Abstract
Dispersal abilities of invading species emerge from the interaction between the species and some features of the target community. Ligustrum lucidum is a tree species invading different ecosystems. Major spatial patterns of Ligustrum invasions and their ecological consequences have been analyzed, but no study addressed the dispersal process at a fine scale, assessing the effects of different biological and environmental factors. Ligustrum lucidum is an ornithochoric species. The structure of the environment determines bird movements and thus affects seed dispersal. We used inverse modeling to analyze bird-mediated dispersal of L. lucidum seeds in a secondary Yungas forest and surrounding crop-fields. We assessed the effects of egestion mode (regurgitation and defecation) and tree density (as an environment character) on seed dispersal. Seed dispersal presented different spatial patterns depending on the egestion mode. Tree density was positively associated with the number of regurgitated dispersed seeds and negatively associated with the number of defecated dispersed seeds. In both cases, dispersal distance increased in open areas, but absence of perches inhibited seed arrival. Thus, spread of L. lucidum is facilitated in open areas with some trees; inside the native forest, short distance dispersal facilitates the gradual invasion by this exotic species. Our results suggest that processes like crop abandonment and forest succession, which are active in subtropical montane systems, may facilitate L. lucidum invasion. Our seed dispersal models should be combined with actual distribution maps of L. lucidum to identify areas vulnerable to new invasions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal patterns of invasive species usually emerge from the interaction between their dispersal abilities and some features of the target community such as distribution of seed sources, forest cover, fruit availability and the presence of dispersal agents (Carlo et al. 2013). It has been observed that some of the most harmful woody species need to establish mutualisms with vertebrates to invade new areas (Richardson and Rejmánek 2011). Usually, seed dispersal constitutes a bottleneck during the spread stage of the invasion process, which involves a lag-phase (when species are dispersed into new territories) and subsequent population growth (Richardson and Pyšek 2013). Understanding the seed dispersal process can be informative to control invasion at this stage. Nevertheless, environmental and biological effects on fine scale seed dispersal (a few 100 m around seed sources) are generally inferred with limited empirical support (Aikio et al. 2010).
The glossy privet (Ligustrum lucidum, W.T. Aiton, Oleaceae) is an aggressive tree species invading ecosystems of different countries, including the United States, New Zealand (Panetta 2000; Aslan et al. 2012), Brazil (Biondi and Pedrosa-Macedo 2008) and Argentina (Steibel et al. 2000; Aragón and Morales 2003; Gavier-Pizarro et al. 2012). The first steps of expansion in the invasion process generally involve a rapid and massive colonization of degraded habitats near introduction points, which are generally areas with some degree of human intervention. For example, in Sierras Chicas, central Argentina, a rapid expansion of L. lucidum was documented. The invaded area increased 50 times in 23 years, from 50 to 2500 ha (Gavier-Pizarro et al. 2012). The first foci of this invasion were associated to urban and peri-urban areas, where seed sources were concentrated, but this constraint was gradually relaxed as seed sources became more abundant and widespread. When massive invasion occurs, L. lucidum may control forest regeneration by forming mono-specific stands, mainly in abandoned fields close to seed sources (Hoyos et al. 2010; Pero et al. 2015). In these cases, the invasion process has evident effects on forest ecology, and the modification of species composition determines shifts in other environmental factors such as soil humidity, light availability and in the disturbance regime (Ayup et al. 2014; Zamora Nasca et al. 2014; Ceballos et al. 2015). Although the major spatial patterns of Ligustrum massive invasion and their ecological consequences have been analyzed, no study addressed the fine scale dispersal process. Such a study would shed light on the process of gradual invasion inside native forests, which are generally incorrectly assumed as resistant to plant invasions (Martin et al. 2009). In contrast with massive invasion, gradual invasion is controlled by a diffusion process from the edge of the range of the invasive species and it is generally limited by the dispersal abilities of the species and the characteristics of the new site (Wilson et al. 2009). Gradual invasions do not form mono-specific stands, thus they do not imply huge ecological effects locally, but individuals distributed within the forest constitute new seed sources that may speed up the invasion process. The fine scale dispersal process leading to gradual invasion emerges from the interaction between L. lucidum and the preexisting community.
The dispersal of the glossy privet is mediated by fruit-eating birds (Montaldo 2000; Panetta 2000; Wilcox 2000; Aragón and Groom 2003; Montaldo 2005). In Yungas forests, fruit consumption of L. lucidum is favored because its fructification occurs during winter, when few native fruits are available (Pacheco and Héctor Ricardo Grau 1997; Rougès and Blake 2001; Blendinger et al. 2012). Bird-mediated seed dispersal depends on the movements of the vectors, which are influenced by the environment (Morales and Carlo 2006). Different biotic characteristics (e.g. plant aggregation, structure and phenology) and abiotic features (e.g. climate, soil and light) influence the behavior of birds and the spatial pattern of the seed rain (Côrtes and Uriarte 2013). Among fruit-eating birds, only those that do not damage seeds while eating fruits are considered legitimate dispersers (Jordano 2000). Seed size influences post-ingestion seed fate: generally, small seeds are eaten and defecated, while bigger seeds are regurgitated (Levey 1987). Both fates involve different time lapses from fruit consumption to seed deposition, which may affect the spatial patterns of seed rain, i.e. regurgitated seeds may fall closer to source trees (Traveset et al. 2007). Other mechanisms of seed consumption (e.g. seed predation) imply seed damage and do not contribute to the invasion process (Heleno et al. 2011).
Most invasibility theories focus on the probability that a propagule of an invading species establishes in a given site. These theories take into account certain features of the invading species and of the pre-existing community but, generally, they do not consider the probability of arrival of each propagule (Lamarque et al. 2011; but see Lonsdale 1999). On the other hand, most studies dealing with biological invasions analyze the spatial pattern of invaders (Lonsdale 1999), which is the result of at least two processes, namely, seed dispersal and plant establishment. It is known that vegetation structure can influence the spatial patterns of propagule dispersal but no general rules of this interaction have been proposed (Schurr et al. 2008; Morales et al. 2012; Carlo et al. 2013). Therefore, it is necessary to use empirical data to analyze the invasion process mediated by birds under different landscape structures to understand how the environment may shape the invasion process. This kind of studies should include abandoned crop-fields and native communities, which may favor massive or gradual invasions, respectively.
In this study, we quantify the contribution of seed dispersal by birds to the fine-scale spread of L. lucidum in a secondary forest of southern Yungas and surrounding crop-fields. We use an inverse modeling technique to make inferences about the dispersal process based on analysis of the spatial pattern of seed rain, and the distribution of seed sources. The study was designed to assess the effect of tree density (an environmental variable) on tree fecundity (i.e. the capacity to produce seeds), and on the dispersal process (dispersal kernel and bird movement). We also evaluated the effect of different egestion modes (regurgitation and defecation) on L. lucidum dispersal. We then investigated i) the influence of tree density (as an environmental variable) on L. lucidum dispersal; and ii) the ecological implications of ornithochoric dispersal of L. lucidum in the invasion process in secondary forests and early successional communities. We evaluated defecated and regurgitated seeds separately because different ecological processes are involved (e.g. different time lapses between fruit consumption and seed deposition) (Levey 1987). Tree density is an environmental variable that affects seed production, dispersal and deposition in different ways. High tree density around a focal tree may have a negative effect on seed production, caused by competition for light, water and nutrients. On the other hand, birds may choose dense areas to forage because they may be less exposed to predators, they may have more fruits available, and thus will need fewer movements to feed. Consequently, it is likely that seeds are dispersed at shorter distances than in open forests. Finally, trees function as perches for birds and their absence may prevent the arrival of ornithochoric seeds. The combination of egestion modes and tree density could affect the invasion process in different ways; as defecation implies longer digestive times than regurgitation, defecated seeds could reach longer distances facilitating L. lucidum spread, whereas regurgitation could contribute to population growth with small advance on new territories. The analysis of the individual contribution of different sources to the spatial pattern of seed rain through inverse modeling can shed some light on the importance of the different ecological mechanisms involved in L.lucidum spread process.
Methodology
Study area and study species
We conducted this study in the Sierra de San Javier (26°47′S 65°20′W), a mid-elevation mountain in Tucumán (Argentina), covered by a subtropical humid montane forest, which belong to the Yungas phytogeographic region (Cabrera 1976). Mean annual temperature is 19 °C, and average annual rainfall is between 1300 and 1500 mm, with 80% of rainfall concentrated between October and April (Minetti et al. 2005a, b). Although most birds migrate altitudinally and latitudinally during the winter, some resident species remain in the forest during the driest and coldest period (Malizia 2001) when available native-species fruits decrease to a minimum (Pacheco and Héctor Ricardo Grau 1997; Rougès and Blake 2001; Blendinger et al. 2012). During the first half of the twentieth century, a significant portion of the forest at lower altitude was replaced by annual crops and fruit orchards, mostly citrus (Grau and Aragón 2000). In the second half of the twentieth century, cultivated lands were abandoned, due to soil fertility loss and other socio-economic processes, and they were progressively and spontaneously re-vegetated (Grau et al. 1997). Some stands of the secondary-growth forests are now dominated by the invasive species under study, L. lucidum (Aragón and Morales 2003; Lichstein et al. 2004).
Ligustrum lucidum is an evergreen tree native of Asia (China, Japan and Korea), which has been introduced for gardening and has subsequently invaded native environments in many regions of the world. It is a fast growing species, which reaches 8–14 m height, and develops a dense canopy (IUCN-SSC 2006). After insect pollination, small and cream colored flowers produce fleshy drupes of about 6 mm in diameter. Each fruit contains one or two seeds of 3.8 ± 0.4 mm in diameter (Montaldo 2005; Aguirre-Acosta et al. 2014). In the study area, fruits ripen during winter and spring (July to October approximately). The main bird species that consume these fruits in southern Yungas is Turdus rufiventris, a resident thrush (Rougès and Blake 2001) that is considered a legitimate seed disperser (Montaldo 1993), and is one of the most important fruit-eating birds of southern Yungas (Malizia 2001; Blendinger et al. 2012). After fruit ingestion, birds digest the exocarp increasing germination probability (Montaldo 1993).
We established a 310 × 110 m rectangular plot (3.41 hectares) in the lower areas of Parque Sierra de San Javier, a protected area owned by Universidad Nacional de Tucumán. The study area is located at 700 m.a.s.l. and it includes an area of secondary forest bounded by a lemon tree plantation and an abandoned orchard with no woody vegetation (Fig. 1). The diversity, density and species composition of trees varied within the forest patch, with certain places exhibiting high densities of L. lucidum, and others dominated by native species (e.g. Cinnamomum porphyrium, Myrcine laetevirens and Parapiptadenia excelsa). During June 2013, we analyzed tree composition of the forest patch. On a pre-established grid, we mapped every tree with at least 10 cm diameter at breast height (dbh), and recorded the species and dbh. For this study, we only distinguished between L. lucidum, (which functioned as seed sources) and the rest of the tree species. To estimate tree density, we considered all the trees measured and mapped in the forest plot. Tree density was estimated as the density of trees (independently of species identity), observed in a moving window represented by a 5-by-5-meter quadrat. The moving window was centered in the intersection of every meter (integer) of the study area.
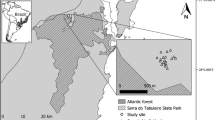
Study area. A Stripped area indicates Yungas extension in Argentina. Little black area within, corresponds to Sierra de Javier location (in Tucumán province, North-west Argentina). Small inset shows the location of Argentina (in grey) within South America. B Google earth image indicating forest area, abandoned orchard and lemon tree plantation. Study area corresponds to the light grey rectangle, where mapped L. lucidum are shown with dots and seed traps on the three different covers are represented with squares
Seed sampling
We analyzed the spatial pattern of seed rain from July to November 2013, when L. lucidum fruit production occurs. To collect data on seed rain we placed 396 seed traps, distributed in the forest plot and in the two adjacent areas, the abandoned orchard (an area with no woody vegetation) and the lemon tree plantation (Fig. 1). In the forest plot, we regularly placed seventy-five traps, forming a grid of 15 rows and 5 columns, separated by 20 m in all directions. In the adjacent abandoned orchard, we placed 56 traps forming a grid of 8 rows and 7 columns, separated by 20 m in all directions. The maximum distance between the forest and the abandoned orchard traps was 180 m, and the minimum distance was 20 m due to the presence of a trail, which also separates lemon tree plantations from the forest. The abandoned orchard had a total surface of 1.26 hectares. In the lemon tree plantation (5.50 hectares) we could not keep the same grid design of traps due to the potential interference with agricultural practices, so we had to follow the spatial arrangement of lemon trees and place the traps under their canopy. We placed 265 traps separated every 15 m within rows, and 20 m between rows. The farthest distance between a trap of lemon trees and the forest was 360 m (Fig. 1).
Each trap consisted in a 0.5 m × 0.5 m square mosquito net. The net was held 0.3 m above the ground with PVC pipes to avoid seed removal or post dispersal seed predation. We censed traps for regurgitated seeds and bird faeces every week from July to November 2013. Seeds found in bird faeces were considered defecated seeds, while undamaged seeds with no exocarp were defined as regurgitated seeds. We removed all the seeds and we cleared all traps after sampling. At each sampling, we recorded the number of regurgitated and defecated Ligustrum seeds.
Data handling
To model both dispersal processes (defecation and regurgitation) we used the cumulative number of seeds within each trap throughout the fruiting period of Ligustrum, because seed counts were too low to analyze each week separately. To model the defecation-mediated dispersal process we excluded 20 traps from the inner forest edge, to reduce the effects of source trees outside the study area.
To model the dispersal of regurgitated seed, we used the same subset of forest traps used for defecated models, but in the other land covers (lemon tree plantation and abandoned orchard), we included only the traps located at a distance up to 60 m from forest edge.
This was due to the absence of regurgitated seeds on those areas, which hindered the convergence and fitting of the models. For the lemon tree plantation and the abandoned orchard we excluded 303 traps, all with zero seed counts.
Seed shadow modeling
We used an inverse modeling (IM) technique to infer ecological processes involved in seed dispersal from the observed spatial pattern of seed sources and seed counts of every trap (Nathan and Muller-Landau 2000). This technique is useful to infer dispersal processes when multiple sources supply seeds to each trap, and it is not possible to identify the origin of each seed. In this sense, we evaluate the likelihood of the emergent spatial pattern of dispersed seeds, rather than the contribution of each source (Nathan and Muller-Landau 2000). Inverse modeling allows evaluation of the dispersal kernel and different environmental effects on the dispersal mechanisms (Schurr et al. 2008). In this study, we used inverse modeling to simultaneously assess the effect of tree density and tree size on seed yield of individual sources, the effects of tree density on the dispersal kernel and the effects of the environment on the seed path from the source to the deposition point.
We assumed that the number of seeds arriving to a site is the sum of the contribution of every individual seed source. We modeled the seed shadow of individual sources as the product of source fecundity Q and a dispersal kernel f (Ribbens et al. 1994; Clark et al. 1998). Thus, seed contribution of every source to every trap can be estimated as a function of the distance between them. The expected number of seeds (S t) in a trap t of area A t is the sum of the seeds of all the sources:
where G is a vector of tree sizes of every source, r t is a vector of the distances between every source and trap t and β is a vector of model parameters (βfec for fecundity parameters and βdisp for dispersal parameters).
Following Clark et al. (1999), we used the circumference of L. lucidum trees (G s) as a proxy to estimate their fecundity Q s. It is known that seed production can be affected by tree density (D s) due to competition for different resources, but that the total yield (the number of dispersed seeds from a source) can increase due to mass effect. We estimated fecundity as:
This expression determines that source size always has a positive effect on fecundity. Tree density may have a positive (e.g. by mass effect) or negative effect (e.g. through competition) on seed yield and when B 1 is zero it has no environmental effect on seed production.
Different dispersal kernels have been proposed to describe seed rain (Ribbens et al. 1994; Clark et al. 1998, 1999). We chose the 2Dt dispersal kernel (Clark et al. 1999) because it presents the flexibility to fit local and long distance seed densities, which is appropriate for animal-mediated seed dispersal (Herrera et al. 2011). In this kernel, the probability density of seeds is a function of the dispersal distance (r), with scale parameter (u) and the shape parameter (p). Parameters u and p represent the dispersal parameters (βdisp) of Eq. 1.
The model distinguishes two types of environmental effects on the dispersal process (Schurr et al. 2008). Source effects describe the influence of the environment surrounding each tree on seed production and dispersal kernel, while path effects describe the influence of environmental heterogeneity on seed movement along a straight path. The path effect can be estimated through an additional parameter (w) which affects the dispersal distance (r). Conceptually, this parameter, which is an indicator of seed permeability through the forest, transforms the spatial distance between traps and seed sources into ‘movement space’. Under this concept, the distances in environments with low seed permeability are increased with respect to distances in more permeable areas. Although this approach only takes into account the straight path between seed sources and traps, it allows considering potential effects of the environment on bird tracks, which may affect the spatial pattern of seed rain. To include the path effect in the modeling process, it is necessary to map a variable that informs about the permeability (or resistance) of the environment to bird and seed movement (Schurr et al. 2008). We considered that tree density is a good indicator of environmental permeability, because birds use trees as perches, canopy cover protects them from predation, and denser areas tend to have more food resources (Carlo et al. 2013). In the models, we assumed that the effect of tree density and the dispersal kernel were isotropic, i.e. they presented no directional bias in the dispersal of seeds.
We defined a series of progressively more complex models. Simplest models considered only the effect of tree size on seed yield (evaluated through fecundity parameter B 0) and single u and p parameters with no effects of seed sources on them. We considered all possible combinations of environmental effects on seed sources (on βdisp and βfec parameters) and path (w). We fitted eight models for each post-ingestion seed fate (Table 2) through maximum likelihood using the simulated annealing method (Bélisle 1992).
We used the Akaike information Criterion (AIC, Akaike 1987) to estimate the performance of each model. Sometimes data support more than one model to different degrees. In these cases, it is a good practice to take into account the relative support of each model to get significant average predictions (Burnham et al. 2011). We used the AIC of the models to estimate their relative weights, which indicate how much data support them. When differences in AIC were small (i.e. below two units of AIC) we used the relative weights of the models to estimate a weighted average model (Burnham et al. 2011). In this case, we only considered the best models that accounted for at least 70% of the total weight, and we calculated the average of the values of the parameters involved. We performed all the quantitative analyses with R software (R Core Team 2015).
Results
Tree density was highly variable within the plot. Mean density was 0.12 trees per m2 but values ranged from 0 to 1.25 trees per m2 and zero was the most frequent value. Circumference (in m) of L. lucidum trees ranged from 0.12 to 3.05, with a mean of 0.69 m.
Most seeds were found in forest traps, where we recorded 100% of the regurgitated seeds and 96% of the defecated seeds. Records of defecated seeds were scarce and isolated in lemon tree areas (single faeces with one or two seeds, only in four sampling dates). No regurgitated seeds were found in this area (Table 1). In the abandoned orchard, only faeces without seeds were found and no regurgitated seed were recorded there.
Inverse modelling
As none of the proposed models clearly outperformed the others neither for regurgitated nor for defecated seeds, we averaged the models that accounted for at least 70% of the support from data. Averages took into account the relative data support of the models assessed through their weight (Table 2). The resulting models for regurgitated and defecated seeds were different (Table 2; Fig. 2). In the defecated seeds model, tree density had effects on seed source fecundity (B 1) and on path effect W. In contrast, in the regurgitated seeds model, tree density affected both fecundity (B 1) and scale parameter of the dispersal kernel (u), but path effect was not included in the best models. Parameter values were also different between models (Table 2).
Expected number of Ligustrum lucidum regurgitated seeds (line) and defecated seeds (dotted line) at different distances from a hypothetical source. The expected number of seeds is the product of individual tree fecundity, the seed dispersal kernel and the path effect. The hypothetical tree is medium-sized (basal area = 0.69 m2) located in a homogeneous density area (0.12 trees m−1). Inset figure represents a detail of the curves at long distances (between 30 and 80 m) from seed sources
Although both models included the fecundity parameter, B 1, their signs were different. For regurgitated seeds, B 1 was positive and, consequently, the number of dispersed seeds (seed yield) from each source increased with tree density. Instead, less defecated seeds were dispersed from sources surrounded by dense forest. The estimates of the remaining parameters (though not their sign) were different between models, (Table 2), which was reflected in the emergent pattern of seed dispersal (Fig. 2). Although defecated seeds were much scarcer than regurgitated seeds, they were dispersed farther (Fig. 2). In both cases, the number of seeds decreased with distance from seed sources, but the decrease was steeper for regurgitated seeds, and the expected number of seeds was close to 0 at 40 m from seed sources, while defecated seeds could be found up to 80 m from seed sources, even if in small numbers.
Seed dispersal was affected by tree density in both models, but the parameters involved in each case were different. In the model of defecated seeds, there was no effect of tree density around the source on the scale parameter of the dispersal kernel, but we found habitat resistance to seed movement (w) (Table 2). This indicates that defecated seeds can be dispersed farther in low-density forests (Fig. 3). In the case of regurgitated seeds, the scale parameter (u) was negative, indicating that seeds from sources in denser areas attain shorter distances (Fig. 4), but no path effect was detected (Table 2). The dispersal of regurgitated seeds was very sensitive to tree density around sources. When seed sources were in dense forests, there was an abrupt decrease in the expected number of seeds at short distances, a pattern that was not observed for seed sources in less dense forests (Fig. 4).
Environmental effect of tree density on the expected number of defecated L. lucidum seeds. Graph shows the expected number of seeds at different distances from seed sources, obtained from weighted average model for defecated seeds. We took as source a medium size tree (basal area = 0.69 m2), in a homogeneous density area: solid line represents an environment with median tree density (0.12 trees per m2), hatched lines show the curve for high tree density (1.25 trees per m2), and dotted line for low tree density (0.01 trees per m2)
Environmental effect of tree density on the expected number of L. lucidum regurgitated seeds. Graph shows the expected number of seeds at different distances from seed sources obtained from weighted average model for regurgitated seeds. We took as source a medium size tree (basal area = 0.69 m2), in a homogeneous density area: solid line represents an environment with median tree density (0.12 trees per m2), hatched line shows the curve for high tree density (1.25 trees per m2), and dotted line for low tree density (0.01 trees per m2)
Discussion
Two processes
Both processes involved in seed dispersal of Ligustrum lucidum by fruit-eating birds (regurgitation and defecation) had different and complementary ecological implications on the spread of glossy privet. Regurgitation is more frequent than defecation but regurgitated seeds are concentrated at short distances from seed sources, while defecated seeds may attain longer distances, though seed densities are low. Thus, legitimate dispersers contribute to the spread of invasive species into new areas, mainly through defecation; defecated seeds can reach more than one hundred meters from seed sources, a trend that was also found by Morales et al. (2012) for other dispersal systems. The differences in dispersal distance between regurgitated and defecated seeds are probably because regurgitation is faster than defecation (Levey 1987).
The passage of seeds through the birds’ gut increases seed viability for many plant species (Traveset and Verdú 2002). Thus, although few defecated seeds achieve long distances, they may be sufficient to colonize new areas. The simple removal of the exocarp from fruits by birds also facilitates germination (Aragón and Groom 2003), so undamaged regurgitated and defecated seeds may have increased germination rate with respect to seeds in whole fruits (Montaldo 1993).
Dispersed seeds constitute new nuclei of invasion because established trees become themselves sources of seeds and regurgitation favors rapid growth of local populations of L. lucidum. This combination of colonization of new areas mediated by defecation and population growth mediated by regurgitation generates positive feedbacks that speed up the invasion process. Bird-mediated seed dispersal of L. lucidum, combined with their high growth rate, resprouting capacity and tolerance to wide environmental ranges (Guilhermetti et al. 2013), contributes to the spread, population growth and persistence in new environments.
Tree density and seed arrival
We recorded few seeds arriving to lemon tree plantations, and L. lucidum invasions generally occur in open areas or in early succession areas (Pero et al. 2015). This suggests that even with low quantities of seeds this invasion process is limited by the availability of resources rather than by the arrival of propagules, which supports the invasibility model based on fluctuating resources (Davis et al. 2000). This model proposes that invasibility is enhanced whenever resources are available (e.g. when resource supply increases or the capacity of resource uptake by the community decreases due to a disturbance). In the study area, space seems to be the resource limiting establishment of the glossy privet. Cropland abandonment opens a time window in which L. lucidum increases the probability of colonizing new areas. Thus, glossy privet can colonize these environments given seed sources are close (Aragón and Morales 2003; Montti et al. 2017), which is relatively frequent in the Yungas, since it is a common species in gardens, both in semi-urban and rural areas. Interspecific competition is reduced in early succession when L. lucidum usually forms monodominant stands, due to its higher survival and growth rates in comparison to the most abundant native species (Aragón and Groom 2003; Malizia et al. 2017). Then L. lucidum can alter composition and functioning of secondary forests (Aragón et al. 2014; Malizia et al. 2017). We found that L. lucidum seeds are dispersed low distances inside the forest, because tree density reduces dispersal distances by birds, suggesting that the invasion that occurs within the forest (Malizia et al. 2017) can be delayed in dense forests. In this way, even undisturbed forests can be invaded, because birds that consume L. lucidum fruits are regular residents of Yungas forests. Similar results were found in tropical forests (Martin et al. 2009).
Environmental effects
Areas with no trees (e.g. abandoned orchard) do not receive L. lucidum seeds due to the lack of fruit-eating birds perching and defecating there (Aragón and Groom 2003; Aragón and Morales 2003). Thus, tree species composition in early successional forests is controlled to some extent by land use history and by differences in the dispersal syndromes of the plant species (Grau et al. 1997; Aragón and Morales 2003). In Yungas forest, during early stages of succession of bare fields pioneer wind-dispersed species arrive first (e.g. the native species Tecoma stans, Heliocarpus papayanensis and Jacaranda mimosifolia) and colonize open areas (Grau et al. 1997). When grown, these pioneers serve as perches for fruit-eating birds. Since L. lucidum is a bird dispersed species, its arrival to open areas is delayed, and hence native pioneers have better opportunities of establishing. By contrast, in lemon plantations Ligustrum invasion may begin immediately after abandonment due to the presence of perches. We did not find seeds in the abandoned orchard area, supporting the idea that woody vegetation serving as perches for birds are necessary to activate the colonization of abandoned fields (Guidetti et al. 2016). This may have important implications for forest management. Lemon plantations are among the most frequent croplands surrounding Yungas forests. When these plantations are abandoned they can be rapidly colonized by L. lucidum because lemon plants serve as perches. Indeed, most areas invaded by L. lucidum in Argentinean Yungas are abandoned lemon plantations (Montti et al. 2017).
As we mentioned before, L. lucidum can spread slowly within dense forest because seeds are transported shorter distances in dense forest than in less dense areas. This pattern could be explained by different mechanisms. Dense tree areas tend to have more food resources, thus birds can forage with little movement between close trees (Carlo et al. 2013), which would maximize their energy budget. Besides, dense tree areas offer protection from some predators, and therefore birds might avoid long-distance flights in open areas during foraging. On the other hand, this pattern of seed dispersal has ecological implications not only for abandoned fields but also for native forests. Mature Yungas forests present low tree density with respect to early successional stages within the same ecoregion (Grau et al. 1997), but they are usually far from L. lucidum seed sources, which are spatially associated with human activity. The distance between invasion foci and mature forests may delay colonization. When glossy privet arrives to native forests and grows to maturity, a change in the species composition of these forests may occur, mainly when L. lucidum increases its canopy cover (Hoyos et al. 2010). However, no generalizations about bird-mediated seed dispersal in response to spatial heterogeneity should be done from single studies. Morales et al. (2013) compared seed dispersal by various species of thrushes and found different behaviors in response to landscape heterogeneity.
Many environmental policies aim at reducing or limiting biological invasions. Most of the times economic resources are scarce, so accurate spatial predictions of the invasion process can be the basis for successful planning and management. Our results suggest that it is difficult to manage L. lucidum once it colonizes the forest. Managing efforts should be concentrated in the massive invasion process, which rapidly expands the range of the species and which locally has huge ecological effects. The combination of the present and potential distribution of glossy privet (Montti et al. 2017) based on our dispersal models are needed in order to identify vulnerable areas. Moreover, land cover maps are useful to identify lemon plantations, where massive invasions occur after abandonment. The elimination of lemon trees in abandoned orchards would favor the arrival of native wind-dispersed species. To further favor the colonization by native species it would be necessary to remove L. lucidum seedlings germinated from the seed bank after lemon tree elimination. This practice would be limited to the first year after crop abandonment because L. lucidum seeds have limited viability (Panetta 2000).
Conclusions
Fruit-eating birds facilitate L. lucidum invasion, through both egestion modes, which contribute differentially to the spread and population growth of the species. Defecation is more relevant in the spread process and regurgitation is more important to population growth. The presence of seed sources and other trees serving as perches determines the arrival of L. lucidum seeds; in low-tree density areas (e.g. mature forests), seeds disperse farther than in high-tree density areas (e.g. secondary forests). Our results are useful to predict which areas near L. lucidum individuals are more vulnerable to be colonized by this invader, and hence, to implement early detection actions. Our results should be combined with present-day distribution maps of L. lucidum and with land cover maps to identify areas that are more vulnerable to massive invasion.
References
Aguirre-Acosta N, Kowaljow E, Aguilar R (2014) Reproductive performance of the invasive tree Ligustrum lucidum in a subtropical dry forest: does habitat fragmentation boost or limit invasion? Biol Invasions 16:1397–1410. doi:10.1007/s10530-013-0577-x
Aikio S, Duncan RP, Hulme PE (2010) Lag-phases in alien plant invasions: separating the facts from the artefacts. Oikos 119:370–378. doi:10.1111/j.1600-0706.2009.17963.x
Akaike H (1987) Factor analysis and AIC. Psychometrika 52:317–332. doi:10.1007/BF02294359
Aragón R, Groom M (2003) Invasion by Ligustrum lucidum (Oleaceae) in NW Argentina: early stage characteristics in different habitat types. Rev Biol Trop 51:59–70
Aragón R, Morales JM (2003) Species composition and invasion in NW Argentinian secondary forests: effects of land use history environment and landscape. J Veg Sci 14:195–204. doi:10.1111/j.1654-1103.2003.tb02144.x
Aragón R, Montti L, Ayup MM, Fernández R (2014) Exotic species as modifiers of ecosystem processes: litter decomposition in native and invaded secondary forests of NW Argentina. Acta Oecol 54:21–28. doi:10.1016/j.actao.2013.03.007
Aslan CE, Rejmanek M, Klinger R (2012) Combining efficient methods to detect spread of woody invaders in urban–rural matrix landscapes: an exploration using two species of Oleaceae. J Appl Ecol 49:331–338. doi:10.1111/j.1365-2664.2011.02097.x
Ayup MM, Montti L, Aragón R, Grau HR (2014) Invasion of Ligustrum lucidum (Oleaceae) in the southern Yungas: changes in habitat properties and decline in bird diversity. Acta Oecol 54:72–81. doi:10.1016/j.actao.2013.03.006
Bélisle CJ (1992) Convergence theorems for a class of simulated annealing algorithms on Rd. J Appl Probab 29:885–895. doi:10.1017/S002190020004376X
Biondi D, Pedrosa-Macedo JH (2008) Plantas invasoras encontradas na área urbana de Curitiba (PR). Floresta 38:129–142. doi:10.5380/rf.v38i1.11034
Blendinger PG, Ruggera RA, Núñez Montellano MG, Macchi L, Zelaya PV, Álvarez ME, Martin E, Osinaga Acosta O, Sanchez R, Haedo J (2012) Fine-tuning the fruit-tracking hypothesis: spatiotemporal links between fruit availability and fruit consumption by birds in Andean mountain forests. J Anim Ecol 81:1298–1310. doi:10.1111/j.1365-2656.2012.02011.x
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background observations and comparisons. Behav Ecol Sociobiol 65:23–35. doi:10.1007/s00265-010-1029-6
Cabrera AL (1976) Regiones fitogeográficas argentinas, 2nd edn. ACME, Buenos Aires
Carlo TA, García D, Martínez D, Gleditsch JM, Morales JM (2013) Where do seeds go when they go far? Distance and directionality of avian seed dispersal in heterogeneous landscapes. Ecology 94:301–307. doi:10.1890/12-0913.1
Ceballos SJ, Malizia A, Chacoff NP (2015) Influencia de la invasión de Ligustrum lucidum (Oleaceae) sobre la comunidad de lianas en la sierra de San Javier (Tucumán–Argentina). Ecol Austral 25:65–74
Clark JS, Macklin E, Wood L (1998) Stages and spatial scales of recruitment limitation in southern Appalachian forests. Ecol Monogr 68:213–235. doi:10.1890/0012-9615(1998)068[0213:SASSOR]2.0.CO;2
Clark JS, Silman M, Kern R, Macklin E, HilleRisLambers J (1999) Seed dispersal near and far: patterns across temperate and tropical forests. Ecology 80:1475–1494. doi:10.1890/0012-9658(1999)080[1475:SDNAFP]2.0.CO;2
Côrtes MC, Uriarte M (2013) Integrating frugivory and animal movement: a review of the evidence and implications for scaling seed dispersal. Biol Rev 88:255–272. doi:10.1111/j.1469-185X.2012.00250.x
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invisibility. J Ecol 88:528–534. doi:10.1046/j.1365-2745.2000.00473.x
Gavier-Pizarro GI, Kuemmerle T, Hoyos LE, Stewart SI, Huebner CD, Keuler NS, Radeloff VC (2012) Monitoring the invasion of an exotic tree (Ligustrum lucidum) from 1983 to 2006 with Landsat TM/ETM+ satellite data and Support Vector Machines in Córdoba Argentina. Remote Sens Environ 122:134–145. doi:10.1016/j.rse.2011.09.023
Grau HR, Aragón R (2000) Árboles Invasores de la Sierra de San Javier Tucumán Argentina. In: Grau HR, Aragón R (eds) Ecología de árboles exóticos en las yungas argentinas. LIEY, Tucumán, pp 5–20
Grau HR, Arturi MF, Brown AD, Aceñolaza PG (1997) Floristic and structural patterns along a chronosequence of secondary forest succession in Argentinean subtropical montane forests. For Ecol Manag 95:161–171. doi:10.1016/S0378-1127(97)00010-8
Guidetti BY, Amico GC, Dardanelli S, Rodriguez-Cabal MA (2016) Artificial perches promote vegetation restoration. Plant Ecol 217:935–942. doi:10.1007/s11258-016-0619-4
Guilhermetti PGC, Vogel GF, Martinkoski L, Mokochinski FM (2013) Aspects of the distribution Ligustrum lucidum ET Ainton in different ecosystems: literature review. Revista Verde de Agroecologia e Desenvolvimento Sustentável 8:171–176
Heleno RH, Ross G, Everard AMY, Memmott J, Ramos JA (2011) The role of avian ‘seed predators’ as seed dispersers. Ibis 153:199–203. doi:10.1111/j.1474-919X.2010.01088.x
Herrera JM, Morales JM, García D (2011) Differential effects of fruit availability and habitat cover for frugivore-mediated seed dispersal in a heterogeneous landscape. J Ecol 99:1100–1107. doi:10.1111/j.1365-2745.2011.01861.x
Hoyos LE, Gavier-Pizarro GI, Kuemmerle T, Bucher EH, Radeloff VC, Tecco PA (2010) Invasion of glossy privet (Ligustrum lucidum) and native forest loss in the Sierras Chicas of Córdoba Argentina. Biol Invasions 12:3261–3275. doi:10.1007/s10530-010-9720-0
IUCN SSC Invasive Specialist Group (2006) Global invasive species database. http://www.issg.org/database/species/ecology.asp?si=621. Accessed 20 June 2016
Jordano P (2000) Fruits and frugivory. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities, 2nd edn. Cabi Publishing, Wallingford, pp 125–166
Lamarque LJ, Delzon S, Lortie CJ (2011) Tree invasions: a comparative test of the dominant hypotheses and functional traits. Biol Invasions 13:1969–1989. doi:10.1007/s10530-011-0015-x
Levey DJ (1987) Seed size and fruit-handling techniques of avian frugivores. Am Nat 129:471–485
Lichstein JW, Grau HR, Aragón R (2004) Recruitment limitation in secondary forests dominated by an exotic tree. J Veg Sci 15:721–728. doi:10.1111/j.1654-1103.2004.tb02314.x
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invisibility. Ecology 80:1522–1536. doi:10.1890/0012-9658(1999)080[1522:GPOPIA]2.0.CO;2
Malizia LR (2001) Seasonal fluctuations of birds, fruits and flowers in a subtropical forest of Argentina. The Condor 103:45–61
Malizia A, Osinaga-Acosta O, Powell PA, Aragón R (2017) Invasion of Ligustrum lucidum (Oleaceae) in subtropical secondary forests of NW Argentina: declining growth rates of abundant native tree species. J Veg Sci. doi:10.1111/jvs.12572
Martin PH, Canham CD, Marks PL (2009) Why forests appear resistant to exotic plant invasions: intentional introductions stand dynamics and the role of shade tolerance. Front Ecol Environ 7:142–149. doi:10.1890/070096
Minetti JL, Acuña LR, Nieva J (2005a) El régimen pluviométrico del Noroeste Argentino. In: Minetti JL (ed) El clima del Noroeste Argentino. Magna, San Miguel de Tucumán, pp 169–186
Minetti JL, Bobba ME, Hernández C (2005b) Régimen espacial de temperaturas en el Noroeste de Argentina. In: Minetti JL (ed) El clima del Noroeste Argentino. Magna, San Miguel de Tucumán, pp 141–161
Montaldo NH (1993) Dispersión por aves y éxito reproductivo de dos especies de Ligustrum (Oleaceae) en un relicto de selva subtropical en la Argentina. Rev Chil Hist Nat 66:75–85
Montaldo NH (2000) Éxito reproductivo de plantas ornitócoras en un relicto de selva subtropical en Argentina. Rev Chil Hist Nat 73:511–524
Montaldo NH (2005) Aves frugívoras de un relicto de selva subtropical ribereña en Argentina: manipulación de frutos y destino de las semillas. El hornero 20:163–172
Montti LF, Piriz-Carrillo V, Gutiérrez-Angonese J, Gasparri NI, Aragón R, Grau HR (2017) The role of bioclimatic features landscape configuration and historical land use in the invasion of an Asian tree in subtropical Argentina. Landsc Ecol. doi:10.1007/s10980-017-0563-2
Morales JM, Carlo TA (2006) The effects of plant distribution and frugivore density on the scale and shape of dispersal kernels. Ecology 87:1489–1496. doi:10.1890/0012-9658(2006)87[1489:TEOPDA]2.0.CO;2
Morales JM, Rivarola MD, Carlo TA (2012) Neighborhood effects on seed dispersal by frugivores: testing theory with a mistletoe-marsupial system in Patagonia. Ecology 93:741–748. doi:10.1890/11-0935.1
Morales JM, García D, Martínez D, Rodriguez-Pérez J, Herrera JM (2013) Frugivore behavioural details matter for seed dispersal: a multi-species model for Cantabrian thrushes and trees. PLoS ONE 8:e65216. doi:10.1371/journal.pone.0065216
Nathan R, Muller-Landau HC (2000) Spatial patterns of seed dispersal their determinants and consequences for recruitment. Trends Ecol Evol 15:278–285. doi:10.1016/S0169-5347(00)01874-7
Pacheco S, Héctor Ricardo Grau HR (1997) Fenología de un arbusto del sotobosque y ornitocoria en relación a claros en una selva subtropical de montaña del Noroeste Argentino. Ecol Austral 7:35–41
Panetta FD (2000) Fates of fruits and seeds of Ligustrum lucidum WT Ait and L sinense Lour maintained under natural rainfall or irrigation. Aust J Bot 48:701–706. doi:10.1071/BT00005
Pero EJ, Busnelli J, Juliá JP (2015) Cambios en la cobertura vegetal y mapeo de un área protegida del NO argentino. Lilloa 52:70–81
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing Vienna Austria. http://www.r-project.org. Accessed 12 June 2015
Ribbens E, Silander JA Jr, Pacala SW (1994) Seedling recruitment in forests: calibrating models to predict patterns of tree seedling dispersion. Ecology 75:1794–1806. doi:10.2307/1939638
Richardson DM, Pyšek P (2013) Plant invasions. In: Levin SA (ed) Encyclopedia of biodiversity, vol 6, 2nd edn. Academic Press, Waltham, pp 90–102. doi:10.1016/B978-0-12-384719-5.00319-1
Richardson DM, Rejmánek M (2011) Trees and shrubs as invasive alien species—a global review. Divers Distrib 17:788–809. doi:10.1111/j.1472-4642.2011.00782.x
Rougès M, Blake JG (2001) Tasas de captura y dietas de aves del sotobosque en el Parque Biológico Sierra de San Javier Tucumán. El Hornero 16:007–015
Schurr FM, Steinitz O, Nathan R (2008) Plant fecundity and seed dispersal in spatially heterogeneous environments: models mechanisms and estimation. J Ecol 96:628–641. doi:10.1111/j.1365-2745.2008.01371.x
Steibel P, Troiani H, Williamson T (2000) Agregados al catálogo de las plantas naturalizadas y adventicias de la provincia de La Pampa Argentina. Revista Fac Agron Univ Nac La Pampa 11:75–90
Traveset A, Verdú M (2002) A meta-analysis of the gut treatment and seed germination. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology evolution and conservation. doi:10.1079/9780851995250.0339
Traveset A, Robertson AW, Rodríguez-Pérez J (2007) A review on the role of endozoochory on seed germination. In: Green RJ, Dennis AJ, Schupp EW (eds) Seed dispersal: theory and its application in a changing world. CABI Publishing Wallingford, pp 78–101. doi:10.1079/9781845931650.0078
Wilcox M (2000) Tree privet (Ligustrum lucidum) a controversial plant. Auckl Bot Soc J 55:72–74
Wilson JR, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM (2009) Something in the way you move: dispersal pathways affect invasion success. Trends Ecol Evol 24:136–144. doi:10.1016/j.tree.2008.10.007
Zamora Nasca L, Montti L, Grau R, Paolini L (2014) Efectos de la invasión del ligustro Ligustrum lucidum en la dinámica hídrica de las Yungas del noroeste Argentino. Bosque 35:195–205. doi:10.4067/S0717-92002014000200007
Acknowledgements
We thank Leonardo Paolini, Lía Montti, Roxana Aragón and anonymous reviewers for valuable comments and suggestions on the manuscript. In addition, we thank Sofía Nanni and Silvia Lomáscolo for improving our written English. Oriana Osinaga Acosta, Román Ruggera and Gabriela Núñez provided valuable assistance in the field. This work was supported by PICT Project n°1382, from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina. P. Powell thanks ANPCyT and CONICET for the post-doctoral fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Powell, P.A., Aráoz, E. Biological and environmental effects on fine-scale seed dispersal of an invasive tree in a secondary subtropical forest. Biol Invasions 20, 461–473 (2018). https://doi.org/10.1007/s10530-017-1548-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1548-4