Abstract
Evaluation of the landscape-level movement of Stenotus rubrovittatus (Matsumura), a major rice pest in Japan, is essential for establishing management strategies. We assessed the immigration timing and the average dispersal distance from a bank slope covered with Italian ryegrass Lolium multiflorum Lam. to paddy fields by periodic sampling in two regions in Chiba Prefecture. The immigrant densities in paddy fields increased exponentially as they approached the bank slope as a large-scale habitat of the insect. The relationship between immigrant density and distance from the source habitat fit an empirical model and theoretical model well. The theoretical model estimated the average dispersal distance during immigration: that of females was 72.0 and 92.1 m and that of males was 122.0 and 112.9 m in the respective regions. In addition, the average dispersal distance of males increased 7 days or later after the rice heading date despite the steady dispersal distance of females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects are constantly moving at various scales, such as from crop fields to non-crop fields or from one plant to another. Non-crop plants such as weeds, either inside or outside crop fields, serve as alternative hosts (Fleischer et al. 1988; Horton et al. 1988), overwintering sites (Cleveland 1982; Negron and Riley 1991), and oviposition sites (Boivin and Stewart 1983; Busching and Turpin 1976) for both insect pests and their natural enemies. Thus, it is well known that there are close interactions between insects in crop fields and those in non-crop habitats (Norris and Kogan 2000). Much knowledge about host manipulation of control pests has been gathered (e.g., Fleischer et al. 1988; Panizzi 1992). Removal of non-crop vegetation around fields has been regarded as an important cultural factor in integrated pest management (IPM) (Coaker 1987). IPM programs can in theory be nested by scale; for example, field < cropping system < landscape < ecoregion. Therefore, understanding how, when, and where insects move is a key component of habitat manipulation strategies at all scales. It also helps the development and deployment of regional-scale IPM programs (Irwin 1999).
The sorghum plant bug, Stenotus rubrovittatus (Matsumura), is a principal pest of rice, Oryza sativa L., in Japan, causing pecky rice (kernel spotting) (Higuchi 2010). It usually breeds and overwinters on heads of gramineous plants in grassy fields or meadows, and migrates to paddy fields just after rice heading (Hayashi and Nakazawa 1988). Weed removal can decrease damage (Teramoto 2003), because the pest population and damage to rice plants can be influenced by non-crop vegetation within and near paddies (Kashin et al. 2009; Ono et al. 2007).
The area of S. rubrovittatus infestation has expanded throughout Japan in recent years (Higuchi 2010; Watanabe and Higuchi 2006). One reason seems to be increases in the abandonment of paddy cultivation and in meadows where host plants, mainly annual or biennial gramineous plants typified by Italian ryegrass Lolium multiflorum Lam. and Digitaria ciliaris (Retz.) Koeler, are dominant (Ito 2004; Niiyama 2000). Takada et al. (2000) broadly separated paddy fields into categories by their conditions of surrounding source habitats of S. rubrovittatus. They reported that the density and damage of S. rubrovittatus were higher in paddy fields close to the large source habitats with mainly Italian ryegrass than in those that had weeds only in the field boundaries. Ecological studies recently began on landscape areas where S. rubrovittatus and its source habitat interact each other (Yasuda 2012; Yasuda et al. 2011; Yoshioka et al. 2011). These reports suggest that the habitat manipulation strategy of this pest needs to be developed from the viewpoint of the landscape or areawide scale, not just of individual paddy fields. Although fundamental understanding of the movement of a pest is a key constraint to developing and deploying larger scale IPM programs (Irwin 1999), little is known about its movement pattern in the field. Here, we analyzed the immigration period and dispersal distance of S. rubrovittatus systematically by using the timing and distance data of its immigration from a large source habitat to paddy fields during the heading to grain-filling period of rice. Insect abundance dispersed from one source is indicated by some equations that have distance as a parameter (Taylor 1978). Kobayashi (2007) reported the flight ability of S. rubrovittatus was different between sexes. We hypothesized that immigration timing and flight distance are influenced mainly by the distance from a large source habitat to paddies and also by the sex of S. rubrovittatus. We fitted the immigrant density in paddy fields to an empirical model and a theoretical model, and tried to analyze the dispersal pattern from source habitat to paddy fields.
Materials and methods
Field sampling
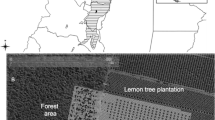
Insects were sampled from early July to mid-August in paddy fields in the Kozaki region (35°54′N, 140°23′E) in 2008 and the Sanno-wakeme region (35°53′N, 140°35′E) in 2009 in Chiba Prefecture, Japan (Table 1). These regions are located in alluvial lowlands of the Tone River and, at the time of the study, comprised homogeneous paddy fields adjacent to belt-like bank slopes (Fig. 1). These bank slopes were covered with grasses, mainly Italian ryegrass, an important host plant for S. rubrovittatus (Hayashi 1986); S. rubrovittatus occurs frequently on these grasses (Katase et al. 2007; Takeda et al. 2008, 2011). We regarded the bank slopes as the main source of the S. rubrovittatus captured in the paddy fields, because there were no other large source habitats nearby.
Sampling site details are shown in Table 1 and Fig. 1. A minimum distance of 10 m to the borders of the field was used for sampling, and each sampling site was 20 m or more apart from other sites. The shortest distance from sampling sites to the bank slope was calculated using ArcGIS version 9.2 (ESRI 2004).
Adult S. rubrovittatus were caught around each sampling site by 20 sweeps with a 36-cm-diameter insect net on a 120-cm-long handle. Sampling was performed twice a week from 2 weeks before the predicted heading date to just before harvest. Heading dates are defined as when heading was observed in 40–50 % of hills.
Immigration period and sex differences
We analyzed how immigration of S. rubrovittatus was influenced by the distance from the source habitat and sex. For such analyses, empirical equations have been advocated. Taylor (1978) showed that the following equation, proposed by Wallace (1966), was appropriate:
where y = abundance, x = distance from the source, and a and b are constants. We added sex as another predictor variable and log-transformed the number of adult S. rubrovittatus captured at 0, 3, 7, 10, 14, 17, and 21 days after the heading date (DAH) as the response variable (log(y + 1)).
We used multivariate analyses of variance (MANOVA) because the response values were collected periodically at the same location. In the design matrix for the multivariate comparison (Eq. 2), the columns indicate the variables themselves, and the rows indicate the combinations of variables to compare. Equation 2 shows a comparison between numbers captured on one date and on the following date (for example, 3 and 7 DAH):
If MANOVA showed that sex or distance had a significant effect on abundance, we performed separate analyses by sampling date to determine when the changes occurred. The level of significance was 0.007 (0.05 ÷ 7 sampling dates). These analyses were performed in JMP v. 10.0.0 software (SAS Institute Inc., Cary, NC, USA).
Dispersal distance
We compared the average dispersal distances between males and females. Yamamura et al. (2003) described the average dispersal distance (\( {1 \mathord{\left/ {\vphantom {1 {\sqrt {\lambda_{\text{D}} } }}} \right. \kern-0pt} {\sqrt {\lambda_{\text{D}} } }} \)) as a parameter in one-dimensional (1D) space (Eq. 3):
where n 0 = the number of individuals released, x = the distance at which they settle, λ D = λ/D, where λ = the rate at which individuals settle at a position, and D = the diffusion coefficient, which measures the dispersal rate (distance2/time).
Yamamura et al. (2003) developed Eq. 3 by releasing marked individuals at a single moment and capturing them continuously in pheromone traps. We considered our experiment to be equivalent to the model of Yamamura et al. (2003), because there was only one source in an otherwise empty area: a paddy field and pest individuals were invaded continuously there (Turchin and Omland 1999). Although Yamamura et al. (2003) defined a model in 2D space as well, we selected the 1D model because we considered the diffusion to occur vertically from the bank slope adjacent to the paddy fields.
We redefined Eq. 3 as:
where g(x) = the number of adults at the time when the effect of distance was significant, \( c = {{n_{0} \sqrt {\lambda_{\text{D}} } } \mathord{\left/ {\vphantom {{n_{0} \sqrt {\lambda_{\text{D}} } } 2}} \right. \kern-0pt} 2} \), and \( d = {1 \mathord{\left/ {\vphantom {1 {\sqrt {\lambda_{\text{D}} } }}} \right. \kern-0pt} {\sqrt {\lambda_{\text{D}} } }} \).
The parameters were derived by maximum likelihood estimation. The G-test examined the deviance of the model by Eq. 4 with the constant versus the empirical model Eq. 1, and rejection was at p < 0.05 significance based on a chi-squared distribution. These analyses were performed using R v. 2.13.2 software (R Foundation for Statistical Computing, Vienna, Austria) and the bbmle library (version 1.0.5.2) (Bolker 2012).
Results
Immigration period and sex differences
In both regions of Japan, few adults were captured in the paddy fields before rice heading (Fig. 2). The density was high at 7 to 14 DAH in Kozaki and at 3 to 10 DAH in Sanno-wakeme (Fig. 2). It then dropped to near zero.
The density decreased clearly with the increase of distance from the bank slope at 7 and 14 DAH in Kozaki (Fig. 3) and at 3 DAH in Sanno-wakeme (Fig. 4). MANOVA showed that both distance and sex had significant effects on density in both regions, indicating these effects differed in their response over time (Table 2). Analyses by sampling date showed that distance had significant effects on density at 7, 14, and 17 DAH in Kozaki, and from 0 to 10 DAH in Sanno-wakeme (Table 3).
The number of males was significantly higher than that of females at 7–17 DAH in Kozaki, while being only 7 DAH in Sanno-wakeme (Table 3). Although significant differences between sexes were not detected at 10 DAH in Sanno-wakeme, more males were captured than females in an area within 100 m from the bank slope (Fig. 4). The patterns of the sex ratio were similar, but the number of males showed a tendency to be higher than that of females after 7 DAH.
Dispersal distance
The total numbers of adult S. rubrovittatus captured fit well to Eqs. 1 and 4 during the period in which the effect of distance was significant (7–17 DAH in Kozaki and 0–10 DAH in Sanno-wakeme). The curves obtained by the two models were very similar (Fig. 5), with no significant differences (Table 4). Equation 4 gives the average distance of a captured male as 122.0 m in Kozaki and 112.9 m in Sanno-wakeme, and of that of a captured female as 72.0 and 92.1 m, respectively.
Relationship between total numbers of adult Stenotus rubrovittatus captured and distance from source habitat. Solid lines, estimates using the model of Yamamura et al. (2003); broken lines, estimates using the model of Wallace (1966). Numbers are totals for invasion period (7–17 days after heading date in Kozaki, 0–10 days after heading date in Sanno-wakeme)
In Sanno-wakeme, the female dispersal distance remained steady from 3 DAH, whereas the male dispersal distance started smaller than the female dispersal distance and increased beyond it (Fig. 6).
Discussion
Although many previous studies reported that numbers of S. rubrovittatus adults peaked around 7 DAH and then decreased in rice paddies (Katase et al. 2007; Nakata 2000; Takeda et al. 2008), the immigration periods differed from 7 DAH in our experimental regions and varied between the regions. In Kozaki, the number of insects captured peaked at 7 and 14 DAH, and in Sanno-wakeme, it reached a peak at 3 DAH. Cultivars seemed to have less influence, because heading dates in Kozaki and Sanno-wakeme were similar to those in the other paddies. The immigration of S. rubrovittatus started and finished later in Kozaki than in Sanno-wakeme. The reason for this difference seems to be the different landscapes between the regions. In Sanno-wakeme, the paddy fields were directly adjacent to the bank slope as a source habitat. In Kozaki, a roadway, a non-habitat area, might delay the S. rubrovittatus invasion from the bank slope to paddies. Kikuchi et al. (2004) reported that the immigrations of S. rubrovittatus were sometimes observed in the later stage of the grain-filling period in case a very large source habitat surrounded paddies.
Immigration timing may affect the amount and character of the damage to rice. The damage caused by S. rubrovittatus differs according to the timing of infestation: infestation during the late grain-filling period caused heavier damage than earlier infestations (Takeda and Shimizu 2009). Although very few comparisons of the population dynamics of S. rubrovittatus between paddy fields and source habitats have been reported (Kikuchi et al. 2004), weeding in the source habitat during the heading period promotes S. rubrovittatus immigration into the paddies (Higuchi 2010). Therefore, the timing of weed control should be tested to reduce the damage to rice.
The flight behavior of S. rubrovittatus or other Miridae has been reported. The mean flight speed of male S. rubrovittatus was measured as 1.20 m/s in the laboratory (Kobayashi 2007). Adult S. rubrovittatus flew continuously for at least 3 h (Ando et al., not published). From these dates, the daily potential flight distance of S. rubrovittatus can be calculated as about 13 km, much more than the average dispersal distance derived from our estimation. The range of dispersal of insects is usually smaller than the flight distance predicted from a straight line because of undirected flight behavior (Kobayashi 2007). Considering the S. rubrovittatus at our study sites flew repeated, undirected, short flights, the much more limited dispersal area in the field than in the laboratory can be explained.
There have been several reports that arthropod communities were influenced by landscape structures such as the location of surrounding habitats. Yasuda et al. (2011) estimated the functional scale of S. rubrovittatus was a radius of 300 m, which reflected the typical dispersal ability and the spatial range of the host plant use. Ichimori et al. (1990) captured a large number of adult S. rubrovittatus during the rice heading period at all distances up to 100 m from the source habitat. Ono et al. (2007) reported damage was detected within 100 m from the source habitat. These reports were comparable to our estimate that the average dispersal distance was around 100 m.
Our results also revealed sex differences in flight performance: male S. rubrovittatus flew in greater numbers and further than females in both regions. Males flew a bit faster than females in a laboratory experiment (Kobayashi 2007). This difference in flight ability may result in the difference in the average dispersal distance between sexes (Figs. 2, 3). Further laboratory studies will reveal the influence of age and physical condition on flight behavior.
In conclusion, the present study has demonstrated the immigration period and dispersal distance of S. rubrovittatus from a large-scale habitat. The immigration period started at the heading date and was prolonged until the late stage of the grain-filling period when the grains of the host plants became suitable food for S. rubrovittatus. The average dispersal distance was around 100 m, and the immigration density increased exponentially as the insects approached a large-scale habitat. These facts suggest that gramineous fields need to be kept in improper conditions for S. rubrovittatus, which means cutting the weeds from before the beginning of heading of rice plants up to the harvest. In addition, the closer a paddy field is to a large-scale habitat, the more actions need to be taken in areas within a few hundred meters from the large-scale habitat.
Reality is more complicated. Paddy fields are surrounded by many small source habitats such as paddy margins, which influence the occurrence of pecky rice by providing a transit site for the bugs’ migration (Yuasa 2006). Not only large- and small-scale source habitats, but also reclaimed land influenced the abundance of S. rubrovittatus (Yasuda et al. 2011). Paddy weeds such as Echinochloa frumentacea Link or Scirpus juncoides Roxb. var. ohwianus also attracted S. rubrovittatus and increased rice grain damage, because their anthesis is usually earlier than that of rice (Goto et al. 2000; Kashin et al. 2009). These results can be used for the development of strategies to estimate the risk posed by various source habitats.
Habitat manipulation at the landscape level will be increasingly important, because the areas of pastures and abandoned paddies have tended to expand in recent years (Ito 2004; Niiyama 2000). Our knowledge will contribute to the development of strategies to decrease pecky rice damaged by S. rubrovittatus through managing local source habitats where grass weeds are dominant.
References
Boivin G, Stewart RK (1983) Seasonal development and interplant movements of Phytophagous Mirids (Hemiptera: Miridae) on alternate host plants in and around an apple orchard. Ann Entomol Soc Am 76:776–780
Bolker B (2012) bbmle: Tools for general maximum likelihood estimation. R package version 1.0.5.2
Busching MK, Turpin FT (1976) Oviposition preferences of black cutworm moths among various crop plants, weeds, and plant debris. J Econ Entomol 69:587–590
Cleveland TC (1982) Hibernation and host plant sequence studies of tarnished plant bugs, Lygus lineolaris, in the Mississippi delta. Environ Entomol 11:1049–1052
Coaker TH (1987) Cultural method: the crop. In: Burn AJ, Coaker TH, Jepson PC (eds) Integrated pest management. Academic press, London, pp 79–80
ESRI (2004) ArcGIS version 9.2. Environmental Systems Research Institute, Redlands
Fleischer SJ, Gaylor MJ, Hue NV (1988) Dispersal of Lygus lineolaris (Heteroptera: Miridae) adults through cotton following nursery host destruction. Environ Entomol 17:533–541
Goto J, Ito Y, Shishido M (2000) Relationship between barn grass in rice paddies and spotted rice caused by Stenotus rubrovittatus (Matsumura). Ann Rept Plant Prot North Jpn 51:62–164 (in Japanese)
Hayashi H (1986) Ecology and control of the sorghum plant bug (Stenotus rubrovittatus Matsumura) causing the pecky rice. Plant Prot 40:21–326 (in Japanese)
Hayashi H, Nakazawa K (1988) Studies on the bionomics and control of the sorghum plant bug, Stenotus rubrovittatus Matsumura (Hemiptera: Miridae) 1. Habitat and seasonal prevalence in Hiroshima prefecture. Bull Hiroshima Agric Exp Stn 51:45–53 (in Japanese with English summary)
Higuchi H (2010) Ecology and management of rice bugs causing pecky rice. Jpn J Appl Entomol Zool 54:171–188. doi:10.1303/jjaez.2010.171 (in Japanese)
Horton DR, Capinera JL, Chapman PL (1988) Local differences in host use by two populations of the Colorado potato beetle. Ecology 69:823–831
Ichimori T, Chiba T, Tanaka H et al (1990) Relation between the content of spotted rice and the distance from the origin place of sorghum plant bug, Stenotus rubrovittatus Matsumura. Ann Rept Plant Prot North Jpn 41:121–124 (in Japanese)
Irwin ME (1999) Implications of movement in developing and deploying integrated pest management strategies. Agric For Meteorol 97:235–248. doi:10.1016/S0168-1923(99)00069-6
Ito K (2004) A possible cause of recent outbreaks of rice-ear bugs-changes in the use of paddy field. Ann Rept Plant Prot North Jpn 55:134–137 (in Japanese)
Kashin J, Hatanaka N, Ono T et al (2009) Effect of Scirpus juncoides Roxb. var. ohwianus on occurrence of sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Hemiptera: Miridae) and pecky rice. Jpn J Appl Entomol Zool 53:7–12. doi:10.1303/jjaez.2009.7 (in Japanese with English abstract)
Katase M, Shimizu K, Siina S et al (2007) Seasonal occurrence of rice bugs in the northern part of Chiba prefecture. Ann Rept Kanto Pl Prot Soc 54:99–104 (in Japanese)
Kikuchi A, Kanno H, Kimura T et al (2004) A survey on the occurrence of rice-ear bugs and their damage in the Tohoku region from 1999 to 2002. Bull Natl Agric Res Cent Tohoku Reg 102:101–180 (in Japanese with English abstract)
Kobayashi T (2007) Flight velocity of rice leaf bug, Trigonotylus caelestialium and sorghum plant bug, Stenotus rubrovittatus (Heteroptera: Miridae). Ann Rept Plant Prot North Jpn 58:96–98 (in Japanese with English abstract)
Nakata K (2000) Occurrence trend of the sorghum plant bug, Stenotus rubrovittatus Matsumura, in grass and rice fields. Plant Prot 54:316–321 (in Japanese)
Negron JF, Riley TJ (1991) Seasonal migration and overwintering of the chinch bug (Hemiptera: Lygaeidae) in Louisiana. J Econ Entomol 84:1681–1685
Niiyama T (2000) Outbreak of rice leaf bug, Trigonotylus caelestialium (Kirkaldy) in 1999. Plant Prot 54:309–312 (in Japanese)
Norris RF, Kogan M (2000) Interactions between weeds, arthropod pests, and their natural enemies in managed ecosystems. Weed Sci 48:94–158
Ono T, Kashin J, Kidokoro T (2007) Control of sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Hemiptera: Miridae), in rice paddy field by grass mowing in relation to migration source. Ann Rept Plant Prot North Jpn 58:75–79 (in Japanese)
Panizzi AR (1992) Performance of Piezodorus guildinii on four species of Indigofera legumes. Entomol Exp Appl 63:221–228
Takada M, Tanaka H, Chiba T (2000) Occurrence of the spotted rice in Iwate prefecture in 1999. Ann Rept Plant Prot North Jpn 51:165–169 (in Japanese)
Takeda A, Shimizu K (2009) Characteristics of the damaged grain caused by the sorghum plant bug, Stenotus rubrovittatus (Hemiptera: Miridae) at different infection periods. Ann Rept Kanto Pl Prot Soc 56:85–87 (in Japanese)
Takeda A, Shimizu K, Shiina S et al (2008) Seasonal prevalence of Stenotus rubrovittatus (Hemiptera: Miridae) in a gramineous weed field and a rice field. Ann Rept Kanto Pl Prot Soc 55:97–102 (in Japanese)
Takeda A, Yasuda M, Tabuchi K et al (2011) Effects of gramineous weed control on the seasonal occurrence of rice bugs in the northern part of Chiba prefecture. CAFRC Res Bull 3:79–84 (in Japanese with English abstract)
Taylor RAJ (1978) The relationship between density and distance of dispersing insects. Ecol Entom 3:63–70. doi:10.1111/j.1365-2311.1978.tb00903.x
Teramoto N (2003) Control of rice sting bug complex (Hemiptera) in paddy fields by gramineous weed control on balks. Bull Shiga Agric Res Cent Exp Stn 43:47–70 (in Japanese with English summary)
Turchin P, Omland KS (1999) Quantitative analysis of insect movement. In: Huffaker CB, Gutierrez AP (eds) Ecological entomology. Wiley, New York, pp 479–488
Wallace B (1966) On the dispersal of Drosophila. Am Nat 100:551–563
Watanabe T, Higuchi H (2006) Recent occurrence and problem of rice bugs. Plant Prot 60:201–203 (in Japanese)
Yamamura K, Kishita M, Arakaki N et al (2003) Estimation of dispersal distance by mark-recapture experiments using traps: correction of bias caused by the artificial removal by traps. Popul Ecol 43:149–155. doi:10.1007/s10144-003-0152-x
Yasuda M (2012) Does surrounding landscape composition affect agricultural pest abundances? Plant Prot 66:366–370 (in Japanese)
Yasuda M, Mitsunaga T, Takeda A et al (2011) Comparison of the effects of landscape composition on two mirid species in Japanese rice paddies. Appl Entomol Zool 46:519–525. doi:10.1007/s13355-011-0071-9
Yoshioka A, Takada M, Washitani I (2011) Facilitation of a native pest of rice, Stenotus rubrovittatus (Hemiptera: Miridae), by the non-native Lolium multiflorum (Cyperales: Poaceae) in an agricultural landscape. Environ Entomol 40:1027–1035. doi:10.1603/EN11032
Yuasa K (2006) Relationships between weeds on levee of paddy fields and incidence of the rice sting bugs and pecky rice. Plant Prot 60:211–214 (in Japanese)
Acknowledgments
We thank the owners of the paddies and the staff of the Katori Agriculture Office. We also thank Dr. Yamamura for explaining the dispersal model. This study was supported by a Grant-in-Aid for Research Projects Utilizing Advanced Technologies in Agriculture, Forestry and Fisheries from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeda, A., Yasuda, M., Tabuchi, K. et al. Pattern of immigration of the sorghum plant bug Stenotus rubrovittatus (Hemiptera: Miridae) into paddy fields from a large source habitat. Appl Entomol Zool 49, 139–147 (2014). https://doi.org/10.1007/s13355-013-0233-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-013-0233-z