Abstract
Diglyphus isaea (Walker) and Neochrysocharis formosa (Westwood) (Hymenoptera: Eulophidae) are common idiobiont parasitoids of leafminers attacking vegetable crops. They exhibit differing levels of synovigeny, and host feeding enhances their fecundity and longevity. The reproductive systems of these two parasitoids are typical of hymenopteran eulophids, consisting of two ovaries, each usually comprising three polytrophic meroistic ovarioles. Diglyphus isaea possesses two obvious oviduct accessory glands, which are absent in N. formosa. Both parasitoids underwent oosorption when starved, while feeding on host larvae promoted oogenesis and egg maturation. In both, oogenesis and vitellogenesis commenced on the first day of the pupal stage rather than after eclosion. Formation of ovarioles in D. isaea commenced 1 day earlier than in N. formosa. Mature eggs were rarely observed in ovaries of newly emerged D. isaea, but usually a few were present in N. formosa. When hosts (second–third instar Liriomyza sativae larvae) were provided, the number of mature eggs in D. isaea ovaries initially increased and then stabilized, while in N. formosa, the number first increased and then decreased. Diglyphus isaea had fewer but larger eggs than N. formosa did. Thus, synovigenic divergence begins at the pupal stage and may result in different life-history traits of adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult parasitoid nutrition is derived from two sources: ‘capital nutrients’ are obtained during growth and development in the host, while ‘income nutrients’ are acquired during adult feeding (Casas et al. 2005; Jervis and Ferns 2004). Capital nutrients of the adult parasitoid are formed in the immature stages (before adult eclosion) and then do not increase after eclosion, as most parasitoid species do not replenish lipid levels after emergence from their host (Visser and Ellers 2008; Visser et al. 2010). Therefore, income nutrients significantly influence life history traits of adult wasps, particularly adult feeding (Heimpel and Collier 1996; Jervis et al. 2008). Heimpel and Collier (1996) divided parasitoid food sources into host food (host hemolymph and tissues) and non-host food (e.g., nectar, fermented fruits, insect honeydew). Female parasitoids that need to feed on the host to meet the nutrient requirements of egg maturation, continuous oviposition and survival are called host-feeding parasitoids. More than 140 species in 17 families of Hymenoptera feed on their host after adult eclosion (Jervis and Kidd 1986). However, the total number of host-feeding parasitoid species is nearly 100,000, about one-third of all parasitoids species (Kidd and Jervis 1991).
Because host-feeding parasitoids often adversely affect their hosts, and may even kill them through oviposition and feeding, they have greater potential as biocontrol agents than non-host-feeding wasps (Heimpel and Collier 1996; Kidd and Jervis 1989, 1991). When host-feeding parasitoids encounter hosts, the females face a trade-off between current and future reproduction (i.e., decreased survival and future reproduction as a function of present reproduction). As both may be determined by a combination of environmental and physiological factors (Bernstein and Jervis 2008; Heimpel and Collier 1996), it is vital that host-feeding parasitoids adjust oogenesis to host availability (Bodin et al. 2009).
In recent years, the relationships between the dynamics of ovarian development (the balance between oogenesis and oosorption) and the life history of female parasitoids have received considerable attentions (Bodin et al. 2007, 2009; Ellers and Jervis 2004; Harvey 2008; Kapranas and Luck 2008; Papaj 2000; Ralec 1995; Richard and Casas 2009). Flanders (1950) divided parasitoids into proovigenic parasitoids and synovigenic parasitoids, based on the number of mature eggs in the ovaries of female adults at emergence. The degree of synovigeny is characterized by an ovigeny index (OI), which is the ratio of the mean egg complement at adult emergence to the mean maximum lifetime egg complement. For a strictly proovigenic species, OI = 1, and for a strictly synovigenic species, OI = 0 (Jervis et al. 2001). Recent evidence supports a continuum of ovigeny indices (the ovigeny index hypothesis) rather than a simple dichotomy (Jervis and Ferns 2011).
The OI of female parasitoids has a negative relationship with longevity or body size (Jervis and Ferns 2011; Jervis et al. 2001). Jervis et al. (2001) and Jervis and Ferns (2011) speculated that parasitoid synovigeny is associated with resource allocation strategies that balance the acquisition of capital versus income nutrients. Strongly synovigenic wasps have relatively low capital nutrients; body maintenance and reproduction of adult female wasps mainly depend on adult feeding. Hence, in female wasps, oogenesis is affected by life-history traits, particularly the way in which parasitoids obtain nutrients. However, relatively few studies have been conducted on the relationship between oogenesis and adult life history traits.
All host-feeding parasitoids are considered to be synovigenic (Heimpel and Collier 1996; Jervis and Ferns 2011; Jervis and Kidd 1986). Most host-feeding parasitoids feed on host tissues and hemolymph, enhancing their reproduction and increasing their longevity (Giron et al. 2004; Heimpel and Collier 1996; Jervis and Ferns 2011). While different host-feeding parasitoids have different levels of synovigeny, corresponding differences in their oogenesis patterns have not been well studied.
As important natural enemies of Liriomyza leafminers, the idiobiont parasitoids Diglyphus isaea (Walker) and Neochrysocharis formosa (Westwood) (Hymenoptera: Eulophidae) have wide distributions and ecological adaptability and strong pest-control potential (Liu et al. 2013; Wang et al. 2012). These two parasitoids are sympatric in some areas of China, e.g., in celery fields in Kunming, Yunnan (Wan-Xue et al., personal observation). Both D. isaea and N. formosa are host-feeding parasitoids. Both of them feed on the host larvae and may kill them. However, their synovigeny indices (OI) differ, being 0.002 and 0.12 for D. isaea and N. formosa, respectively. The two species also have different developmental duration, body size, longevity, reproductive capability, and host-feeding capacity (Chien et al. 2005; Liu et al. 2013; Wang et al. 2012; Zhang et al. 2011).
The objective of this study was to determine the oogenesis and maturation patterns of D. isaea and N. formosa, and to examine the effects of host feeding on oogenesis based on the structure of their reproductive systems. This should lead to a better understanding of the resource allocation strategies of these two parasitoids where they coexist.
Materials and methods
Both D. isaea and N. formosa were obtained by collecting Liriomyza huidobrensis (Blanchard) hosts in celery fields in Gongxian, Yunnan, China, in April 2011. Subsequently, parasitoids were reared on second or third instars of L. sativae Blanchard in a greenhouse. Host larvae for this rearing colony were collected from a bean field at the Langfang Experimental Station of the Institute of Plant Protection, Chinese Academy of Agricultural Sciences. Parasitoid colonies were maintained on L. sativae reared on dwarf oil-bean plants kept at 25–30 °C, 70–80 % relative humidity, and natural light conditions.
Freshly emerged D. isaea and N. formosa adults were provided with excess second or third instar L. sativae for host feeding and oviposition. Twenty 2- to 3-day-old D. isaea and N. formosa females were randomly selected, held at −20 °C for 20 min, and then dissected on a slide with PBS buffer (pH 7.2), where the structure of the reproductive system was observed and photographed under a stereoscopic microscope (LX73, Olympus, Tokyo, Japan). Twenty adult females of D. isaea and N. formosa were provided with late second to early third instar L. sativae larvae for 2 h to stimulate oviposition in order to obtain pupae and, later, adults at the same developmental stage. Parasitized host larvae were transferred to an artificial climate chamber and kept at 27 ± 1 °C, 70 ± 5 % RH, and a 14:10 L:D photoperiod. Based on the results of preliminary experiments, mature parasitoid larvae at the same developmental stage could be collected on the fifth morning after the hosts were parasitized. These immature parasitoids were then transferred for further development under the above conditions.
Ovarian development and oogenesis were observed at the pupal and adult stages. To study ovarian development during the pupal stage, cohorts of pupae of equal size were selected under a stereomicroscope (SZ61, Olympus) and dissected every 24 h after pupation. Male pupae were discarded, and up to ten female pupae were dissected at each sampling time. To study oogenesis in the adult stage, individual newly emerged adult parasitoids were exposed daily to one bean leaf with about 20 late second to early third instar L. sativae larvae in a glass petri dish (9 cm diameter, 2.2 cm height). Each day, the parasitoids were transferred to a new dish with a fresh supply of host larvae. The dishes were kept at 25 ± 1 °C, 70 ± 5 % RH, and 14:10 L:D photoperiod. Within 48 h, oviposition and host feeding of each parasitoid were recorded under a binocular stereomicroscope. Ten females were randomly selected at times 0, 6, 12, 24, 48, 96, 120, 144, 168, 192, 216, and 240 h after provision of the host larvae. Control adult female parasitoids were provided with water over the same time period, and ten females were randomly selected at each of the 6, 12, 24, 48, and 96 h time points. The wasps were frozen for 5 min at −20 °C and dissected immediately.
Ovarian development and oogenesis were observed during the dissection; stages of oocyte development were categorized and their numbers recorded using the scheme of Bodin et al. (2009). Oocytes were classified into three stages of development: group I, when the area of yolk deposition occupied less than half of the whole volume of the egg chamber; group II, when the area of yolk deposition occupied half or more of the egg chamber but did not completely fill it; group III, when oocytes had their full complement of yolk. We measured the length (L) and width (W) of two mature eggs (group III) on each side of the ovary with a micrometer. When there were fewer than four group III eggs, the lengths and widths of all group III eggs were measured. The volume of eggs (V) was calculated as V = (π × L × W 2)/6 (Avelar 1993). Measurements were compared using SAS (Version 12.0), while mean oogenesis as well as ovarian development was subjected to one-way analysis of variance. Mean values in the text are given ± SEM.
Results
Reproductive morphology
The reproductive systems of D. isaea and N. formosa each consisted of one pair of ovaries (Fig. 1). Of the 520 adult females of D. isaea tested, 99.2 % had six ovarioles (three in each ovary). The remaining 0.8 % had five ovarioles. Of 520 N. formosa, 97.7 % had six ovarioles, while a few had four, five, or seven ovarioles, accounting for 0.2, 1.9, and 0.2 % of the total, respectively.
The left and right ovaries of both species extended from the genitalia to the anterior wall of abdomen (Fig. 1). A yellow spermatheca (Fig. 1E) was located on the dorsal side of the junction between the two lateral oviducts, and, at their junction with the common oviduct, three protruding accessory glands and two spherical sacs containing transparent fluid were observed (Fig. 1). The larger sac was identified as a mucous gland, and the smaller one, in the form of along curved tube, as Dufour’s gland (Fig. 1G). In D. isaea, an accessory gland also protruded from both sides of the common oviduct (Fig. 1a).
We observed no obvious difference in the number of mature eggs on the two sides of the oviduct in either of the two wasps. While mature unlaid eggs were present in the ovaries, the wasps continued to host-feed for a short time (1–2 h) and new eggs continued to develop. The maximum number of mature eggs held in one ovariole by D. isaea was six and by N. formosa was five. At the onset of oosorption, mature eggs in the midsections of the ovarioles on both sides were affected first, followed by those further along the ovarioles (toward the common oviduct). The most mature eggs in the ovaries of both species were found in the lateral oviducts.
Ovarian development and oogenesis
The durations of the pupal stages of D. isaea and N. formosa were 5.2 ± 0.03 days (n = 100) and 5.5 ± 0.05 days (n = 100), respectively, with a significant difference between the two (F 1,199 = 48.31, p < 0.0001). Ovary formation commenced 24 h after pupation in both species. Initially, the ovaries appeared as thin threads, with no differentiation of the ovarioles or egg chamber. Ovarioles and egg chambers started to form in D. isaea at 96 h after pupation and yolk deposition started at 120 h (Fig. 2a). In N. formosa, ovarioles started to form at 48 h and were clearly visible 72 h after pupation. Formation of the egg chambers and yolk deposition began at 96 h.
The ovarioles of both wasps progressively increased in length, starting 24 h after pupation (Fig. 3). However, the ovarioles of D. isaea increased at a faster rate and were larger overall than those of N. formosa. Ovariole length of in N. formosa increased rapidly from 96–120 h after pupation.
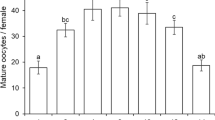
Numerous group I and a few group II eggs were present in the ovaries of D. isaea between 96 and 120 h after pupation (Fig. 4). Many group I eggs had formed in the ovaries of N. formosa 72 h after pupation, but their numbers decreased after 96 h, and many group II eggs were present at 120 h after pupation (Fig. 4). At emergence, few group III eggs were found in D. isaea (mean 0.1 ± 0.07, n = 20, Fig. 5a), while in N. formosa, 5.6 ± 0.5 (n = 20) group III eggs were found (Fig. 5b).
Diglyphus isaea adults fed on host larvae soon after emergence and the number of group III eggs in their ovaries increased within 48 h, peaking at 17.0 ± 0.8 eggs/female (n = 10) at 96 h, after which the number remained relatively stable (Fig. 6a). The number of group II eggs stabilized within 48 h, and the number of group I eggs increased steadily until 120 h. The total number of eggs exhibited a time course similar to that of the group III eggs.
When newly emerged N. formosa adults were provided with host larvae, the number of group III eggs in their ovaries increased steadily for the first 120 h, decreased somewhat after that, increased again to a maximum of 27.5 ± 2.1 eggs/female at 168 h (significantly higher than the maximum egg load of D. isaea, F 1,19 = 175.5, p < 0.0001), after which the number again decreased (Fig. 6b). The number of group II eggs in the ovaries increased sharply for the first 12 h, after which the number remained relatively stable. The number of group I eggs increased sharply in the first 6 h and then continued to increase at a relatively constant rate. In general, the total number of eggs in the ovaries of N. formosa adults exhibited a similar time course to that of group III eggs. The total number of eggs in N. formosa was clearly greater than in D. isaea.
Newly emerged D. isaea females provided only with water had a maximum longevity of 4 days and mean longevity of 3.3 ± 0.9 days (n = 20) (Fig. 7a). The number of group III eggs in the ovaries increased gradually for the first 12 h and peaked at 2.8 ± 0.9 eggs/female (n = 10), after which oosorption commenced. The number of group II eggs decreased steadily, and all groups II and III eggs were reabsorbed. The number of group I eggs increased during the first 48 h and decreased after that.
Newly emerged N. formosa females provided only with water had a maximum longevity of 4 days and a mean longevity of 2.6 ± 0.2 days (n = 20) (Fig. 7b). The number of group III eggs increased somewhat during the first 12 h and then decreased sharply. The number of group II eggs increased during the first 24 h and then decreased. All group II and III eggs had been reabsorbed 72 h after emergence. The number of group I eggs decreased steadily from 6 h after emergence.
Change in volume of group III eggs in the ovaries of adults
The volume of group III eggs in D. isaea ovaries feeding on host larvae was greatest within 12 h of emergence; it then decreased and remained stable from 24 h (Fig. 8). The volume of group III eggs in the ovaries of D. isaea provided with only water increased throughout the trial, but the eggs were smaller than those in ovaries of D. isaea feeding on host larvae. The volume of group III eggs in the ovaries of N. formosa increased for the first 6 h after emergence and then essentially stabilized, peaking at 48 h. The volume of group III eggs in the ovaries of N. formosa provided with only water decreased after 6 h, and the eggs were smaller than those in the ovaries of N. formosa feeding on host larvae.
Discussion
While the ovaries of most parasitoid wasps share a similar anatomical structure, the number of ovarioles varies greatly among families and genera. For example, although most parasitoids possess 2–20 pairs of ovarioles (Iwata 1959), Aphidius spp. possess a single pair, and Euceros spp. possess more than 200 pairs. In Ichneumonidea, the number of ovarioles is associated with life-history traits. Generally, koinobiont parasitoids have more ovarioles than idiobionts (Iwata 1960). The number of ovarioles among individuals varies little within species with few ovarioles, but in species with many ovarioles it may vary greatly and can even differ between the left and right ovaries of the same individual (Iwata 1959, 1960).
Although D. isaea and N. formosa are sympatric parasitoids of different subfamilies (Eulophinae and Entedoninae, respectively) and both are idiobionts, the former is ectoparasitic, while the latter is endoparasitic. The ovarian structures of the two parasitoids were quite similar, with three pairs of meristic polytrophic ovarioles in most of the ovaries dissected. This agrees with the pattern described by Iwata (Iwata 1959, 1960). For example, Brachymeria intermedia (Nees) (Hymenoptera: Chalcididae) has two ovaries, each with three polytrophic ovarioles. Each ovariole contains eight to ten follicles and one or two ripe eggs (Dindo et al. 1996).
We also found that the number of ovarioles per female did not differ among females of different sizes, but larger females carried more mature and immature eggs, which is also the case in the ectoparasitoid Agrothereutes lanceolatus Walker (Hymenoptera: Ichneumonidae) (Ueno 1999). Not all synovigenic endoparasitoids display the same pattern. In Venturia canescens (Gravenhorst), there is a positive and significant positive correlation between wasp size and egg load as well as ovariole number; large wasps possess significantly more ovarioles and mature eggs than do small ones (Eliopoulos et al. 2003).
No eggs were found in the oviducts of either D. isaea or N. formosa, suggesting that both species laid eggs rapidly. However, a pair of oviduct accessory glands was clearly visible in female D. isaea but absent in N. formosa. The eggs of D. isaea were nearly twice as large as those of N. formosa, and it is possible that the accessory glands of D. isaea may secrete mucus to lubricate the oviducts or facilitate further absorption of nutrients by the eggs in the oviducts (Flanders 1942). These accessory glands may also secrete host-marking pheromones concerned with host recognition and parasitization by female wasps (Nacro and Nenon 2008; Rosi et al. 2001).
Since the work of Flanders (1942, 1950), host feeding in female parasitic Hymenoptera has been associated with the production of large, yolk-rich eggs that have been termed ‘anhydropic’ because they do not swell during embryogenesis. Such host feeding has also been linked to synovigeny. Yolkless eggs, in contrast, are termed ‘hydropic,’ and these are generally laid by proovigenic parasitoids. Proovigenic parasitoids generally lay relatively fewer hydropic eggs than anhydropic eggs compared with synovigenic parasitoids on the same diet. However, proovigenic parasitoids are able to mature their anhydropic eggs in a shorter time after encountering hosts, decreasing the chance that adult parasitoids will be preyed upon by natural enemies while searching for food. The eggs of both parasitoids studied here were of the anhydropic type. Eggs laid by D. isaea were large and yellow and probably contained more yolk than eggs of N. formosa, which were smaller and white. Mature eggs swell slightly when held in water for at least 5 min, but the size of mature eggs held in PBS (pH 7.2) for a long time showed no obvious change (Wan-Xue et al., personal observation).
Most host-feeding parasitoids (especially destructive host-feeding parasitoids) produce anhydropic eggs, e.g., Tetrastichus incertus (Ratz.) (Eulophidae) (Dowell 1978), Aphelinus asychis (Walker) (Aphelinidae) (Bai and Mackauer 1990), Epidinocarsis lopezi (De Santis) (Encyrtidae), Leptomastix dactylopii Howard (Encyrtidae), Asaphes vulgaris Walker (Pteromalidae) (Ralec 1995), Pimpla nipponica Uchida (Ichneumonidae) (Ueno 1999), Aphelinus abdominalis (Dalman) (Aphelinidae) (Couty and Poppy 2001; Ralec 1995), Encarsia formosa Gahan (Aphelinidae) (Burger et al. 2004), and Eretmocerus eremicus Rose and Zolnerowich (Aphelinidae) (Asplen and Byrne 2006).
Eggs laid by host feeding, but non-destructive, koinobiont parasitoids such as Opius caricivorae Fischer (Hymenoptera: Braconidae), an endoparasitoid of dipteran leafminers, tend to be hydropic (Chien and Chang 2012; Xu et al. 2007). Some non-host-feeding synovigenic parasitoids, including the synovigenic, secondary ectoparasitoid Dendrocerus carpenteri (Curtis) (Megaspilidae) (Ralec 1995), Coccophagus atratus Compere (Aphelinidae), and several heteronomous hyperparasitoids (Donaldson and Walter 1988), also produce anhydropic eggs. However, some non-host-feeding synovigenic parasitoids, such as V. canescens (Hymenoptera: Ichneumonidae), produce micro-type hydropic eggs (Harvey et al. 2001). It therefore seems possible that destructive host feeding has evolved to enable females to sustain the production of high-quality anhydropic eggs. Oosorption is a common process in insects where anhydropic (high yolk content) eggs are broken down into their constituents, which are returned to the somatic nutrient pool for future reproduction (Bell and Bohm 1975; Papaj 2000). This is usually an active female response to resource deprivation and may be triggered by dietary stress, lack of available oviposition sites, or the absence of males (Bell and Bohm 1975).
Generally, oosorption occurs in anhydropic eggs of synovigenic parasitoids. However, there are exceptions, including the synovigenic, secondary ectoparasitoid Dendrocerus carpenteri (Megaspilidae) (Ralec 1995) and Eulophus pennicornis (Nees) (Eulophidae), a gregarious ectoparasitoid of the tomato moth Lacanobia oleracea (Linnaeus) (Wakefield et al. 2010), in which anhydropic eggs are stored. Similarly, egg resorption does not occur in the synovigenic endoparasitoid, V. canescens, in which egg production stops in the absence of food (Eliopoulos et al. 2003).
Oosorption occurs in all host-feeding parasitoids reported to date (Asplen and Byrne 2006; Bodin et al. 2009; Liu and Ueno 2012; Takasu et al. 2012; Tschudi-Rein and Dorn 2001). Our study revealed that oosorption occurred in both of our study species and that their oosorption pattern was consistent with that in other insects, i.e., a tendency for absorption of mature or nearly mature eggs (Bell and Bohm 1975). Under a starvation diet (only water provided), female parasitoids are able of utilizing capital nutrients to continue egg maturation for the first 12 h after emergence. After this length of time, oosorption occurs. On a starvation diet, within 48 h after emergence, group I eggs of D. isaea continued to develop to group II eggs, while group III eggs continued to be produced, and the total number of eggs increased. After 48 h, the number of eggs laid decreased. On a starvation diet, although group I eggs of N. formosa continued to develop to group II eggs, group III eggs were no longer laid. Therefore, the total number of eggs produced decreased from 12 h after emergence.
Oosorption differed between the two parasitoids. Both wasps retained a number of immature (group I) eggs, allowing the rapid resumption of oviposition and reproduction upon obtaining host food. When host larvae were provided, the ovaries of D. isaea and N. formosa contained at most 34 and 30 mature oocytes, respectively, and each ovariole contained a maximum of six and five mature oocytes, respectively. In another host-feeding parasitoid, Eupelmus vuilleti, in the presence of a host, each ovariole generally contained three oocytes: one fully mature egg, one nearly mature, and one immature. Host-deprived females reabsorbed their most mature and their smallest oocytes, keeping just one almost mature oocyte per ovariole (Bodin et al. 2009). Host-feeding parasitoids are synovigenic, and host feeding contributes to their longevity and reproductive capability (Heimpel and Collier 1996; Jervis and Ferns 2011; Jervis and Kidd 1986).
Our previous studies (Zhang et al. 2011) and this research have shown that D. isaea and N. formosa are strongly synovigenic parasitoids in which host feeding facilitates oogenesis and egg maturation and increases their longevity. Of the two, D. isaea had greater synovigeny (OI = 0.002) than N. formosa (OI = 0.12) (Zhang et al. 2011; Yi-Bo et al., unpublished data); mature eggs were rarely found in newly emerged D. isaea females, but a few mature eggs were found in newly emerged N. formosa.
Yolk deposition occurred at the pupal stage in both wasps, suggesting that oogenesis in females was initiated at this stage. However, the two wasps showed obvious differences in the dynamics of ovarian development and oogenesis. The formation of the ovarian chamber, oogenesis, and egg maturation was slower in D. isaea than in N. formosa. At the adult stage, the number of mature eggs peaked in D. isaea and then remained stable for a long period before decreasing. In contrast, mature egg numbers of N. formosa first increased and then immediately decreased.
Under the same conditions, the total number of eggs produced by the two wasps was similar but, in D. isaea, there were fewer larger eggs than in N. formosa, possibly reflecting different nutrient restraints. When the parasitoids were provided with the same nutrients, larger but fewer eggs were produced. Therefore, it appears that D. isaea needs more nutrients to complete egg development than does N. formosa. This could explain why D. isaea has a stronger synovigenic level than N. formosa. In addition, the larger body size of D. isaea may provide a greater range of host-feeding and non-host-feeding opportunities. Although these two idiobiont host-feeding parasitoids are sympatric, belong to the same family, and parasitize the same hosts, differences in their oogenesis could be related to differences in their life history characteristics, e.g., D. isaea is larger and lives longer than N. formosa (Wan-Xue et al., unpublished results). This is consistent with the “oogenesis hypothesis” suggested by Jervis and Ferns (2011).
Because both wasps are idiobionts, they cannot gain host resources by altering host metabolism. However, such constraints may be reduced as they obtain resources for oogenesis and somatic maintenance from host feeding. Because these parasitoids feed on and kill the host by stinging without oviposition, their potential use as control agents is enhanced, and they are now used around the world as important natural enemies of leafminer pests (Liu et al. 2013; Wang et al. 2012; Zhang et al. 2011).
The sympatricity of these species is especially important for their combined use as biological control agents. For example, they co-occur in celery fields damaged by L. huidobrensis in Kunming, Yunnan, China (Wan-Xue et al., unpublished observations). This coexistence results from niche partitioning and allows increased resource exploitation because of differences in their life histories and oogenesis. Because of these differences in the strategies for resource allocation and exploitation, D. isaea and N. formosa could be released concurrently to control leafminer pests. D. isaea plays a steady long-term role in control because of its greater longevity and low mature egg number in early adult life after emergence. In contrast, N. formosa could be used for more immediate control because of its shorter longevity in adult life and more rapid egg maturation in the early days after emergence. A further advantage of the combined release of these two parasitoids may be that their host-killing behaviors are selective for different sizes of host larvae.
References
Asplen MK, Byrne DN (2006) Quantification and ultrastructure of oosorption in Eretmocerus eremicus (Hymenoptera: Aphelinidae). J Morphol 267:1066–1074
Avelar T (1993) Egg size in Drosophila: standard unit of investment or variable response to environment? The effect of temperature. J Insect Physiol 39:283–289
Bai B, Mackauer M (1990) Oviposition and host-feeding patterns in Aphelinus asychis (Hymenoptera: Aphelinidae) at different aphid densities. Ecol Entomol 15:9–16
Bell WJ, Bohm MK (1975) Oosorption in insects. Biol Rev 50:373–396
Bernstein C, Jervis MA (2008) Food-searching in parasitoids: the dilemma of choosing between ‘intermediate’ or future fitness gains. In: Wajnberg E, Bernstein C, van Alphen JJM (eds) Behavioral ecology of insect parasitoids: from theoretical approaches to field applications. Blackwell Publishing Ltd., Oxford, pp 129–171
Bodin A, Jaloux B, Mandon N, Vannier F, Delbecque JP, Monge JP, Mondy N (2007) Host-induced ecdysteroids in the stop-and-go oogenesis in a synovigenic parasitoid wasp. Arch Insect Biochem Physiol 65:103–111
Bodin A, Jaloux B, Delbecque JP, Vannier F, Monge JP, Mondy N (2009) Reproduction in a variable environment: how does Eupelmus vuilleti, a parasitoid wasp, adjust oogenesis to host availability? J Insect Physiol 55:643–648
Burger JMS, Reijnen TM, van Lenteren JC, Vet LEM (2004) Host feeding in insect parasitoids: why destructively feed upon a host that excretes an alternative? Entomol Exp Appl 112:207–215
Casas J, Pincebourde S, Mandon N, Vannier F, Poujol R, Giron D (2005) Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology 86:545–554
Chien CC, Chang SC (2012) Morphology and life history of Opius caricivorae (Hymenoptera: Braconidae). J Taiwan Agric Res 61:144–157 (in Chinese)
Chien CC, Ku SC, Chang SC (2005) Study of the storage and oviposition-regulating capability of Neochrysocharis formosa (Hymenoptera: Eulophidae). Plant Prot Bull 47:213–227 (in Chinese)
Couty A, Poppy GM (2001) Does host-feeding on GNA-intoxicated aphids by Aphelinus abdominalis affect their longevity and/or fecundity? Entomol Exp Appl 100:331–337
Dindo ML, Gardenghi G, Grasso M (1996) Notes on the anatomy and histology of the female reproductive system of Brachymeria intermedia (Nees) (Hymenoptera Chalcididae) reared in vivo and in vitro. Bollettino dell’Istituto di Entomologia ‘Guido Grandi’ della Universita degli Studi di Bologna 50:5–13 (in Italian)
Donaldson JS, Walter GH (1988) Effects of egg availability and egg maturity on the ovipositional activity of the parasitic wasp, Coccophagus atratus. Physiol Entomol 13:407–417
Dowell R (1978) Ovary structure and reproductive biologies of larval parasitoids of the alfalfa weevil (Coleoptera: Curculionidae). Can Entomol 110:507–512
Eliopoulos PA, Harvey JA, Athanassiou CG, Stathas GJ (2003) Effect of biotic and abiotic factors on reproductive parameters of the synovigenic endoparasitoid Venturia canescens. Physiol Entomol 28:268–275
Ellers J, Jervis MA (2004) Why are so few parasitoid wasp species pro-ovigenic? Evol Ecol Res 6:993–1002
Flanders SE (1942) Oosorption and ovulation in relation to oviposition in the parasitic Hymenoptera. Ann Entomol Soc Am 35:251–266
Flanders SE (1950) Regulation of ovulation and egg disposal in the parasitic Hymenoptera. Can Entomol 82:134–140
Giron D, Pincebourde S, Casas J (2004) Lifetime gains of host-feeding in a synovigenic parasitic wasp. Physiol Entomol 29:436–442
Harvey JA (2008) Comparing and contrasting development and reproductive strategies in the pupal hyperparasitoids Lysibia nana and Gelis agilis (Hymenoptera: Ichneumonidae). Evol Ecol 22:153–166
Harvey JA, Harvey IF, Thompson DJ (2001) Lifetime reproductive success in the solitary endoparasitoid, Venturia canescens. J Insect Behav 14:73–593
Heimpel GE, Collier TR (1996) The evolution of host-feeding behaviour in insect parasitoids. Biol Rev 71:373–400
Iwata K (1959) The comparative anatomy of the ovary in Hymenoptera. Part III. Braconidae (including Aphidiidae) with descriptions of ovarian eggs. Kontyu 27:31–238
Iwata K (1960) The comparative anatomy of the ovary in Hymenoptera. Part V. Ichneumonidae. Acta Hymenopterol 1:155–169
Jervis MA, Ferns PN (2004) The timing of egg maturation in insects: ovigeny index and initial egg load as measures of fitness and of resource allocation. Oikos 107:449–460
Jervis M, Ferns P (2011) Towards a general perspective on life-history evolution and diversification in parasitoid wasps. Biol J Linn Soc 104:443–461
Jervis MA, Kidd NAC (1986) Host-feeding strategies in hymenopteran parasitoids. Biol Rev 61:395–434
Jervis MA, Heimpel GE, Ferns PN, Harvey JA, Kidd NAC (2001) Life-history strategies in parasitoid wasps: a comparative analysis of ‘ovigeny’. J Anim Ecol 70:442–458
Jervis MA, Ellers J, Harvey JA (2008) Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Ann Rev Entomol 53:61–385
Kapranas A, Luck RF (2008) Egg maturation, host feeding, and longevity in two Metaphycus parasitoids of soft scale insects. Biol Control 47:147–153
Kidd NAC, Jervis MA (1989) The effects of host-feeding behaviour on the dynamics of parasitoid-host interactions, and the implications for biological control. Res Popul Ecol 31(2):235–274
Kidd NAC, Jervis MA (1991) Host-feeding and oviposition strategies of parasitoids in relation to host stage. Res Popul Ecol 33:13–28
Liu HY, Ueno T (2012) The importance of food and host on the fecundity and longevity of a host-feeding parasitoid wasp. J Fac Agric Kyushu Univ 57:121–125
Liu WX, Wang WX, Wang W, Zhang YB, Wan FH (2013) Characteristics and application of Diglyphus parasitoids (Hymenoptera: Eulophidae: Eulophinae) in controlling the agromyzid leafminers. Acta Entomol Sin 56:427–437 (in Chinese)
Nacro S, Nenon JP (2008) Female reproductive biology of Platygaster diplosisae (Hymenoptera: Platygastridae) and Aprostocetus procerae (Hymenoptera: Eulophidae), two parasitoids associated with the African rice gall midge, Orseolia oryzivora (Diptera: Cecidomyiidae). Entomol Sci 11:231–237
Papaj DR (2000) Ovarian dynamics and host use. Ann Rev Entomol 45:423–448
Ralec AL (1995) Egg contents in relation to host-feeding in some parasitic Hymenoptera. Entomophaga 40:87–93
Richard R, Casas J (2009) Stochasticity and controllability of nutrient sources in foraging: host-feeding and egg resorption in parasitoids. Ecol Monogr 79:465–483
Rosi MC, Isidoro N, Colazza S, Bin F (2001) Source of the host marking pheromone in the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). J Insect Physiol 47:989–995
Takasu KSDA, Ueno K, Takagi T (2012) Effect of host-feeding on reproduction in Ooencyrtus nezarae (Ishii) (Hymenoptera: Encyrtidae), an egg parasitoid of the bean bug Riptortus clavatus. J Fac Agric Kyushu Univ 57:115–120
Tschudi-Rein K, Dorn S (2001) Reproduction and immature development of Hyssopus pallidus (Hymenoptera: Eulophidae), an ectoparasitoid of the codling moth. Eur J Entomol 98:41–45
Ueno T (1999) Adult size and reproduction in the ectoparasitoid Agrothereutes lanceolatus Walker (Hym. Ichneumonidae). J App Entomol 123:357–361
Visser B, Ellers J (2008) Lack of lipogenesis in parasitoids: a review of physiological mechanisms and evolutionary implications. J Insect Physiol 54:1315–1322
Visser B, Lann CL, den Blanken FJ, Harvey JA, van Alphen JJM, Ellers J (2010) Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. PNAS 107:8677–8682
Wakefield ME, Bell HA, Gatehouse AMR (2010) Longevity and fecundity of Eulophus pennicornis, an ectoparasitoid of the tomato moth Lacanobia oleracea, is affected by nutritional state and diet quality. Agric For Entomol 12:19–27
Wang W, Wang WX, Liu WX, Cheng LS, Wan FH (2012) Research advances on biological characteristics and application of Neochrysocharis formosa (Westwood) (Hymenoptera: Eulophidae). Chin J Biol Control 28:575–582 (in Chinese)
Xu P, Wan ZW, Chen XX, Liu SS, Feng MG (2007) Immature morphology and development of Opius caricivorae (Hymenoptera: Braconidae), an endoparasitoid of the leafminer Liriomyza sativae (Diptera: Agromyzidae). Ann Entomol Soc Am 100:425–432
Zhang YB, Liu WX, Wang W, Wan FH, Li Q (2011) Lifetime gains and patterns of accumulation and mobilization of nutrients in females of the synovigenic parasitoid, Diglyphus isaea Walker (Hymenoptera: Eulophidae), as a function of diet. J Insect Physiol 57:1045–1052
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 30771448, 31372005), the National Basic Research Program of China (Grant No. 2013CB127605), and the Beijing Municipal Natural Science Foundation (Grant No. 6052022). We thank G.L. Lovei (Aarhus University, Denmark) for linguistic corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, WX., Wang, W., Cheng, LS. et al. Contrasting patterns of ovarian development and oogenesis in two sympatric host-feeding parasitoids, Diglyphus isaea and Neochrysocharis formosa (Hymenoptera: Eulophidae). Appl Entomol Zool 49, 305–314 (2014). https://doi.org/10.1007/s13355-014-0251-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-014-0251-5