Abstract
We previously reported that blue light is lethal to various insect species. However, it was also revealed that effective blue light wavelength is species and growth-stage specific. We, therefore, investigated the lethal effects of blue light on booklice, Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae), which frequently occur in food processing and storage facilities, where insecticides cannot often be used because of the risk of their contamination on the food products. Liposcelis bostrychophila eggs were killed by irradiation with 408–462-nm blue light and 378-nm UVA at 5 × 1018 photons·m−2·s−1, with 100% mortality. In particular, 420-nm blue light had a strong lethal effect, showing 96.5% mortality at 1.5 × 1018 photons·m−2·s−1. The adults were killed by irradiation with 378–494-nm light at 5 × 1018 photons·m−2·s−1. Irradiation with 378–440-nm and 462-nm light showed 96%–100% mortality at this photon flux density. In particular, 378 and 408-nm light notably exhibited strong lethal effects, showing 100% and 87% mortality, respectively, at 3 × 1018 photons·m−2·s−1. These results show that blue light irradiation is useful for controlling booklice occurrence in food facilities. Additionally, this study revealed for the first time that blue-light irradiation is lethal to hemimetabolous insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Booklice, Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae), frequently occur in food processing and storage facilities, causing significant damage to stored commodities (Ahmedani et al. 2010). However, insecticides cannot often be used in these facilities because of contamination risk to the food products. In addition, stored product insect pests including the booklice, usually inhabit inside equipment such as food processing machines and switchboards, as well as in narrow spaces under the machines (Bell and Edwards 1999). It is, therefore, difficult to constantly recognize and effectively control their occurrence. Presently, insect pests in these facilities are controlled mainly by sanitation to remove their infestation source. However, sanitation is time consuming and labor intensive. Thus, there is a need to develop safer and cleaner alternatives such as physical pest control methods.
In recent years, the use of light for pest control has been studied along with the development and popularization of light-emitting diodes (LEDs). Light has been used for controlling insect pests for many years, given the attraction of insects to light sources and suppression of their nocturnal activities by yellow light (Shimoda and Honda 2013). Most practical light techniques utilize insect behavioral reactions to light and do not directly kill them (Hori 2016). The use of ultraviolet B (UVB, 280–315 nm) as a control technique in spider mites to directly kill them is currently in the preliminary stage (Tanaka et al. 2016). The lethal effect of ultraviolet C (UVC, 100–280 nm) on insects has been reported (Beard 1972; Bruce 1975; Calderon et al. 1985; Faruki et al. 2007; Ghanem and Shamma 2007; Nakajima and Yoshida 1971; Wharton 1971); however, UVC is yet to be practically used on them.
UVB and UVC are highly toxic to organisms, because they are absorbed by the DNA, thereby directly damaging it (Beggs 2002; Pfeifer 1997). Therefore, it is difficult to use UVB and UVC to kill insect pests in food processing and storage facilities because of the risks of safety hazards posed to humans. The toxicity of UVA (315–400 nm) is much lower than that of UVB and UVC, because UVA does not directly damage the DNA (Rastogi et al. 2010; Sinha and Häder 2002). Furthermore, no reports, with the exception of a recent report on Dialeurodes citri (Ashmead) (Hemiptera: Aleyrodidae) (Tariq et al. 2015), have described the lethal effects of UVA on insects, except for a slight decrease in adult longevity of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) following UVA irradiation (Zhang et al. 2011). It is well known that light with shorter wavelengths carry higher energies and therefore are more harmful to organisms (Clark 1922; McMillan et al. 2008; Reed 2010). Therefore, it had been considered that visible light (400–780 nm) does not have a lethal effect on complex animals, including insects (Hori et al. 2014). We discovered that short-wavelength visible light (blue light, 400–500 nm) can kill some insect species (Hori et al. 2014). To date, we have confirmed the lethal effect of blue light on more than ten insect species, including the fruit fly, Drosophila melanogaster Meigen (Diptera: Drosophilidae), the confused flour beetle, Tribolium confusum Duval (Coleoptera: Tenebrionidae) (Hori et al. 2014), the strawberry leaf beetle, Galerucella grisescens (Joannis) (Coleoptera: Chrysomelidae) (Hori and Suzuki 2017), the urban mosquito Culex pipiens pipiens form molestus Forskål (Diptera: Culicidae) (Taniyama et al. 2021), and the Asian tiger mosquito, Aedes albopictus (Skuse) (Diptera: Culicidae) (Taniyama and Hori 2022). These studies revealed that the highly toxic wavelengths and effective photon flux density of blue light vary depending on the insect species (Hori et al. 2014). Therefore, it is necessary to identify a highly effective wavelength and photon flux density of blue light for each target insect species to utilize the lethal effects of blue light for pest control in food facilities. Therefore, in this study, we investigated the effective wavelength and photon flux density of blue light to kill L. bostrychophila to develop practical blue light techniques for controlling the species.
Materials and methods
Insects
Liposcelis bostrychophila was supplied by Earth Environmental Service Co., Ltd. (Tokyo, Japan) in 2017 and was maintained in our laboratory. The booklice were reared in a plastic container (137 × 137 × 61 mm) on 50 g of a powdered rodent diet (CE-2, CLEA Japan, Inc., Tokyo, Japan). An absorbent cotton pad (0.2 g) soaked in water (2 mL) was placed in an aluminum foil cup (34 × 32 mm) and kept in the container to maintain a relative humidity of ~ 80%. Water (2 mL) was added to the cotton pad every 4 d. The cup with the cotton pad was replaced with a new one every 7 d. All stages of the booklice were maintained in an incubator (MIR-153, Sanyo Electric Co., Ltd., Osaka, Japan) at 27 ± 1 °C under dark conditions.
Light-emitting diode (LED) light radiation
LED lighting units (IS-mini®, ISL-150 × 150 Series; CCS Inc., Kyoto, Japan; light emission surface: 150 × 150 mm; arrangement: 360 LEDs equally arranged on a panel; LED type: φ 3 mm plastic mold) with power supply units (ISC-201-2; CCS Inc., Kyoto, Japan) were used for light radiation. The insects were irradiated with LED light in a multi-room incubator (LH-30CCFL-8CT; Nippon Medical & Chemical Instruments Co., Ltd., Osaka, Japan). The emission spectra and the light intensity (photons·m−2·s−1) were measured using a high-resolution spectrometer (HSU-100S; Asahi Spectra Co., Ltd., Tokyo, Japan; numerical aperture of the fiber: 0.2) in a dark room. The number of photons was adjusted using a power supply unit. During the measurements, the distance between the LED lighting unit and the spectrometer sensor was set to be approximately equal to the distance between the insects and LED lighting unit in the incubator. Because the tested insects were irradiated through the glass lid of a Petri dish, the same lid was placed between the light source and sensor during measurement. The number of photons was measured five times, and the mean values of each experiment are listed in Tables S1 and S2.
Lethal effect of blue-light irradiation on L. bostrychophila eggs
The eggs of L. bostrychophila were collected from stock cultures within 2 d of deposition. Ten eggs were placed on a sheet of filter paper (ADVANTEC®, No. 1, 55 mm in diameter, Toyo Roshi Kaisha, Ltd., Tokyo, Japan) impregnated with 30 μL of water in a lid of a glass Petri dish (50 mm in diameter) with a small amount (~ 10 mg) of powdered rodent diet (CE-2). The lid, with its base inverted, was placed over the Petri dish and sealed with Parafilm. The Petri dishes were placed in a multi-room incubator (25 ± 1 °C) and irradiated with LED light at peak wavelengths of 378 (UVA), 408, 420, 440, 456, 462, 494, 508 (blue green), 532 (green), or 594 nm (yellow), with set values of 1.5 × 1018 photons·m−2·s−1, 3 × 1018 photons·m−2·s−1, and 5 × 1018 photons·m−2·s−1. The irradiation period was set at 7 d, following which the Petri dishes were maintained under continuous darkness (DD) for 9 d (25 ± 1 °C), because their egg period was approximately 1–2 w (Wang et al. 2000). The number of hatchlings was then counted, and egg mortality was calculated. In the control treatment, the eggs were maintained under DD (i.e., no irradiation), as indicated previously (Hori and Suzuki 2017; Shibuya et al. 2018; Taniyama et al. 2021), at 25 ± 1 °C for 16 d, and mortality was calculated. Ten replicates (Petri dishes) were performed for each light dose of every wavelength. The effects of 494–594 nm light at 1.5 × 1018 photons·m−2·s−1 were not investigated, because their mortalities at 3 × 1018 photons·m−2·s−1 were low. The effects of 532 and 594 nm light at 5 × 1018 photons·m−2·s−1 were also not investigated, because the maximum photon flux density of the unit of these wavelengths was low.
Lethal effect of blue-light irradiation on L. bostrychophila adults
A sheet of filter paper (ADVANTEC®, No. 1, 55 mm in diameter, Toyo Roshi Kaisha, Ltd., Tokyo, Japan) impregnated with 40 μL of water was placed at the bottom of a glass Petri dish lid (50 mm in diameter), and a small amount (~ 10 mg) of powdered rodent diet (CE-2) was placed on the filter paper. Ten adults of L. bostrychophila from 1 to 7 d after eclosion were collected from the stock cultures and released on the filter paper. After releasing the insects, the lid, with its base inverted, was placed over the Petri dish and sealed with Parafilm. The Petri dishes were placed in a multi-room incubator (25 ± 1 °C) and irradiated with LED light at peak wavelengths of 378, 408, 420, 440, 456, 462, 494, 508, 532, or 594 nm, with a set value of 3 × 1018 photons·m−2·s−1 or 5 × 1018 photons·m−2·s−1. The irradiation period was set at 6 d, because in the preliminary tests, 99% mortality was obtained by irradiation with 462-nm blue light at 5 × 1018 photons·m−2·s−1 for 6 d. After irradiation, we counted the number of dead adults and calculated the mortality. In the control treatment, the eggs were maintained under DD (i.e., no irradiation) at 25 ± 1 °C for 6 d, and mortality was calculated. An adult booklouse was regarded as dead if the appendages did not move when touched with a calligraphy brush. Ten replicates (Petri dishes) were performed for each light dose of each wavelength. The effects of 532 and 594 nm light at 5 × 1018 photons·m−2·s−1 were not investigated, because the maximum photon flux density of the unit of these wavelengths was low.
Statistical analysis
Irradiation treatments and controls were compared using the Steel test. Mortality rates in irradiation treatments were corrected using Abbott’s formula (Abbott 1925) and compared among wavelengths using the Steel–Dwass test. Calculations were performed using R version 4.0.3 (R Development Core Team 2020).
Results
Lethal effect of blue-light irradiation on L. bostrychophila eggs
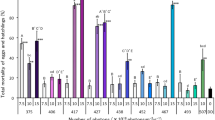
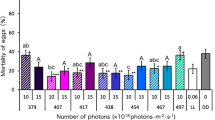
Continuous irradiation with 408–462-nm blue light, as well as 378-nm UVA, at 5 × 1018 photons·m−2·s−1 remarkably increased booklice eggs mortality compared with the control (DD) mortality (Fig. 1). All the eggs were killed by irradiation in the above wavelength range at this photon flux density. In contrast, irradiation with 494 and 508 nm light at this photon flux density did not show a significant lethal effect (p > 0.05). Irradiation with wavelengths of 378–440 nm and 462 nm at 3 × 1018 photons·m−2·s−1 also exhibited a strong lethal effect on the eggs, with more than 90% mortality. More precisely, irradiation with 378–440 nm light resulted in 100% mortality. Irradiation with 420-nm blue light, of which the lethal effect on the eggs was highest among the tested blue light wavelengths, showed 96.5% mortality even at a low photon flux density of 1.5 × 1018 photons·m−2·s−1. The lethal effect of 408-nm blue light on the eggs was also relatively high at low photon flux density, showing 78.8% mortality. The lethal effect of 378-nm UVA was higher than that of any blue light wavelength, showing 100% mortality even at 1.5 × 1018 photons·m−2·s−1.
Comparison of the lethal effects of light irradiation on Liposcelis bostrychophila eggs using various wavelengths of light. Data represent the means ± standard errors. Mortality rates in treatments (irradiation) were corrected using Abbott’s formula (Abbott 1925). Asterisks above the bars indicate significant differences between the treatments and control (DD) (Steel test: * p < 0.05, ** p < 0.01). Mortality under DD was 16.3 ± 1.5 (%). Bars with same letters are not significantly different among the wavelengths (Steel–Dwass test, p > 0.05). Ten replicates (10 eggs per replicate) were maintained
Lethal effect of blue-light irradiation on L. bostrychophila adults
Continuous irradiation with 378–494-nm light at 5 × 1018 photons·m−2·s−1 significantly increased booklouse adult mortality compared with the control (DD) mortality (p < 0.05) (Fig. 2). More precisely, 378–440-nm and 462-nm light showed 96%–100% mortality at this photon flux density. In contrast, irradiation with 508-nm light did not show a significant lethal effect. Irradiation with 378, 408, 420, 456, and 462-nm light showed significant lethal effects, even at 3 × 1018 photons·m−2·s−1 (p < 0.05). Irradiation with 378 and 408-nm light notably exhibited strong lethal effects, showing 100% and 87% mortality, respectively, at this photon flux density.
Comparison of the lethal effects of light irradiation on Liposcelis bostrychophila adults using various wavelengths of light. Data represent the means ± standard errors. Mortality rates in treatments (irradiation) were corrected using Abbott’s formula (Abbott 1925). Asterisks above the bars indicate significant differences between the treatments and control (DD) (Steel test: * p < 0.05, ** p < 0.01). No adult died under DD. Bars with the same letters are not significantly different among the wavelengths (Steel–Dwass test, p > 0.05). Ten replicates (10 adults per replicate) were maintained
Discussion
Liposcelis bostrychophila is a common domestic pest of stored food products that can cause serious damage to products (Turner 1994). In food processing and storage facilities, insecticides cannot often be used because of the risk of contamination of the food products. In addition, the rapid development of chemical resistance by L. bostrychophila has also been reported (Huang et al. 2009; Wang et al. 2004). Because L. bostrychophila is difficult to control using conventional methods, alternative approaches are expected to be developed (Diaz-Montano et al. 2014). Attractants and light attractions have been studied for the development of traps or lures as an alternative approach (Diaz-Montano et al. 2014, 2016; Green and Turner 2005). However, these control methods can be incorporated into integrated pest management for monitoring this pest (Diaz-Montano et al. 2014) but cannot directly kill them. However, the lethal effect of blue light can be useful as a direct killing method for this pest. In previous papers, we reported that photon flux densities of 3.0 × 1018, 15.0 × 1018, and 10.0 × 1018 photons·m−2·s−1 are effective in killing D. melanogaster, G. grisescens, and C. pipiens f. molestus, respectively. The results obtained in this study show that L. bostrychophila eggs can be killed by blue-light irradiation at a relatively low photon flux density, that is, 1.5 × 1018 photons·m−2·s−1, in comparison with that used to kill the above-mentioned pests. It is relatively easy to irradiate the inside of equipment, such as food processing machines and switchboards, with blue light at this photon flux density. We have developed an LED device that can irradiate ~ 50 cm square space with 470-nm blue light at more than 5.0 × 1018 photons·m−2·s−1, to kill small bugs such as D. melanogaster (Hori 2018). This device has already been commercialized and is used in a number of food processing and storage facilities in Japan. It is likely that the occurrence of L. bostrychophila inside the above-mentioned equipment can be prevented using this device.
In previous studies, we demonstrated the lethal effect of blue light on several species of insects, including D. melanogaster and T. confusum (Hori et al. 2014), G. grisescens (Hori and Suzuki 2017), C. pipiens f. molestus (Taniyama et al. 2021), and A. albopictus (Taniyama and Hori 2022). Although these previously studied insects are holometabolous, we revealed for the first time that blue-light irradiation is also lethal to hemimetabolous insects in this study; that is, it is thought that blue-light irradiation is lethal to various species of insects, regardless of the type of metamorphosis.
Currently, we are investigating the mechanisms underlying the lethal effects of blue light in insects. In a previous study, we reported that the amount of H2O2, a reactive oxygen species (ROS), in the whole body of Drosophila pupae was increased by blue light irradiation (Shibuya et al. 2018). In addition, we confirmed that the growth of cultivated cells of Drosophila embryos was suppressed by blue-light irradiation. Furthermore, we revealed that the highly toxic blue light wavelengths are species- and growth stage-specific in insects (Hori et al. 2014; Shibuya et al. 2018). Therefore, we hypothesized that the ROS produced by the absorption of specific blue light wavelengths in insect tissues damage them, thereby killing the insects. Kam et al. (2021) reported that 420-nm blue-light irradiation has a negative effect on the function of mitochondria, which specifically absorbs this wavelength because of the presence of porphyrins, and reduces the climbing mobility of adult Drosophila. In the current study, the effective lethal wavelength for L. bostrychophila eggs had two peaks at 420 and 462 nm in the blue light region. Therefore, under irradiation at ~ 420 nm, lethal effects may result from mitochondrial damage. However, the lethal effect of 408-nm light on adults was higher than that of 420-nm light; additionally, the lethal effect of 462-nm light on eggs and adults was relatively high. Therefore, damage to other sites besides the mitochondria, which absorb 408 and 462-nm blue lights, may have induced the lethal effect on L. bostrychophila. Currently, we are investigating the above absorption sites.
In conclusion, blue light irradiation is a safe and clean technique for killing insect pests. The lethal effect of blue light on insects infesting stored food products is evident in this study. Thus, blue light irradiation could be a useful tool in the integrated pest management of food processing and storage facilities.
Data Availability
The datasets are available from the corresponding author on reasonable request.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267. https://doi.org/10.1093/jee/18.2.265a
Ahmedani MS, Shagufta N, Aslam M, Hussnain SA (2010) Psocid: a new risk for global food security and safety. Appl Entomol Zool 45:89–100. https://doi.org/10.1303/aez.2010.89
Beard RL (1972) Lethal action of UV irradiation on insects. J Econ Entomol 65:650–654. https://doi.org/10.1093/jee/65.3.650
Beggs CB (2002) A quantitative method for evaluating the photoreactivation of ultraviolet damaged microorganisms. Photochem Photobiol Sci 1:431–437. https://doi.org/10.1039/B202801H
Bell HA, Edwards JP (1999) The activity of (S)-hydroprene space spray against three stored products pests in a simulated food production environment. J Stored Prod Res 35:117–126. https://doi.org/10.1016/S0022-474X(98)00037-X
Bruce WA (1975) Effect of UV radiation on egg hatch of Plodia interpunctella (Lepidoptera: Pyralidae). J Stored Prod Res 11:243–244. https://doi.org/10.1016/0022-474X(75)90038-7
Calderon M, Bruce WA, Leesch JG (1985) Effect of UV radiation on eggs of Tribolium castaneum. Phytoparasitica 13:179–183. https://doi.org/10.1007/BF02980666
Clark JH (1922) The physiological action of light. Physiol Rev 2:277–309. https://doi.org/10.1152/physrev.1922.2.2.277
Diaz-Montano J, Campbell JF, Phillips TW, Throne JE (2014) Evaluation of potential attractants for Liposcelis bostrychophila (Psocoptera: Liposcelididae). J Econ Entomol 107:867–874. https://doi.org/10.1603/EC13427
Diaz-Montano J, Campbell JF, Phillips TW, Cohnstaedt LW, Throne JE (2016) Evaluation of light attraction for the stored-product psocid, Liposcelis bostrychophila. J Pest Sci 89:923–930. https://doi.org/10.1007/s10340-015-0724-5
Faruki SI, Das DR, Khan AR, Khatun M (2007) Effects of ultraviolet (254nm) irradiation on egg hatching and adult emergence of the flour beetles, Tribolium castaneum, T. confusum and the almond moth Cadra cautella. J Insect Sci 7:36. https://doi.org/10.1673/031.007.3601
Ghanem I, Shamma M (2007) Effect of non-ionizing radiation (UVC) on the development of Trogoderma granarium Everts. J Stored Prod Res 43:362–366. https://doi.org/10.1016/j.jspr.2006.09.002
Green PWC, Turner BD (2005) Food-selection by the booklouse, Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae). J Stored Prod Res 41:103–113. https://doi.org/10.1016/j.jspr.2004.01.002
Hori M (2016) Lethal effects of blue light on insects. J Illum Eng Inst Jpn 100:478–482 ((in Japanese with English abstract))
Hori M (2018) Lethal effect of blue light on insects and its application to pest control. Jpn J Pestic Sci 43:109–116. https://doi.org/10.1584/jpestics.W18-32(inJapanese)
Hori M, Suzuki A (2017) Lethal effect of blue light on strawberry leaf beetle, Galerucella grisescens (Coleoptera: Chrysomelidae). Sci Rep 7:2694. https://doi.org/10.1038/s41598-017-03017-z
Hori M, Shibuya K, Sato M, Saito Y (2014) Lethal effects of short-wavelength visible light on insects. Sci Rep 4:7383. https://doi.org/10.1038/srep07383
Huang F, Subramanyam B, Roesli R (2009) Comparative susceptibility of Liposcelis bostrychophila Badonnel and Liposcelis decolor (Pearman) (Psocoptera: Liposcelididae) to spinosad on wheat. Biopestic Int 5:106–113
Kam JH, Hogg C, Fosbury R, Shinhmar H, Jeffery G (2021) Mitochondria are specifically vulnerable to 420nm light in drosophila which undermines their function and is associated with reduced fly mobility. PLoS ONE 16:e0257149. https://doi.org/10.1371/journal.pone.0257149
McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR (2008) Cellular effects of long wavelength UV light (UVA) in mammalian cells. J Pharm Pharmacol 60:969–976. https://doi.org/10.1211/jpp.60.8.0004
Nakajima M, Yoshida H (1971) Studies on ultraviolet sensitivity in the silkworm, with special reference to variations in its killing effect during the larval instar stage. Jpn J Appl Entomol Zool 15:17–22. https://doi.org/10.1303/jjaez.15.17
Pfeifer GP (1997) Formation and processing of UV photoproducts: effects of DNA sequence and chromatin environment. Photochem Photobiol 65:270–283. https://doi.org/10.1111/j.1751-1097.1997.tb08560.x
R Development Core Team (2020) The R Project for Statistical Computing. http://www.r-project.org. Accessed 19 Jan 2021
Rastogi RP, Richa KA, Tyagi MB, Sinha RP (2010) Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids 2010:592980. https://doi.org/10.4061/2010/592980
Reed NG (2010) The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep 125:15–27. https://doi.org/10.1177/003335491012500105
Shibuya K, Onodera S, Hori M (2018) Toxic wavelength of blue light changes as insects grow. PLoS ONE 13:e0199266. https://doi.org/10.1371/journal.pone.0199266
Shimoda M, Honda K (2013) Insect reactions to light and its applications to pest management. Appl Entomol Zool 48:413–421. https://doi.org/10.1007/s13355-013-0219-x
Sinha RP, Häder DP (2002) UV-induced DNA damage and repair: a review. Photochem Photobiol Sci 1:225–236. https://doi.org/10.1039/b201230h
Tanaka M, Yase J, Aoki S, Sakurai T, Kanto T, Osakabe M (2016) Physical control of spider mites using ultraviolet-B with light reflection sheets in greenhouse strawberries. J Econ Entomol 109:1758–1765. https://doi.org/10.1093/jee/tow096
Taniyama K, Hori M (2022) Lethal effect of blue light on Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae). Sci Rep 12:10100. https://doi.org/10.1038/s41598-022-14096-y
Taniyama K, Saito Y, Hori M (2021) Lethal effect of blue light on the developmental stages of the urban mosquito, Culex pipiens form molestus (Diptera: Culicidae). Appl Entomol Zool 56:319–325. https://doi.org/10.1007/s13355-021-00737-7
Tariq K, Noor M, Saeed S, Zhang H (2015) The effect of ultraviolet-A radiation exposure on the reproductive ability, longevity, and development of the Dialeurodes citri (Homoptera: Aleyrodidae) F1 generation. Environ Entomol 44:1614–1618. https://doi.org/10.1093/ee/nvv133
Turner BD (1994) Liposcelis bostrychophila (Psocoptera: Liposcelididae), a stored food pest in the UK. Int J Pest Manag 40:179–190. https://doi.org/10.1080/09670879409371879
Wang J-J, Tsai JH, Zhao Z-M, Li L-S (2000) Development and reproduction of the psocid Liposcelis bostrychophila (Psocoptera: Liposcelididae) as a function of temperature. Ann Entomol Soc Am 93:261–270. https://doi.org/10.1603/0013-8746(2000)093[0261:DAROTP]2.0.CO;2
Wang J-J, Cheng W-X, Ding W, Zhao Z-M (2004) The effect of the insecticide dichlorvos on esterase activity extracted from the psocids, Liposcelis bostrychophila and L. entomophila. J Insect Sci 4:1–5. https://doi.org/10.1673/031.004.2301
Wharton DRA (1971) Ultraviolet repellent and lethal action on the American cockroach. J Econ Entomol 64:252–255. https://doi.org/10.1093/jee/64.1.252
Zhang CY, Meng JY, Wang XP, Zhu F, Lei CL (2011) Effects of UV-A exposures on longevity and reproduction in Helicoverpa armigera, and on the development of its F1 generation. Insect Sci 18:697–702. https://doi.org/10.1111/j.1744-7917.2010.01393.x
Acknowledgements
We wish to thank Earth Environmental Service Co., Ltd. for providing cultures of L. bostrychophila.
Funding
Part of this work was carried out with financial support from Earth Environmental Service Co., Ltd.
Author information
Authors and Affiliations
Contributions
MH conceived the study, designed the experiments, and wrote the manuscript. NO performed the experiments. MH and NO analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hori, M., Oyama, N. Lethal effect of blue light on Liposcelis bostrychophila (Psocoptera: Liposcelididae). Appl Entomol Zool 58, 133–138 (2023). https://doi.org/10.1007/s13355-022-00814-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-022-00814-5