Abstract
We previously reported that short-wavelength visible light (blue light: 400–500 nm) has a lethal effect on various insect species and that the most toxic wavelength to the pupae of the hygiene pest, the mosquito, Culex pipiens form molestus Forskål (Diptera: Culicidae), is 417 nm. However, previous reports on Drosophila melanogaster Meigen (Diptera: Drosophilidae), and Galerucella grisescens (Joannis) (Coleoptera: Chrysomelidae) demonstrated that the most harmful wavelengths of blue light differed among different developmental stages. The most toxic wavelengths to the developmental stages of C. pipiens f. molestus, besides the pupal stage, remain unclear. We investigated the lethal effect of various wavelengths of the blue-light spectrum on the eggs, larvae, and adults of C. pipiens f. molestus using light-emitting diodes (LEDs). Blue light irradiation had a lethal effect on all life stages tested. Furthermore, our results reaffirmed the results of previous studies, where 417 nm light had a strong effect on all life stages. To our knowledge, this is the first report of an insect species where the most effective wavelength does not vary among developmental stages. In addition, our findings indicate that ~ 420 nm is the most promising wavelength to control C. pipiens f. molestus populations using blue-light irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosquitoes are transporters and transmitters of a variety of diseases and are the most important hygiene pests worldwide. Currently, mosquitoes are mainly controlled by chemical insecticides. However, insecticide resistance has been observed in several mosquito species (Casimiro et al. 2006; Hamdan et al. 2005; Kasai et al. 2007). Therefore, alternative control methods are required.
Culex pipiens form molestus Forskål (Diptera, Culicidae) is an urban mosquito that occurs in underground areas (Byrne and Nichols 1999). Culex pipiens f. molestus is an autogenous and strongly anthropophilic species (Byrne and Nichols 1999; Osório et al. 2014; Vinogradova 2003) that mediates West Nile, Japanese encephalitis, and Rift Valley fever viruses (Tahori et al. 1955; Turell et al. 2006; Zakhia et al. 2018). This species does not diapause and is active throughout year (Nelms et al. 2013; Vinogradova 2003), and can, thus, potentially transmit viruses year-round. Therefore, C. pipiens f. molestus is one of the most important hygiene mosquito species.
Recently, light-based pest control techniques have attracted attention as alternative methods to insecticides. For example, the phototaxis of insects (i.e., attraction to light sources) has been used in pest control methods, such as light traps and electric insect killers. Nighttime lighting with yellow lamps has also been used to suppress the behaviors of fruit-piercing moths (Meyer-Rochow 1974; Nomura 1966; Nomura et al. 1965). Recently, red light has been shown to inhibit the attraction of thrips to host plants (Katai et al. 2015; Murata et al. 2018a, b). Despite the numerous light-based insect-pest control techniques available, most of these techniques involve the behavioral responses of insects to a light source.
Over the last 5 decades, numerous studies have reported the lethal effects of ultraviolet C (100–280 nm), and B (280–315 nm) on insects (Cohen et al. 1973; Ghanem and Shamma 2007; Gingrich 1975; Okamoto 1989, 1992; Suzuki et al. 2014). Recently, we found that short-wavelength visible light (blue light: 400–500 nm) has lethal effects on Drosophila melanogaster Meigen (Diptera: Drosophilidae), C. pipiens f. molestus, Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae), and Galerucella grisescens (Joannis) (Coleoptera: Chrysomelidae) (Hori et al. 2014; Hori and Suzuki 2017; Shibuya et al. 2018). These studies showed that the most toxic wavelengths differ among the developmental stages as well as among insect species. In a previous study, we revealed that the most toxic wavelength to C. pipiens f. molestus pupae is ~ 420 nm and that this blue-light wavelength is also lethal to the eggs (Hori et al. 2014). However, the most lethal wavelength to the eggs, and the lethal effects of blue light on larvae and adults remain unclear. Such information is necessary for the development of effective control methods of C. pipiens f. molestus mosquitoes using blue light.
Here, we investigated the lethal effect of blue light on the eggs, larvae, and adults of C. pipiens f. molestus to clarify the most toxic wavelengths on the developmental stages of this species of mosquitoes.
Materials and methods
Insects
Culex pipiens f. molestus mosquitoes were provided by Earth Chemical Co., Ltd. (Tokyo, Japan) and maintained in our laboratory. Eggs, larvae, and pupae were reared in a plastic container (150 mm diameter × 91 mm height) containing 250 mL of tap water decalcified for 48 h, with a constant supply of fishery feed for trout juveniles (EC 1C, Feed One Co., Ltd., Yokohama, Japan). Adults were maintained in a plastic cage (340 × 250 × 340 mm) containing an Erlenmeyer flask (50 mL), and a plastic cup (30 mm diameter × 35 mm height). Cotton wool soaked with 10% honey solution (50 mL) was placed in the flask (food substitute) and cotton wool soaked with water was placed in the cup (oviposition substrate). Adult females were never fed blood because of the autogenic nature of this species. All stages were maintained in a constant-temperature room at 25 ± 1 °C and ~ 60% relative humidity under a 16 h:8 h (L:D) photoregime.
Light-emitting diode (LED) light radiation
LED lighting units (IS-mini®, ISL-150 × 150 Series; CCS Inc., Kyoto, Japan; light emission surface: 150 × 150 mm; arrangement: 360 LEDs were equally arranged on a panel; LED type: ϕ 3 mm plastic mold) with power supply units (ISC-201–2; CCS Inc., Kyoto, Japan) were used for light radiation. Insects were irradiated with LED light in a multi-room incubator (LH-30CCFL-8CT; Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan). The emission spectra and the number of photons (photons∙m−2∙s−1) were measured using a high-resolution spectrometer (HSU-100S; Asahi Spectra Co., Ltd., Tokyo, Japan; numerical aperture of the fiber: 0.2) in a dark room. The number of photons was adjusted by current control using the power supply unit. During measurements, the distance between the LED lighting unit and the spectrometer sensor was set to be approximately equal to the same distance between the insects and the LED lighting unit in the incubator. Since the mosquitoes were irradiated through a glass plate or glass lid, the same plate or lid was placed between the light source and sensor during measurement. The number of photons was measured five times before and after the experiments. The average values of the number of photons in each experiment are shown in Tables S1–S3. Comparisons of the emission spectra used in the experiments are shown in Fig. S1.
Lethal effect of blue-light irradiation on the egg stage of C. pipiens f. molestus
Eggs were collected from the stock cultures within 1 h of deposition. Eggs (n = 30) were placed in water (50 mL) in polyethylene-terephthalate ice-cream cups (60 mm diameter × 38 mm height, Risupack, Co., Ltd., Aichi, Japan), which were each covered with a glass plate. Cups were placed in the multi-room incubator (25 ± 1 °C) and irradiated with LED light at wavelengths of 375 (UVA), 406, 417, 427, 438, 452, 467, 493, or 507 nm (blue green) with set values of 7.5 × 1018 photons∙m−2∙s−1, 10 × 1018 photons∙m−2∙s−1, or 15 × 1018 photons∙m−2∙s−1 for 48 h. The set values were the same as the tests for the pupae of the mosquito in our previous report (Hori et al. 2014). After irradiation, the cups were maintained under continuous dark (DD) for 72 h (25 ± 1 °C). The number of surviving hatchlings (larvae) was then counted to determine the total mortalities of eggs and hatchlings because of the difficulty in counting unhatched eggs and dead hatchlings. In the control treatment, mosquitoes were maintained under DD (i.e., no irradiation) for 120 h, and the total mortalities were determined using the same method. Ten replications (cups) were performed for each light dose of each wavelength.
Lethal effect of blue-light irradiation on the larval to pupal stages of C. pipiens f. molestus
Hatchlings were collected from the stock cultures within 12 h of hatching. Hatchlings (n = 10) were maintained in water in cups under the same conditions described above. Fishery feed for trout juveniles (EC 1C, Feed One Co., Ltd., Yokohama, Japan) (0.01 g) was supplied in the cups on the first day of irradiation and subsequently, 0.03 g was added every 5 days. The cups, which were each covered with a glass plate, were placed in a multi-room incubator and irradiated with LED light at the same wavelengths described above, with set values of 5 × 1018 photons∙m−2∙s−1, 10 × 1018 photons∙m−2∙s−1, or 15 × 1018 photons∙m−2∙s−1. In the larval to pupal test, a relatively high lethal effect was obtained at 10 × 1018 photons∙m−2∙s−1, and therefore, the effect at 5 × 1018 photons∙m−2∙s−1, but not 7.5 × 1018 photons∙m−2∙s−1, was investigated. The larvae in the cups were irradiated until they had all died or developed to the adult stage. Similarly, the number of pupated larvae and emerging adults were counted to calculate the larval and pupal mortalities, respectively. In the control treatment, mosquitoes were maintained under DD (no irradiation) until they had all died or developed to the adult stage, after which the total mortalities were determined using the method described previously. Ten replications (cups) were performed for each light dose of each wavelength.
Lethal effect of blue-light irradiation on the adult stage of C. pipiens f. molestus
Adults were collected from the plastic rearing boxes within 12 h of emergence. Pairs of adults (n = 5 pairs) were released onto a circular cotton pads (10 g, diameter 90 mm) soaked with 5% honey solution (10 mL) in a glass Petri dish (60 mm diameter × 90 mm height). The Petri dish was placed in the multi-room incubator equipped with the LED lighting unit. The adults were irradiated with different wavelengths of light at 25 ± 1 °C until all of them died. We counted the number of dead adults every day and we replaced the Petri dish containing the honey water every 2 days. Lethal effects of irradiation at the set values of 10 and 15 × 1018 photons∙m−2∙s−1 were compared among the nine wavelengths [375 nm (UVA), 406, 417, 427, 438, 452, 467, 493, and 507 nm (blue green)]. In the adult test, a high lethal effect was not obtained even at 15 × 1018 photons∙m−2∙s−1, and the effect at 7.5 × 1018 photons∙m−2∙s−1 was, therefore, not investigated. In the control treatment, the longevities of the mosquitoes maintained under DD (no irradiation) were determined. The lethal effect was determined using the reduction rate of longevity (RRL). The RRL was calculated as follows:
where, LD and LI represent the average longevities of nonirradiated and irradiated adults, respectively. Fifty replications (five adult’s × ten Petri dishes) were performed for each light dose of each wavelength for each sex.
Statistical analysis
Comparisons of mortalities and RRLs between irradiations and control treatments, and among wavelengths were analyzed using the Steel and Steel–Dwass tests, respectively. The calculations were performed using R version 4.0.2. (R Development Core Team 2020).
Results
Lethal effect of blue-light irradiation on the egg stage of C. pipiens f. molestus
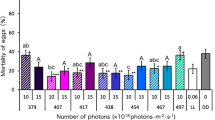
Irradiation at wavelengths of 375, 417, 427, 438, 467, and 507 nm at 10 or 15 × 1018 photons∙m−2∙s−1 during the egg stage significantly increased the mortalities of eggs and hatchlings (Fig. 1). The strongest lethal effect was observed after irradiation with 417 nm blue light at 10 and 15 × 1018 photons∙m−2∙s−1 (92.0 and 99.7% mortality, respectively). In these photon flux densities, the lethal effect of 417 nm blue light was significantly stronger than that of 375 nm UVA light (34.3 and 56.7% mortality at 10 and 15 × 1018 photons∙m−2∙s−1, respectively). Although 467 nm blue light also had a strong lethal effect at 15 × 1018 photons∙m−2∙s−1 (92.7% mortality), it did not show the same lethal effect at 10 × 1018 photons∙m−2∙s−1 (11.7% mortality). The lethal effect of 427 nm blue light was relatively strong at 10 × 1018 photons∙m−2∙s−1 (71.3% mortality), and at 15 × 1018 photons∙m−2∙s−1 (75.0% mortality). The 438 nm blue light only had a lethal effect at 15 × 1018 photons∙m−2∙s−1 (36.3% mortality). The other wavelengths of blue light did not have a lethal effect. At 7.5 × 1018 photons∙m−2∙s−1, only the 375 nm UVA light had a lethal effect on the mosquitoes (54.0% mortality). However, a dose–response relationship was not observed. Although the effect of 507 nm blue–green light at 15 × 1018 photons∙m−2∙s−1 was not investigated because the maximum photon flux density of the unit of this wavelength was low, the 507 nm light did have a lethal effect at 10 × 1018 photons∙m−2∙s−1 (38.0% mortality).
Mortality of Culex pipiens f. molestus irradiated with blue light during the egg stage. Data represent the means ± standard errors. Asterisks above the bars indicate significant differences between the treatments (irradiation) and control [dark condition (DD)] (Steel test: **p < 0.01, ***p < 0.001). Bars with same letters are not significantly different (Steel–Dwass test, p > 0.05). Ten replications (30 eggs per replicate) were conducted
Lethal effect of blue-light irradiation on the larval to pupal stages of C. pipiens f. molestus
Irradiation with 375 nm UVA light and the 406–467 nm blue light during the larval to pupal stages had significant lethal effects at 10 or 15 × 1018 photons∙m−2∙s−1 (Fig. 2). Irradiation with 417 nm blue light had the strongest lethal effect with 100% larval mortality at 15 × 1018 photons∙m−2∙s−1, 94.0% total (total of larval and pupal) and 81.0% larval mortality at 10 × 1018 photons∙m−2∙s−1, and 33.0% total and 19.0% larval mortality at 5 × 1018 photons∙m−2∙s−1. Irradiation with 427 and 467 nm blue light also resulted in 100% larval mortality at 15 × 1018 photons∙m−2∙s−1. However, the 467 nm blue light did not have significant lethal effect on larvae at 10 × 1018 photons∙m−2∙s−1 (Fig. S2). Irradiation with 406 nm blue light also exhibited a strong lethal effect at 15 × 1018 photons∙m−2∙s−1 (98.0% larval mortality), but a mild effect at 10 × 1018 photons∙m−2∙s−1 (29.0% larval mortality). Irradiation with 493 nm blue-light did not have any lethal effect (16.0% and 11.0% total mortality at 10 and 15 × 1018 photons∙m−2∙s−1, respectively). Although the 375 nm UVA light had a strong lethal effect at 10 and 15 × 1018 photons∙m−2∙s−1 (76.0% total and 100% larval mortality, respectively), it did not have a significant effect at 5 × 1018 photons∙m−2∙s−1 (14.0% total mortality).
Mortality of Culex pipiens f. molestus irradiated with blue light during the larval to pupal stages. Data represent the means ± standard errors. Asterisks above the bars indicate significant differences between the treatments (irradiation) and control [dark condition (DD)] (Steel test: **p < 0.01, ***p < 0.001). Bars with the same letters are not significantly different (Steel–Dwass test, p > 0.05). Ten replications (10 larvae per replicate) were conducted
Lethal effect of blue-light irradiation on the adult stage of C. pipiens f. molestus
The longevities of nonirradiated male and female adults were 20.9 and 32.7 days, respectively (Fig. S3). Compared with nonirradiated adults, the longevities of both male and female adults were reduced by irradiation with all tested wavelengths of lights (Steel test, p < 0.001) (Fig. 3). The RRLs were similar between males and females. The RRLs under the 375–467 nm treatments were higher than those under the 493 and 507 nm treatments. The RRLs of males irradiated with 375 to 467 nm light were 67.9–70.6% and 73.8–80.2% at 10 and 15 × 1018 photons∙m−2∙s−1, respectively, whereas those at 493 and 507 nm were 41.4–48.6% and 51.0% at 10 and 15 × 1018 photons∙m−2∙s−1, respectively (Fig. 3a). The RRLs of females irradiated with 375–467 nm light were 71.2–75.9% and 78.3–83.8% at 10 and 15 × 1018 photons∙m−2∙s−1, respectively, whereas those with 493 and 507 nm were 47.9–52.8% and 54.9% at 10 and 15 × 1018 photons∙m−2∙s−1, respectively (Fig. 3b).
Reduction rate of longevity (RRL) of Culex pipiens f. molestus adults irradiated with blue light. RRLs of males (a) and females (b). Data represent the means ± standard errors. Significant differences were obtained for all wavelengths between the treatments (irradiation) and control (dark condition) (Steel test: p < 0.001). Bars with same letters are not significantly different (Steel–Dwass test, p > 0.05). Fifty replications (five adult’s × ten Petri dishes) were conducted for each light dose of each wavelength for each sex
Discussion
In the present study, we revealed that blue-light irradiation during the egg, larval, and adult stages can kill C. pipiens f. molestus. Although our study further confirmed our previous findings that blue-light irradiation has a lethal effect on C. pipiens f. molestus pupae (Hori et al. 2014), we also demonstrated that blue-light irradiation has a lethal effect on all developmental stages of C. pipiens f. molestus.
The most toxic wavelength of blue light to C. pipiens f. molestus pupae was previously found to be 417 nm (Hori et al. 2014). In the present study, the 417 nm wavelength was not only confirmed to be the most toxic wavelength to C. pipiens f. molestus pupae, but also to the eggs and larvae of this species. In D. melanogaster and G. grisescens, effective lethal wavelengths were shown to differ among insect growth stages (Hori and Suzuki 2017; Shibuya et al. 2018). Therefore, changes in the effective wavelengths of blue light among insect growth stages may be species dependent.
Previously, we indicated that highly toxic wavelengths of blue light are species- and growth stage-specific in insects (Hori et al. 2014; Shibuya et al. 2018). In addition, we showed that reactive oxygen species (ROS) may induce the lethal effects of blue light on insects (Shibuya et al. 2018). In the primary retinal cells of mice, cell injury by blue-light irradiation was shown to be induced by ROS (Kuse et al. 2014). Therefore, we propose that the highly lethal effects of specific wavelengths of blue light are the result of the ROS generated by the absorption of specific wavelengths of light by the species, and that this absorption potential can vary according to the growth stage-specific photoreceptive parts in insect tissues (Hori et al. 2014; Shibuya et al. 2018). In addition to previous studies, the present study demonstrated that specific wavelengths of blue light can exhibit especially high toxicity effects on the eggs, larval, and pupal stages of C. pipiens f. molestus. However, in the adult stage, no specific effective wavelength of blue light was identified. It is possible that continuous lighting may influence the flight behavior of adult insects, which may account for the observed reduction in the longevity of C. pipiens f. molestus adults. However, the RRLs under 375–467 nm light were significantly higher than those under 493 and 507 nm light, and the RRLs increased with increasing photon density. Similar to our findings regarding C. pipiens f. molestus adults, Shibuya et al. (2018) reported that no specific wavelength of blue light had a lethal effect on the eggs and larvae of D. melanogaster. Therefore, the reduction in the longevity of adult mosquitoes is presumably not due to the influence on their flight behavior. The photoreceptive parts that absorb a broad (not specific) range of wavelengths, in addition to the amount of photon energy and transmittance of wavelength, might be involved in the lethal effects on C. pipiens f. molestus adults, and the eggs and larvae of D. melanogaster.
In C. pipiens f. molestus, the biological implications of different action spectra between the adults and the other stages are unclear. However, the differences in habitats between adult and the other stages may be involved in the different action spectra. Adult mosquitoes live on the ground whereas egg to pupal stages exist near the surface of water. Elucidation of the mechanisms of the lethal effect of blue light is necessary to clarify the biological implications.
Irradiation with 507 nm blue–green light at 10 × 1018 photons∙m−2∙s−1 had a significant lethal effect on the eggs of C. pipiens f. molestus. The eggs may have photoreceptive tissue that is able to absorb relatively high amounts of light at ~ 507 nm wavelength. It is necessary to investigate the lethal effect of light with wavelengths longer than 507 nm.
The present study demonstrated that light irradiation at ~ 420 nm at 10 × 1018 photons∙m−2∙s−1 or higher was effective in killing the eggs, larvae, and pupae of C. pipiens f. molestus. Culex pipiens f. molestus often occurs in water storage containers and septic tanks (Moriya et al. 1967; Noguchi 1962; Noguchi et al. 1965). The eggs of this species form rafts and float on the surface of the water (Harbach et al. 1984; Kassim et al. 2012), and the larvae and pupae also breathe at the water surface using respiratory siphons. Therefore, irradiation of the water surface with ~ 420 nm blue light may be used to kill the eggs, larvae, and pupae of C. pipiens f. molestus mosquitoes and thus suppress the occurrence of this hygiene pest to reduce associated viral transmissions (e.g., West Nile virus, and Japanese encephalitis virus). Since blue light has been shown to also have a lethal effect on various insect species such as D. melanogaster, T. confusum, and G. grisescens (Hori et al. 2014; Hori and Suzuki 2017), it is possible that blue-light irradiation may be effective in controlling populations of other mosquito species besides C. pipiens f. molestus. In the near future, we aim to examine the efficacies of ~ 420 nm blue light against C. pipiens f. molestus under field conditions. The control of mosquitoes using the lethal effect of blue light is a promising, safe and clean technique and is an alternative to the use of pesticides.
References
Byrne K, Nichols RA (1999) Culex pipiens in London underground tunnels: differentiation between surface and subterranean populations. Heredity 82:7–15. https://doi.org/10.1038/sj.hdy.6884120
Casimiro S, Coleman M, Mohloai P, Hemingway J, Sharp B (2006) Insecticide resistance in Anopheles funestus (Diptera: Culicidae) from Mozambique. J Med Entomol 43:267–275. https://doi.org/10.1093/jmedent/43.2.267
Cohen SH, Sousa JA, Roach JF (1973) Effects of UV irradiation on nymphs of five species of cockroaches. J Econ Entomol 66:859–862. https://doi.org/10.1093/jee/66.4.859
Ghanem I, Shamma M (2007) Effect of non-ionizing radiation (UVC) on the development of Trogoderma granarium Everts. J Stored Prod Res 43:362–366. https://doi.org/10.1016/j.jspr.2006.09.002
Gingrich JB (1975) Ultraviolet-induced histological and histochemical changes in the integument of newly molted American cockroaches, Periplaneta americana (Dictyoptera: Blattaria: Blattidae). Can J Zool 53:154–159. https://doi.org/10.1139/z75-018
Hamdan H, Sofian-Azirun M, Nazni WA, Lee HL (2005) Insecticide resistance development in Culex quinquefasciatus (Say), Aedes aegypti (L.) and Aedes albopictus (Skuse) larvae against malathion, permethrin and temephos. Trop Biomed 22:45–52
Harbach RE, Harrison BA, Gad AM (1984) Culex (Culex) molestus Forskål (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proc Entomol Soc Wash 86:521–542
Hori M, Shibuya K, Sato M, Saito Y (2014) Lethal effects of short-wavelength visible light on insects. Sci Rep 4:7383. https://doi.org/10.1038/srep07383
Hori M, Suzuki A (2017) Lethal effect of blue light on strawberry leaf beetle, Galerucella grisescens (Coleoptera: Chrysomelidae). Sci Rep 7:2697. https://doi.org/10.1038/s41598-017-03017-z
Kasai S, Shono T, Komagata O, Tsuda Y, Kobayashi M, Motoki M, Kashima I, Tanikawa T, Yoshida M, Tanaka I, Shinjo G, Hashimoto T, Ishikawa T, Takahashi T, Higa Y, Tomita T (2007) Insecticide resistance in potential vector mosquitoes for West Nile virus in Japan. J Med Entomol 44:822–829. https://doi.org/10.1093/jmedent/44.5.822
Kassim NFA, Webb CE, Russell RC (2012) Culex molestus Forskal (Diptera: Culicidae) in Australia: colonisation, stenogamy, autogeny, oviposition and larval development. Aust J Entomol 51:67–77. https://doi.org/10.1111/j.1440-6055.2011.00834.x
Katai Y, Ishikawa R, Doi M, Masui S (2015) Efficacy of red LED irradiation for controlling Thrips palmi in greehouses melon cultivation. Jpn J Appl Entomol Zool 59:1–6. https://doi.org/10.1303/jjaez.2015.1 (in Japanese with English summary)
Kuse Y, Ogawa K, Tsuruma K, Shimazawa M, Hara H (2014) Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci Rep 4:5223. https://doi.org/10.1038/srep05223
Meyer-Rochow VB (1974) Fine structural changes in dark-light adaptation in relation to unit studies of an insect compound eye with a crustacean-like rhabdom. J Insect Physiol 20:573–589. https://doi.org/10.1016/0022-1910(74)90164-4
Moriya K, Harada F, Yabe T (1967) Some observations on mosquitoes occurring in the septic tanks in Kanagawa prefecture II. Jpn J Sanit Zool 18:247–255. https://doi.org/10.7601/mez.18.247 (in Japanese with English abstract)
Murata M, Hariyama T, Yamahama Y, Toyama M, Ohta I (2018a) Effects of the range of light wavelengths on the phototactic behaviour and biological traits in the melon thrips, Thrips palmi karny (Thysanoptera Thripidae). Ethol Ecol Evol 20:101–113. https://doi.org/10.1080/03949370.2017.1320688
Murata M, Hariyama T, Yamahama Y, Toyama M, Ohta I (2018b) In the presence of red light, cucumber and possibly other host plants lose their attractability to the melon thrips Thrips palmi (Thysanoptera: Thripidae). Appl Entmol Zool 53:117–128. https://doi.org/10.1007/s13355-017-0537-5
Nelms BM, Kothera L, Thiemann T, Macedo PA, Savage HM, Reisen WK (2013) Phenotypic variation among Culex pipiens complex (Diptera: Culicidae) populations from the Sacramento Valley, California: horizontal and vertical transmission of West Nile virus, diapause potential, autogeny, and host selection. Am J Trop Med Hyg 89:1168–1178. https://doi.org/10.4269/ajtmh.13-0219
Noguchi Y (1962) Studies on the autogenous Culex mosquitoes of Japan 1. Mosquitoes prevalent during early winter season in Tokyo area. Jpn J Sanit Zool 13:185–189. https://doi.org/10.7601/mez.13.185 (in Japanese with English abstract)
Noguchi Y, Ogata K, Kazama T, Imai S (1965) Seasonal prevalence of “Culex molestus” breeding in “septic tanks.” Jpn J Sanit Zool 16:133–137. https://doi.org/10.7601/mez.16.133 (in Japanese with English abstract)
Nomura K (1966) Some considerations on the effect of orchard illumination against fruit-piercing moths. Tech Bull Fac Hort Chiba Univ 14:27–34 (in Japanese with English summary)
Nomura K, Oya S, Watanabe I, Kawamura H (1965) Studies on orchard illumination against fruit-piercing moths. Jpn J Appl Entmol Zool 9:179–186. https://doi.org/10.1303/jjaez.9.179 (in Japanese with English summary)
Okamoto K (1989) Test for cockroach control with UV radiation: 1. Entrance of cockroaches into the UV radiation field of germicidal lamps. Jpn J Sanit Zool 40:259–267. https://doi.org/10.7601/mez.40.259
Okamoto K (1992) The lethal effect of UV radiation on the adult German cockroach: 1. Difference in the lethal effect by irradiation regimes. Jpn J Sanit Zool 43:235–241. https://doi.org/10.7601/mez.43.235
Osório HC, Zé-Zé L, Amaro F, Nunes A, Alves MJ (2014) Sympatric occurrence of Culex pipiens (Diptera, Culicidae) biotypes pipiens, molestus and their hybrids in Portugal, Western Europe: feeding patterns and habitat determinants. Med Vet Entomol 28:103–109. https://doi.org/10.1111/mve.12020
R Development Core Team (2020) The R project for statistical computing. http://www.r-project.org. Accessed 26 Aug 2020
Shibuya K, Onodera S, Hori M (2018) Toxic wavelength of blue light changes as insects grow. PLoS ONE 13:e0199266. https://doi.org/10.1371/journal.pone.0199266
Suzuki T, Yoshioka Y, Tsarsitalidou O, Ntalia V, Ohno S, Ohyama K, Kitashima Y, Gotoh T, Takeda M, Koveos DS (2014) An LED-based UV-B irradiation system for tiny organisms: system description and demonstration experiment to determine the hatchability of eggs from four Tetranychus spider mite species from Okinawa. J Insect Physiol 62:1–10. https://doi.org/10.1016/j.jinsphys.2014.01.005
Tahori AS, Strek VV, Goldblum N (1955) Studies on the dynamics of experimental transmission of West Nile virus by Culex molestus. Am J Trop Med Hyg 4:1015–1027. https://doi.org/10.4269/ajtmh.1955.4.1015
Turell MJ, Mores CN, Dohm DJ, Komilov N, Paragas J, Lee JS, Shermuhemedova D, Endy TP, Kodirov A, Khodjaev S (2006) Laboratory transmission of Japanese encephalitis and West Nile viruses by molestus form of Culex pipiens (Diptera: Culicidae) collected in Uzbekistan in 2004. J Med Entomol 43:296–300. https://doi.org/10.1093/jmedent/43.2.296
Vinogradova EB (2003) Ecophysiological and morphological variations in mosquitoes of the Culex pipiens complex (Diptera: Culicidae). Acta Soc Zool Bohem 67:41–50
Zakhia R, Mousson L, Vazeille M, Haddad N, Failloux AB (2018) Experimental transmission of West Nile virus and Rift Valley fever virus by Culex pipiens from Lebanon. PLOS Negl Trop Dis 12:e0005983. https://doi.org/10.1371/journal.pntd.0005983
Acknowledgements
We wish to thank Earth Chemical Co., Ltd. for providing cultures of C. pipiens f. molestus. This study was supported by Japan Society for the Promotion of Science (JSPS) through KAKENHI Grant Number 25660261, 17K19254, and 18H03946, and the JSPS Core-to-Core Program (Advanced Research Networks), titled “Establishment of international agricultural immunology research-core for a quantum improvement in food safety”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Taniyama, K., Saito, Y. & Hori, M. Lethal effect of blue light on the developmental stages of the urban mosquito, Culex pipiens form molestus (Diptera: Culicidae). Appl Entomol Zool 56, 319–325 (2021). https://doi.org/10.1007/s13355-021-00737-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-021-00737-7