (e.g., play, teaching) in the case of house cats. Due to the different motivations, predation is not always followed by consumption (e.g., teaching and playing) or bringing home of the prey (in the case of playing).
From studies on cats wearing video cameras on collars (Loyd et al. 2013), it is demonstrated that almost half of the prey (49%) was left on the predation area, only 28% was consumed, and 23% was carried home. If the prey brought home by the house cat is involved in the analysis (our hierarchical cluster analysis), the difference between the dietary patterns of the cat types decreases. The differences found depending on the method confirm the fourth prediction, but contradict the first one. This indicates the limitations of the diet composition analysis based on indirect (stomach and scat analysis) methods in the case of the house cat. Furthermore, a comparison of the results obtained using different methods, i.e., diet analyses with questionnaire surveys (Krauze-Gryz et al. 2012) supplemented with application of telemetry (Biró et al. 2004) and/or animal-borne video systems (Loyd et al. 2013) can be realistic in a year-round study, so seasonal variations may also be detectable.
In conclusion, based on a comparative analysis of European studies on the diet of domestic (house and feral) cats and wildcats, the hypothesis that dietary differences originate from varying resources was supported in the case of the diet composition and the niche breadth of house cats and wildcats. The diet of feral cats, considering that they consume their prey, seems to be similar to wildcats. The “latitude—trophic niche breadth interrelation” prediction was supported, as the niche breadth decreased with increasing latitude. The results of surveys concerning prey brought home by house cats indicate that their prey composition is close to the diet composition of feral and wildcats. Due to the worldwide high numbers and population densities of house cats as well as the similarity of their diet to the wildcat, the house cat should be considered a threat for wild-living animals and therefore their predation pressure needs to be further investigated.
References
Achterberg H, Metzger R (1980) Neue Untersuchungen und Erkentnisse zur Bedeutung der Hauskatze (Felis silvestris f. catus) für die Niederwildhege. Jahresschrift des Kreismuseums Haldensleben 21:74–83
Apostolico F, Vercillo F, La Porta G, Ragni B (2016) Long-term changes in diet and trophic niche of the European wildcat (Felis silvestris silvestris) in Italy. Mammal Res 61:109–119
Biró Z, Szemethy L, Heltai M (2004) Home range sizes of wildcats (Felis silvestris) and feral domestic cats (Felis silvestris f. catus) in a hilly region of Hungary. Mamm Biol 69:302–310
Biró Z, Lanszki J, Szemethy L, Heltai M, Randi E (2005) Feeding habits of feral domestic cats (Felis catus), wild cats (Felis silvestris) and their hybrids: trophic niche overlap among cat groups in Hungary. J Zool 266:187–196
Blancher P (2013) Estimated number of birds killed by house cats (Felis catus) in Canada. Avian Conserv Ecol 8(2). https://doi.org/10.5751/ACE-00557-080203
Bonnaud E, Zarzoso-Lacoste D, Bourgeois K, Ruffino L, Legrand J, Vidal E (2010) Top-predator control on islands boosts endemic prey but not mesopredator. Anim Conserv 13:556–567
Bonnaud E, Medina FM, Vidal E, Nogales M, Tershy B, Zavaleta E, Donlan CJ, Keitt B, Le Corre M, Horwath SV (2011) The diet of feral cats on islands: a review and a call for more studies. Biol Invasions 13:581–603
Bradshaw JWS, Goodwin D, Legrand-Defrétin V, Nott HMR (1996) Food selection by the domestic cat, an obligate carnivore. Comp Biochem Physiol 114A:205–209
Churcher PB, Lawton JH (1987) Predation by domestic cats in an English village. J Zool 212:439–455
Clavero M, Prenda J, Delibes M (2003) Trophic diversity of the otter (Lutra lutra L.) in temperate and Mediterranean freshwater habitats. J Biogeogr 30:761–769
Colwell RK, Futuyma DJ (1971) On the measurement of niche breadth and overlap. Ecology 52:567–576
Corbett LK (1979) Feeding ecology and social organization of wildcats (Felis silvestris) and domestic cats (Felis catus) in Scotland. PhD Thesis. University of Aberdeen, UK
Courchamp F, Langlais M, Sugihara G (1999) Cats protecting birds: modelling the mesopredator release effect. J Anim Ecol 68:282–292
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566
Daniels MJ, Beaumont MA, Johnson PJ, Balharry D, MacDonald DW, Barratt E (2001) Ecology and genetics of wild-living cats in the north-east of Scotland and the implications for the conservation of the wildcat. J Appl Ecol 38:146–161
Díaz-Ruiz F, Delibes-Mateos M, García-Moreno JL, López-Martín JM, Ferreira C, Ferreras P (2013) Biogeographical patterns in the diet of an opportunistic predator: the red fox Vulpes vulpes in the Iberian Peninsula. Mammal Rev 43:59–70
Dickman CR (1996) Impact of exogenic generalist predators on the native fauna of Australia. Wildl Biol 2:185–195
Dickman CR, Newsome TM (2015) Individual hunting behaviour and prey specialisation in the house cat Felis catus: implications for conservation and management. Appl Anim Behav Sci 173:76–87
Doherty TS, Bengsen AJ, Davis RA (2014) A critical review of habitat use by feral cats and key directions for future research and management. Wildl Res 41:435–446
Doherty TS, Davis RA, van Etten EJB, Algar DA, Collier N, Dickman CR, Edwards G, Masters P, Palmer R, Robinson S (2015) A continental-scale analysis of feral cat diet in Australia. J Biogeogr 42:964–975
Driscoll CA, Macdonald DW, O'Brien SJ (2009) From wild animals to domestic pets, an evolutionary view of domestication. Proc Natl Acad Sci U S A 106:9971–9978
Fitzgerald BM (1988) Diet of domestic cats and their impact on prey populations. In: Turner DC, Bateson P (eds) The domestic cat: the biology of its behaviour. Cambridge Univ Press, Cambridge, pp 123–144
Fitzgerald BM, Karl BJ, Veitch CR (1991) The diet of feral cats (Felis catus) on Raoul Island, Kermadec Group. New Zeal J Zool 15:123–129
Gil-Sánchez JM, Valenzuela G, Sánchez JF (1999) Iberian wild cat Felis silvestris tartessia predation on rabbit Oryctolagus cuniculus: functional response and age selection. Acta Theriol 44:421–428
Hall SL, Bradshaw JWS (1998) The influence of hunger on object play by adult domestic cats. Appl Anim Behav Sci 58:43–150
Hall SL, Bradshaw JWS, Robinson IH (2002) Object play in adult domestic cats: the roles of habituation and disinhibition. Appl Anim Behav Sci 79:263–271
Hervías S, Oppel S, Medina FM, Pipa T, Diez A, Ramos JA, Ruiz de Ybánez R, Nogáles M (2014) Assessing the impact of introduced cats on island biodiversity by combining dietary and movement analysis. J Zool 292:39–47
Kilshaw K, Montgomery RA, Campbell RD, Hetherington DA, Johnson PJ, Kitchener AC, Macdonald DW, Millspaugh JJ (2016) Mapping the spatial configuration of hybridization risk for an endangered population of the European wildcat (Felis silvestris silvestris) in Scotland. Mammal Res 61:1–11
Kozená I (1990) Contribution to the food of wild cats (Felis silvestris). Folia Zool 39:207–212
Krauze-Gryz D, Gryz J, Goszczyński J (2012) Predation by domestic cats in rural areas of central Poland: an assessment based on two methods. J Zool 288:260–266
Krebs CJ (1989) Ecological methodology. Harper Collins, New York
Lanszki J, Kletečki E, Trócsányi B, Mužinić J, Széles GL, Purger JJ (2016) Feeding habits of house and feral cats (Felis catus) on small Adriatic islands (Croatia). North-West J Zool 12:336–348
Lepczyk CA, Flather CH, Radeloff VC, Pidgeon AM, Hammer RB, Liu J (2008) Human impacts on regional avian diversity and abundance. Conserv Biol 22:405–416
Levins R (1968) Evolution in changing environments: some theoretical explorations (No. 2). Princeton Univ Press, Princeton
Liberg O (1984) Food habits and prey impact by feral and house-based domestic cats in a rural area in southern Sweden. J Mammal 65:424–432
Liberg O, Sandell M (1988) Spatial organisation and reproductive tactics in the domestic cat and other felids. In: Turner DC, Bateson P (eds) The domestic cat: the biology of its behaviour. Cambridge Univ Press, Cambridge, pp 83–98
Loss SR, Will T, Marra PP (2013) The impact of free-ranging domestic cats on wildlife of the United States. Nat Commun 4:1396
Lowe S, Browne M, Boudjelas S, De Poorte M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. IUCN, Gland
Loyd KAT, Hernandez SM, Carroll JP, Abernathy KJ, Marshall GJ (2013) Quantifying free-roaming domestic cat predation using animal-borne video cameras. Biol Conserv 160:183–189
Lozano J, Virgós E, Malo AF, Huertas DL, Casanovas JG (2003) Importance of scrub-pastureland mosaics for wild-living cats occurrence in a Mediterranean area: implications for the conservation of the wildcat (Felis silvestris). Biodivers Conserv 12:921–935
Lozano J, Moleón M, Virgós E (2006) Biogeographical patterns in the diet of the wildcat, Felis silvestris Schreber, in Eurasia: factors affecting the trophic diversity. J Biogeogr 33:1076–1085
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Malo AF, Lozano J, Huertas DL, Virgós E (2004) A change of diet from rodents to rabbits (Oryctolagus cuniculus). Is the wildcat (Felis silvestris) a specialist predator? J Zool 263:401–407
Matias R, Catry P (2008) The diet of feral cats at New Island, Falkland Islands, and impact on breeding seabirds. Polar Biol 31:609–616
McDonald RA (2002) Resource partitioning among British and Irish mustelids. J Anim Ecol 71:185–200
McOrist S, Kitchener AC (1994) Current threats to the European wildcat, Felis silvestris, in Scotland. Ambio 23:243–245
Medina FM, Nogales M (2009) A review on the impact of feral cats (Felis silvestris catus) in the Canary Islands: implications for the conservation of its endangered fauna. Biodivers Conserv 18:829–846
Medina FM, Bonnaud E, Vidal E, Tershy BR, Zavaleta ES, Donlan JC, Keitt BS, Le Corre M, Horwath SV, Nogales M (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Chang Biol 17:3503–3510
Millán J (2010) Feeding habits of feral cats Felis silvestris catus in the countryside of Majorca Island, Spain. Wildl Biol Pract 6:32–38
Moleon M, Gil-Sánchez JM (2003) Food habits of the wildcat (Felis silvestris) in a peculiar habitat: the Mediterranean high mountain. J Zool 260:17–22
Niewold FJJ (1986) Voedselkeuze, terreingebruik en aantalsregulatie van in het veld opererende huiskatten Felis catus L., 1758. Lutra 29:145–187
Nogales M, Martín A, Tershy BR, Donlan JC, Veitch D, Puerta N, Wood B, Alonso J (2004) A review of feral cat eradication on islands. Conserv Biol 18:310–319
Ozella L, Cecchetti M, Pessani D (2016) Diet of feral cats during the Scopoli’s shearwater breeding season on Linosa Island, Mediterranean Sea. Ital J Zool 83:589–599
Pearre S, Maass R (1998) Trends in the prey size-based trophic niches of feral and house cats Felis catus L. Mammal Rev 28:125–139
Peck DR, Faulquier L, Pinet P, Jaquemet S, Le Corre M (2008) Feral cat diet and impact on sooty terns at Juan de Nova Island, Mozambique Channel. Anim Conserv 11:65–74
Pianka ER (1973) The structure of lizard communities. Annu Rev Ecol Syst 4:53–74
Pierpaoli M, Biró Z, Herrmann M, Hupe K, Fernandes M, Ragni B, Szemethy L, Randi E (2003) Genetic distinction of wildcat (Felis silvestris) populations in Europe, and hybridization with domestic cats in Hungary. Mol Ecol 12:2585–2598
Randi E, Ragni B (1991) Genetic variability and biochemical systematics of domestic and wildcat populations (Felis silvestris, Felidae). J Mammal 72:79–88
Reynolds JC, Aebischer NJ (1991) Comparison and quantification of carnivore diet by faecal analysis: a critique, with recommendations, based on a study of the fox Vulpes vulpes. Mammal Rev 21:97–122
Sarmento P (1996) Feeding ecology of the European wildcat Felis silvestris in Portugal. Acta Theriol 41:409–414
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Soe E, Davison J, Süld K, Valdmann H, Laurimaa L, Saarma U (2017) Europe-wide biogeographical patterns in the diet of an ecologically and epidemiologically important mesopredator, the red fox Vulpes vulpes: a quantitative review. Mammal Rev 50:59–14
Stahl P, Artois M (1994) Status and conservation of the wildcat (Felis silvestris) in Europe and around the Mediterranean rim. Nature and Environment Series, n° 69. Council of Europe Press, Strasbourg
Tranchant A, Vidal E, Kayser Y (2003) Premieres données sur le régime alimentaire du chat haret Felis catus en situation micro-insulaire Méditerranéenne. Rev Ecol – Terre Vie 58:411–418
Turner DC, Bateson PB (1988) The domestic cat: the biology of its behaviour. Cambridge Univ. Press, Cambridge
Turner DC, Meister O (1988) Hunting behaviour of the domestic cat. In: Turner DC, Bateson P (eds) The domestic cat: the biology of its behaviour. Cambridge Univ Press, Cambridge, pp 111–121
Weber J-M, Dailly L (1998) Food habits and ranging behaviour of a group of farm cats (Felis catus) in a Swiss mountainous area. J Zool 245:234–237
Woods M, McDonald RA, Harris S (2003) Predation of wildlife by domestic cats Felis catus in Great Britain. Mammal Rev 33:174–188
Yamaguchi N, Kitchener A, Driscoll C, Nussberger B (2015) Felis silvestris. In: IUCN 2015. The IUCN red list of threatened species. e.T60354712A50652361. http://www.iucnredlist.org. Accessed 22 Apr 2017
Zhou YB, Newman C, Xu WT, Buesching CD, Zalewski A, Kaneko Y, Macdonald DW, Xie ZQ (2011) Biogeographical variation in the diet of Holarctic martens (genus Martes, Mammalia: Carnivora: Mustelidae): adaptive foraging in generalists. J Biogeogr 38:137–147
Acknowledgements
Thanks to Grace Yoxon for revising the English of the manuscript and two reviewers for helpful advice and comments on early drafts of the manuscript. This study was supported by the EFOP-3.6.1-16-2016-00007 project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
This article does not contain any studies with animals performed by any of the authors.
Additional information
Communicated by: Krzysztof Schmidt
Electronic supplementary material
ESM 1
(DOC 351 kb).
Rights and permissions
About this article
Cite this article
Széles, G.L., Purger, J.J., Molnár, T. et al. Comparative analysis of the diet of feral and house cats and wildcat in Europe. Mamm Res 63, 43–53 (2018). https://doi.org/10.1007/s13364-017-0341-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-017-0341-1