Abstract
Allotropa subclavata Muesebeck (Hymenoptera: Platygasteridae) is one of the most effective natural enemies of the Japanese mealybug Planococcus kraunhiae (Kuwana) (Hemiptera: Pseudococcidae), a serious pest in Japanese orchards. We examined the developmental and reproductive traits including host deprivation effect on oviposition of A. subclavata to evaluate its potential as a biological control agent for P. kraunhiae. The developmental zero and effective accumulative temperature of A. subclavata females and males reared on second-instar nymphs, a suitable host stage for this parasitoid, were 12.3 °C and 514.3 degree days and 12.8 °C and 432.8 degree days, respectively. The longevity of A. subclavata maintained with honey was longer than that of those maintained with water or no feeding. The average numbers of mature eggs in ovaries of the 0- and 30-day-old A. subclavata females were 301.1 and 290.4, respectively, and were not significantly different. Offspring production of 30-day-old A. subclavata females was approximately 80% that of 0-day-old female parasitoids. On average, 50% of all eggs were laid within 24 h of the start of oviposition. The intrinsic rate of increase at 25 °C was 0.115, which is considered to be approximate to that of the host, P. kraunhiae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Japanese mealybug, Planococcus kraunhiae (Kuwana) (Hemiptera: Pseudococcidae) is one of the most serious pests of fruit trees, including persimmon Diospyros kaki Thunberg, in Japan (Kawai 1980). The conventional control of P. kraunhiae in Japan depends only on scheduled applications of chemical insecticides. However, the efficacy of chemical pesticides is limited because many P. kraunhiae enter confined spaces such as the bud, bark, hull, and so on. Therefore, attention has focused on integrated pest management practices incorporating natural enemies of this pest. Indeed, some success was achieved previously using natural enemies to control mealybugs (Beltrà et al. 2013a, b, 2015; Spodek et al. 2018). Especially, parasitoid wasps are effective natural enemies of various mealybugs, including invasive ones and serious pests of many crops (Boavida et al. 1995; Löhr and Varela 1990; Moore 1988; Muniappan et al. 2006). Among eight species of parasitoid wasps attacking P. kraunhiae in Fukuoka Prefecture, Allotropa subclavata Muesebeck (Hymenoptera: Platygastridae) contributes to the suppression of the density of P. kraunhiae because large numbers of A. subclavata individuals have been observed in persimmon orchards where pesticides had not been applied (Teshiba and Tsutsumi 2004). Allotropa subclavata is an arrhenotokous solitary parasitoid (Tanaka and Kobayashi 1968) with a body length of approximately 1 mm and black body color. Tanaka and Kobayashi (1971) succeeded in controlling P. kraunhiae through mass release of A. subclavata adults in a persimmon orchard, further suggesting that the wasp could be used as a biological control agent for this pest.
Only a few studies have investigated A. subclavata biology (Tanaka and Kobayashi 1968; Teshiba et al. 2015). To utilize natural enemies of insects in pest control, it is important to study the biology and reproductive characteristics of these enemies in detail (Doutt 1964). Therefore, the objective of the current study was to understand the developmental biology and reproductive potential of A. subclavata, such as longevity, daily number of mature eggs produced after emergence, development at different host stages, developmental rate, lifetime fecundity, and the intrinsic rate of increase of A. subclavata.
Planococcus kraunhiae produces three generations per year with first- and second-instar nymphs occurring in June, August, and October. Allotropa subclavata occurs simultaneously with P. kraunhiae (Teshiba and Tsutsumi 2004) and attacks first- and second-instar nymphs (Teshiba et al. 2015). Allotropa subclavata appears unable to parasitize third-instar nymphs or adult females (Teshiba et al. 2015) so that female wasps may have to wait until the next generation of the host if host density is low. Thus, we clarified the effect of host deprivation on oviposition by A. subclavata. This information is important to estimate the availability of A. subclavata for use as a biological control agent against P. kraunhiae.
Materials and methods
Origin and rearing conditions of the insects
The host insect, P. kraunhiae, was originally collected from Japanese persimmon trees in Chikushino City and Asakura City, Fukuoka Prefecture, in 1999 to initiate a laboratory culture. The culture was maintained on pumpkin fruit at a constant temperature of 25 °C under a 16-h light:8-h dark photoperiod in a wooden box (25 cm × 25 cm × 25 cm) with a glass roof and wooden sides. One of the walls had an opening and another wall was covered with fine mesh netting for ventilation.
Allotropa subclavata was also collected from a Japanese persimmon orchard in Asakura City, Fukuoka Prefecture, in 1999 and used to develop a laboratory culture. The culture was maintained on P. kraunhiae in a wooden box similar to that described above. To obtain newly emerged adult wasps for the experiments, A. subclavata mummies were placed into individual polyethylene tubes (0.2 ml) at a constant temperature of 25 °C under a 16-h light:8-h dark photoperiod and emergence was checked daily. All females other than those from Experiment 4 (2) were tested after the confirmation of mating on the day of emergence.
Experiment 1: development of A. subclavata at different host stages
We examined the development of A. subclavata reared on first- or second-instar P. kraunhiae nymphs. First- and second-instar nymphs were obtained as follows: a first-instar nymph that hatched within a 24-h period was placed in a glass tube (5 mm diameter × 8 mm height). One side of the tube was directly attached to a kabosu fruit, Citrus sphaerocarpa Tanaka, and fixed to the fruit surface with paraffin. The other side of the glass tube was covered with vinyl film (Asahi Kasei Corporation Tokyo, Japan). Nymphs were checked daily for molting and reared to each stage at a constant temperature of 25 °C under a 16-h light: 8-h dark photoperiod. Females of A. subclavata that emerged within a 24-h period and had never encountered any hosts were put into individual glass tubes containing either a first- or second-instar nymph host. The A. subclavata female with ovipositor inserted into the host was removed promptly after oviposition. The host was reared at a constant temperature of 25 °C under a 16-h light: 8-h dark photoperiod after parasitoid oviposition. Time (days) from egg to emergence was observed once daily.
Experiment 2: developmental rates of A. subclavata at different constant temperatures
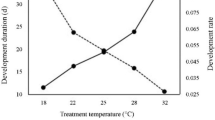
As in experiment 1, A. subclavata females that had emerged within a 24-h period were put into individual glass tubes on a kabosu fruit containing a second-instar host nymph. The A. subclavata female with ovipositor inserted into the host was removed promptly after the oviposition. The hosts were reared at 20, 22.5, 25, 27.5, and 30 °C under a 16-h light:8-h dark photoperiod. Allotropa subclavata emergence was checked daily for 100 days.
Experiment 3: effect of food type on the longevity of A. subclavata adults
We investigated the effect of providing honey, water, or no feeding on the longevity of A. subclavata adults. Males and females were mated on the day of emergence and they were individually released in a polyethylene tube (0.2 ml). Three different treatments were performed with approximately 15 replicates for each sex and treatment: honey, water, or no feeding. Honey drops were provided ad libitum on the wall of the polyethylene tube and added as necessary. For providing water, a piece of tissue (2 mm × 2 mm) soaked with water was placed in the tube and replaced daily to keep the insects from drying out. Adults were reared at a constant temperature of 25 °C under a 16-h light: 8-h dark photoperiod and their survival was checked daily until death. Specimens that drowned in honey drops or in the moistened tissue were excluded from the analysis.
Experiment 4: effect of host deprivation on the oviposition of A. subclavata
Fecundity
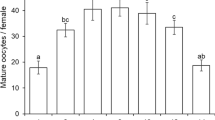
We investigated the effect of host deprivation period on the oviposition of A. subclavata using the females that were at 0 day after emergence (0-day-old female) and 30 days after emergence (30-day-old female). Both 0- and 30-day-old females were mated on the day of emergence and did not experience oviposition prior to the experiment. Nine and seven replicates were performed for 0- and 30-day-old females, respectively. Females were reared in individual polyethylene tubes at a constant temperature of 25 °C under a 16-h light:8-h dark photoperiod with honey as their food source. Females were dissected in water under a stereomicroscope (80 × magnification, Carl Zeiss, Oberkochen, Germany) to count the number of mature eggs.
Parasitization ability
We investigated the effect of host deprivation on the parasitization ability of female A. subclavata using 0- or 30-day-old mated females. Adult females with no oviposition experience were reared on honey in individual polyethylene tubes until the beginning of the test. A kabosu fruit infested with 400 individuals of second-instar P. kraunhiae nymphs was placed in a plastic container (9 cm × 9 cm × 10 cm) with the lid opening (3 cm × 3 cm) covered with a fine net for ventilation. The container was streaked with honey, an adult male was A. subclavata introduced, and then an adult female was released into the container. The container was left at a constant temperature of 25 °C under a 16-h light:8-h dark photoperiod for 24 h to allow the female to lay eggs. Then the male and female were removed from the container and placed into a new plastic container with a new infested kabosu fruit (the number of infested hosts was reduced to 200 starting from the second day). This operation was repeated until the female died. Dead male A. subclavata was removed and replaced as needed. The numbers of progeny were compared between 0- and 30-day-old A. subclavata female. Because the A. subclavata eggs could not be identified in the host’s body, the number of adults in the next generation was used as a measure instead of the number of eggs laid. The kabosu fruit with parasitized hosts was kept at a constant temperature of 25 °C under a 16-h light:8-h dark photoperiod and the number of emerged A. subclavata was counted. The tests were replicated 14 and 15 times for 0- and 30-day-old females, respectively.
Statistical analysis, life table, and demographic characteristics
The developmental period of A. subclavata at different host stages and in different sexes (Experiment 1), the effect of food type on the longevity of A. subclavata adults (Experiment 3), and the effect of host deprivation on the oviposition and longevity of A. subclavata adults (Experiment 4) were analyzed using a generalized linear model (GLM) with a Poisson distribution and a log link function. The effect of food type was compared using Tukey–Kramer’s highly significant difference (HSD) test. These statistical analyses were performed using R ver. 3.5.1 (R Core Team 2018). We also examined the developmental rate of A. subclavata at different constant temperatures in Experiment 2. From the results of this experiment, we calculated the developmental zero (t) and effective accumulative temperature (k) using the following relationship between temperature (T) and developmental rate (1/D, where D is the number of days until development).
The mean generation time (Tc), net reproductive rate (R0), and intrinsic rate of increase (rm) were calculated from these results. Survival rate by age (days) of females (lx) and fecundity age (days) of females (mx) were calculated using the following formulas, from Birch (1948):
Results
Experiment 1: development of A. subclavata at different host stages
Table 1 shows the developmental periods of A. subclavata on first- and second-instar nymph hosts. Both host stage and the sex of the emerging A. subclavata did not significantly affect the developmental period (GLM; host stage: 2 = 1.09, p = 0.296; sex: 2 = 1.49, p = 0.222; host stage × sex: 2 = 0.028, p = 0.866).
Experiment 2: developmental rate of A. subclavata at different constant temperatures
The developmental period of A. subclavata at constant temperatures of 22.5, 25, 27.5, and 30 °C is shown in Table 2. During the experimental period of 100 days, no females or males emerged at 20 °C. The developmental zero and the effective accumulative temperature were calculated from the developmental periods at all rearing temperatures except 20 °C. The developmental zero and the effective accumulative temperature from egg to emergence were 12.3 °C and 514.3 degree days, and 12.8 °C and 432.8 degree days in females and males, respectively (Table 3).
Experiment 3: effect of food type on the longevity of A. subclavata adults
The longevity of adult A. subclavata under different food treatments is shown in Table 4. The longevity of A. subclavata females and males maintained with honey was more than 40 days and significantly longer than that of those maintained with water or no feeding (GLM; treatment: 2 = 1411.4, p < 0.001; sex: 2 = 1.83, p = 0.176; treatment × sex: 2 = 0.74, p = 0.691; Tukey–Kramer’s HSD, p < 0.001). This shows that providing food, such as honey, is important for the longevity.
Experiment 4: effect of host deprivation on the oviposition of A. subclavata
Fecundity
The number of mature eggs in ovaries was not significantly different between 0- and 30-day-old A. subclavata females (Table 5, GLM, z = 1.231, p = 0.218). These results indicate that A. subclavata is a pro-ovigenic wasp that contains many mature eggs when it emerges.
Parasitization ability
Figure 1 shows the daily mean fecundity and survival curves of A. subclavata females. The mean longevity of 0- and 30-day-old A. subclavata females was 6.0 and 4.7 days, respectively. No significant difference was found between the two groups (GLM, z = 1.553, p = 0.120). All tested females started to oviposit on the day the host was provided. The highest mean parasitization rate was recorded on the first day with the hosts, with 97.86 progeny/female (range 66–133 eggs) produced by 0-day-old A. subclavata females and 79.40 progeny/female (range 34–111 eggs) produced by 30-day-old A. subclavata females. On subsequent days, parasitization gradually decreased. On average, 50% of all eggs were laid within 24 h of the start of oviposition. Within the first 4 days of the experiment, 90% of all eggs had been laid regardless of the period of host deprivation. During the experiment, 0- and 30-day-old A. subclavata females produced 2746 and 2294 offsprings, respectively, in total. The mean number of progeny produced by 0- and 30-day old A. subclavata females was 196.1 (range 115–250) and 157.7 (range 72–237), respectively, and was significantly different (GLM, z = 7.770, p < 0.001). Offspring production of 30-day-old A. subclavata females was about 80% that of 0-day-old A. subclavata females.
Fecundity and survival curves of Allotropa subclavata females starting from: a 0-day-old and b 30-day-old. Experiments were conducted at 25 °C under a 16-h light:8-h dark photoperiod. The open circles show daily survival rates (%) of females and the bars indicate the number of adult offspring per A. subclavata
Life table parameters
According to our calculations using lx and mx of the 0-day-old female (Fig. 1) and sex ratio and developmental period (days) of female at 25 °C (Table 2), R0 (female progeny/female), TC, and rm were 105.1, 40.5 days, and 0.115 at 25 °C, respectively.
Discussion
The parasitoid A. subclavata is one of the most effective natural enemies of P. kraunhiae, a serious pest of Japanese persimmon. Allotropa subclavata mainly parasitizes first- or second-instar nymphs and no significant differences were found in host stage preference (Teshiba et al. 2015). No significant difference was observed between the developmental periods on first- and second-instar nymphs (Experiment 1).
Additionally, A. subclavata can produce three generations per year, the same number as its host (Teshiba and Tsutsumi 2004). In the current study, we found that rm was 0.115 at 25 °C. Sawamura and Narai (2008) reported that the natural rm for P. kraunhiae was 0.117 and 0.093 at 24 °C and 28 °C, respectively. These values are considered to be approximate to the rate we observed for A. subclavata. Thus, we surmise that A. subclavata could control P. kraunhiae populations at 25 °C.
The longevity of A. subclavata demonstrated in this study was much greater than that of Allotropa japonica Ashmead (Hymenoptera: Platygastridae) (Mani and Krishnamoorthy 1989) or Allotropa burrelli Muesebeck (Hymenoptera: Platygastridae) (Clancy 1944). From the results of Experiment 3, honey was shown to elongate the longevity of A. subclavata. We showed that A. subclavata can lay eggs even after 30 days of host deprivation. Planococcus kraunhiae takes approximately 30 days from the third-instar nymph to the egg hatching stage in the next generation (Sawamura and Narai 2008). A previous study suggested that adults of A. subclavata occur at almost the same time as the occurrence of young instar nymphs of P. kraunhiae in the field (Teshiba and Tsutsumi 2004). However, if there are few host individuals, A. subclavata may be able to wait until development of the next host generation. This feature would be advantageous if A. subclavata was released as a biological control agent. It must to be inspected. In another parasitic wasp Pseudaphycus maculipennis Mercet (Hymenoptera: Encyrtidae), adult lifespan was longer when they fed on honeydew from Pseudococcus viburni Signoret (Hemiptera: Pseudococcidae) (Sandanayaka et al. 2009). In the field, A. subclavata might also consume honeydew, instead of honey, secreted by P. kraunhiae, which supports the growth of sooty mold on fruits.
The conventional control of P. kraunhiae in Japan depends only on scheduled applications of chemical insecticides. However, the effects of chemical pesticides are limited because many P. kraunhiae enter confined spaces and avoid sprays. However, we observed that A. subclavata regularly explored gaps, such as the edge of the plastic cage. Thus, we expect that A. subclavata could act as an effective natural enemy suppressing the density of survivor P. kraunhiae in these spaces.
Eight species of parasitoid wasps are reported from P. kraunhiae in Fukuoka Prefecture. Among them, Teshiba and Tsutsumi (2004) previously suggested that Anagyrus fujikona Tachikawa (Hymenoptera: Encyrtidae) might also be an effective parasitoid because it was more frequently caught than other parasitoid wasps. Anagyrus fujikona has a higher emergence rate when grown on host adults than on second-instar nymphs (Ryohei Inoue, personal communication). Thus, A. subclavata and A. fujikona might suppress P. kraunhiae populations by efficiently partitioning host resources. Further research is required to clarify the interactions between them.
References
Beltrà A, Tena A, Soto A (2013a) Fortuitous biological control of the invasive mealybug Phenacoccus peruvianus in Southern Europe. Biocontrol 58:309–317
Beltrà A, Tena A, Soto A (2013b) Reproductive strategies and food sources used by Acerophagus n. sp. near coccois, a new successful parasitoid of the invasive mealybug Phenacoccus peruvianus. J Pest Sci 86:253–259
Beltrà A, Soto A, Tena A (2015) How a slow-ovipositing parasitoid can succeed as a biological control agent of the invasive mealybug Phenacoccus peruvianus: implications for future classical and conservation biological control programs. Biocontrol 60:473–484
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Boavida C, Neuenschwander P, Herren HR (1995) Experimental assessment of the impact of the introduced parasitoid Gyranusoidea tebygi Noyes on the mango mealybug Rastrococcus invadens Williams, by physical exclusion. Biol Control 5:99–103
Clancy DW (1944) Biology of Allotropa burrelli, a gregarious parasite of Pseudococcus comstocki. J Agric Res 69:159–167
Doutt RL (1964) Biological characteristics of entomophagous adults. In: DeBach P (ed) Biological control of insect pests and weeds. Chapmann & Hall, London, pp 145–167
Kawai S (1980) Scale insects of Japan in colors. Zenkoku-Noson-Kyouiku-Kyoukai, Tokyo, pp 105–106 (In Japanese)
Löhr B, Varela AM (1990) Exploration for natural enemies of the cassava mealybug, Phenacoccus manihoti (Homoptera Pseudococcidae), in South America for the biological control of this introduced pest in Africa. Bull Entomol Res 80:417–425
Mani M, Krishnamoorthy A (1989) Life cycle, host stage suitability and pesticide susceptibility of the grape mealybug parasitoid, Allotropa japonica sp. n. J Biol Control 3:7–9
Moore D (1988) Agent used for biological control of mealybugs (Pseudococcidae). Biol News Inf 9:209–225
Muniappan R, Meyerdirk DE, Sengebau FM, Berringer DD, Reddy GVP (2006) Classical biological control of the papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae) in the Republic of Palau. Florida Entomol 89:212–217
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Austria. http://www.R-project.org/
Sandanayaka WRM, Charles JG, Allan DJ (2009) Aspects of the reproductive biology of the Pseudaphycus maculipennis (Hym: Encyrtidae), a parasitoid of obscure mealybug, Pseudococcus viburni (Hem; Pseudococcidae). Biol Control 48:30–35
Sawamura N, Narai Y (2008) Effect of temperature on development and reproductive potential of two mealybug species Planococcus kraunhiae (Kuwana) and Pseudococcus comstocki (Kuwana) (Homoptera: Pseudococcidae). Jpn J Appl Entomol Zool 52:113–121 (In Japanese with english summary)
Spodek M, Ben-Dov Y, Mondaca L, Protasov A, Erel E, Mendel Z (2018) The cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) in Israel: pest status, host plants, and natural enemies. Phytoparasitica 46:45–55
Tanaka M, Kobayashi M (1968) Oviposition and emergence of Allotropa sp. (Hymenoptera, Platygasteridae), a parasite of persimmon and citrus pest, Planococcus kraunhiae Kuwana (Hemiptera, Pseudococcidae). Proc Assoc Plant Prot Kyushu 14:58–61 (In Japanese with english summary)
Tanaka M, Kobayashi M (1971) Liberation of Allotropa subclavata (Hymenoptera, Platygasteridae) at the second generation of Planococcus kraunhiae (Hemiptera, Pseudococcidae) in a persimmon grove. Proc Assoc Plant Prot Kyushu 17:74–77 (In Japanese with english summary)
Teshiba M, Tsutsumi T (2004) The natural enemy complex of Planococcus kraunhiae injuring Japanese persimmons. Bull Fukuoka Agric Res Center 23:68–72 (In Japanese with english summary)
Teshiba M, Kawano S, Takagi M (2015) Host stage preference of Allotropa subclavata Muesebeck (Hymenoptera: Platygasteridae), a parasitoid of Planococcus kraunhiae Kuwana (Homoptera: Pseudococcidae). Kyushu Plant Prot Res 61:68–72 (In Japanese with english summary)
Acknowledgements
We thank Dr. Takafumi Tsutsumi for his useful comments on the experiments. We thank Dr. Takatoshi Ueno, Dr. Midori Tsuda, and all the staff members of the Laboratory of Insect Natural Enemies, Kyushu University. We are grateful to the anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teshiba, M., Kawano, S., Tokuda, M. et al. Development and reproduction of the parasitic wasp Allotropa subclavata (Hymenoptera: Platygastridae) on the Japanese mealybug Planococcus kraunhiae (Hemiptera: Pseudococcidae). Appl Entomol Zool 54, 313–318 (2019). https://doi.org/10.1007/s13355-019-00628-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-019-00628-y