Abstract
Under laboratory conditions, we investigated the host suitability of 24 poaceous plants for Trigonotylus caelestialium (Kirkaldy) and Stenotus rubrovittatus (Matsumura). More than 50 % of the nymphs of both bugs reached the adult stage on Poa annua L., Alopecurus aequalis Sobol. var. amurensis (Komar.), Poa sphondylodes Trin., Hordeum murinum L., Agrostis clavata Trin. ssp. matsumurae (Hack. ex Honda), and Lolium multiflorum Lam. In addition, a similar number of S. rubrovittatus nymphs reached the adult stage on Dactylis glomerata L. and Digitaria violascens Link. However, a high percentage of T. caelestialium adults emerged on both spikelets and leaves of the host plants, whereas a lower percentage of S. rubrovittatus adults emerged on leaves than on spikelets. While the numbers of T. caelestialium adults that emerged on spikelets and leaves were similar, those reared on spikelets had a shorter developmental period and longer forewings than those reared on leaves, indicating that spikelets were more suitable for growth. In addition, more adults of both species emerged on plants with ears that arise during the spring and early summer than on plants with ears that arise during the summer and fall. Therefore, the development of these bugs is dependent on the season of ear emergence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grain-feeding bugs cause major problems in rice (Oryza sativa L.) and other economically important grains on a global scale. When rice plants in paddy fields are attacked by heteropteran bugs, rice grains (at the milk and dough stages) are susceptible to invasion by bacteria and fungi, resulting in spotted rice. These spotted rice grains are termed pecky rice (Douglas and Tullis 1950; Ingram 1927; Ito 1978). Currently, heteropteran bugs are a major problem for rice farmers in Japan because pecky rice reduces the commercial value of the crops. The rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Hemiptera: Miridae), and the sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Hemiptera: Miridae), both of which are found throughout Japan, specifically cause pecky rice (Watanabe and Higuchi 2006). Poaceous plants are the primary hosts of these rice bugs (Hachiya 1999; Hayashi 1986), which grow on the poaceous plants around the paddy fields and move into the paddy fields during the emergence of rice panicles (i.e., the heading stage).
The identification of plants suitable for the growth of pecky rice bugs might allow their occurrence to be predicted and densities to be suppressed. Monma and Kikuchi (2004) demonstrated that the addition of non-host ground-cover plants could reduce the occurrence of the bugs, while other studies have also indicated that the type of surrounding vegetation can influence the density of bug populations. For example, several studies have suggested that removal of weeds could reduce the occurrence of the bugs (Kikuchi and Kobayashi 2001; Niiyama and Itoyama 2006; Ono et al. 2007; Yokota and Suzuki 2008), and therefore it is important to identify the plant species that host them.
Hosts of T. caelestialium in Japan include ladino clover (Trifolium repens L. var. giganteum Lagreze-Fossat), timothy (Phleum pratense L.), bentgrass (Agrostis palustris Hud.), wheatgrass (Agropyron cristatum (L.) Gaertn.), Kentucky bluegrass (Poa pratensis L.), bermudagrass (Cynodon dactylon (L.) Pers.), rice, Italian ryegrass (Lolium multiflorum Lam.), wiregrass (Eleusine indica (L.) Gaertn.), southern crabgrass (Digitaria ciliaris (Retz.) Koel.), common barnyardgrass (Echinochloa crus-galli (L.) Beauv.), redtop (Agrostis gigantea Roth), annual bluegrass (Poa annua L.), orange foxtail (Alopecurus aequalis Sobol.), and wheat (Triticum aestivum L.) (Hachiya 1999; Kawasawa and Kawamura 1977; Watanabe et al. 2002). Likewise, the hosts of S. rubrovittatus include rice, corn (Zea mays L.), Sudan grass (Sorghum sudanense (Piper) Stapf.), Johnson grass (Sorghum halepense (L.) Pers.), pearl millet (Pennisetum americanum (L.) Leeke.), sweet sorghum (Sorghum saccharatum Pers.), foxtail millet (Setaria itarica (L.) P. Beauv.), rescue grass (Bromus catharticus Vahl), foxtail grass (Setaria viridis (L.) Beauv.), Italian ryegrass, orchard grass (Dactylis glomerata L.), A. aequalis, P. annua, Japanese paspalum (Paspalum thunbergii Kunth), wheat, early barnyardgrass (Echinochloa oryzicola Vasing.), D. ciliaris, and daisy fleabane (Erigeron annuus (L.) Pers.) (Hayashi 1986; Hayashi and Nakazawa 1988; Kato and Hasegawa 1950; Kawasawa and Kawamura 1977). However, as this information is based on field observations or sweep-net sampling, the suitability of these plants for nymphal growth remains unclear. Under laboratory conditions, T. caelestialium grows well on L. multiflorum, P. annua, A. aequalis, A. gigantea, P. pratensis, Eragrostis ferruginea (Thunb.) Beauv., and violet crabgrass (Digitaria violascens Link) (Kikuchi and Kobayashi 2004). Comparable studies of the suitability of various plant hosts for S. rubrovittatus are lacking. Therefore, we investigated the suitability of 24 poaceous plants as hosts for these two species of pecky rice bugs. In addition, we separately tested nymphal growth of the two species on both spikelets and leaves because the presence of ears may influence their growth (Kikuchi and Kobayashi 2004; Sato et al. 2009; Yokota and Suzuki 2007).
Materials and methods
Insects
Trigonotylus caelestialium and S. rubrovittatus were collected from fields at the Hokuriku Research Center, National Agricultural Research Center, Niigata Prefecture, Japan. These insects were successively reared on young wheat seedlings under laboratory conditions (L:D photoperiod, 16:8 h; 25 °C) as described previously (Higuchi and Takahashi 2000; Nagasawa and Higuchi 2008, 2010).
Plants
We used 24 poaceous plants in this study: P. annua, A. aequalis var. amurensis, Anthoxanthum odoratum L., Poa sphondylodes Trin., Hordeum murinum L., D. glomerata, Festuca arundinacea Schreb., Vulpia myuros (L.) C. C. Gmel., Agrostis clavata Trin. ssp. matsumurae (Hack. ex Honda) T. Tateoka, L. multiflorum, Elymus tsukushiensis Honda var. transiens (Hack.) Osada, A. gigantea, D. ciliaris, Eragrostis multicaulis Steud., Setaria faberi Herrm., E. crus-galli, E. indica, Echinochloa crus-galli (L.) Beauv. var. aristata S. F. Gray, O. sativa [cultivar Koshiibuki], Panicum dichotomiflorum Michx., Setaria glauca (L.) Beauv., E. ferruginea, D. violascens, and Pennisetum alopecuroides (L.) Spreng. All plants were growing collected from wild populations growing in or around the experimental fields of the Hokuriku Research Center, except for rice, which was cultivated in the paddy fields of the Hokuriku Research Center. We divided these plants into 2 groups, according to the time in the season when ears appeared and disappeared (Fig. 1). Spring plants, which had ears that appeared before June and disappeared by July, were as follows: P. annua, A. aequalis var. amurensis, A. odoratum, P. sphondylodes, H. murinum, D. glomerata, F. arundinacea, A. clavata ssp. matsumurae, V. myuros, L. multiflorum, E. tsukushiensis var. transiens, and A. gigantea. Summer–fall plants, which had ears that appeared between June and November, were as follows: D. ciliaris, E. multicaulis, S. faberi, E. crus-galli, E. indica, E. crus-galli var. aristata, P. dichotomiflorum, S. glauca, E. ferruginea, D. violascens, and P. alopecuroides.
The sequence of emergence of pecky rice bugs Trigonotylus caelestialium and Stenotus rubrovittatus nymphs and adults, and of poaceous plant ears in Joetsu, Niigata, Japan. The solid and broken lines represent the time when most and some plant ears, respectively, are present. The solid and dashed arrows represent the time when pecky rice bug adults and nymphs, respectively, are present on the plants
Nymphal development
We reared newly hatched T. caelestialium and S. rubrovittatus nymphs (<24 h after hatching) individually in Petri dishes (height 15 mm, diameter 50 mm). Nymphs were provided with either leaves or spikelets from a single plant species until they either emerged as adults or died. A piece of filter paper was placed at the bottom of the Petri dishes and moistened with water to maintain humidity. The expanded leaves or spikelets were obtained from plants of each poaceous species during the flowering stage. We cut the leaves or ears into appropriate sizes and exposed the nymphs to the plant parts (1 or 2 pieces) immediately after preparation. We replaced leaves or spikelets with fresh ones every 1–2 days. To estimate body size, we measured the length of the forewing of newly emerged adults by using a stage micrometer on a microscope. Fifty nymphs of each bug species were reared on the leaves of each plant species and 50 nymphs of each bug were reared on the spikelets of each plant species. We conducted all tests under controlled conditions in the laboratory (L:D cycle, 16:8 h; 25 °C).
Statistical analysis
We performed statistical analyses using R v.2.11.1 for Mac OS X (R Development Core Team 2010). We compared the number of adults that emerged on spring and summer–fall plants using the Wilcoxon rank-sum test. To compare adult emergence between plant species, we used the value from the portion of the plant (leaves or spikelets) that produced the greatest number of adults to represent the species. We compared the number of adults that emerged on the leaves and spikelets of the same plant using Fisher’s exact test. To compare the performance of the host plant, the duration of the nymphal period (days) and the size (forewing length) of emerged T. caelestialium adults on leaves and spikelets were compared using the Wilcoxon rank-sum test. Mean values were significantly different when P < 0.05.
Results
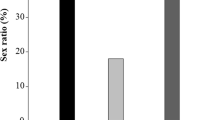
The percentages of T. caelestialium and S. rubrovittatus adult emergence are shown in Figs. 2 and 3, respectively. More than 50 % of T. caelestialium nymphs emerged as adults when reared on the leaves or spikelets of the following spring plants: P. annua, A. aequalis, P. sphondylodes, H. murinum, D. glomerata, A. clavata, and L. multiflorum. However, the number of emerging T. caelestialium adults on summer–fall plants was significantly lower than that on spring plants (Wilcoxon rank-sum test, P < 0.01; Fig. 2). Among those plant species with >50 % adult emergence, the number of emerging T. caelestialium adults on the leaves differed significantly from those on the spikelets of D. glomerata and A. clavata (Fisher’s exact test, P < 0.001). However, the number of emerging T. caelestialium adults did not differ significantly between the leaves and spikelets of P. annua, A. aequalis, P. sphondylodes, H. murinum, and L. multiflorum (Fisher’s exact test, P > 0.05). To compare the leaf and spilkelet performance of these 5 suitable plants, the duration of the nymphal period and the length of the forewing of T. caelestialium were measured. The duration of the nymphal period on the 5 plant species was significantly shorter on spikelets than on leaves (Wilcoxon rank-sum test, P < 0.05; Table 1). The length of the forewing of T. caelestialium adults was significantly longer when nymphs were reared on spikelets than on leaves, except for males and females on A. aequalis and males on H. murinum (Wilcoxon rank-sum test, P < 0.01; Table 2).
More than 50 % of all S. rubrovittatus nymphs emerged as adults when reared on the spikelets of P. annua, A. aequalis, P. sphondylodes, H. murinum, D. glomerata, A. clavata, L. multiflorum, and D. violascens, which are all spring plants (except for D. violascens). However, the percentage of adult emergence of S. rubrovittatus was significantly lower on summer–fall plants than on spring plants (Wilcoxon rank-sum test, P < 0.05; Fig. 3). In addition, there were significantly fewer emergent S. rubrovittatus adults on the leaves than on the spikelets of plant species with >50 % adult emergence (Fisher’s exact test, P < 0.001; Fig. 3).
Discussion
The development and ear emergence times of poaceous plants vary depending on the species; therefore, species compositions change across seasons (Osada 1993). As a result, T. caelestialium and S. rubrovittatus, which have 4–5 generations per year (Ishimoto 2004; Sato et al. 2009), use a succession of host plants. Our field observations showed the heading stage of potential host plants of two rice bugs (Fig. 1). A high percentage of both T. caelestialium and S. rubrovittatus emerge as adults when reared on several spring plants. In contrast, a relatively low percentage of these two species emerge as adults when reared on summer–fall plants. This indicates that overwintering insects and early generations of these insects that occur during the heading stage of spring plants will perform very well on highly suitable host plants, while the growth of subsequent generations will be poorer and on less suitable host plants. This is consistent with previous studies that showed that densities of both insects decrease in summer (Sato et al. 2009; Takita 2005).
Our results demonstrate that the development of S. rubrovittatus depends on the presence of spikelets of poaceous plants. Removing ears of weedy grasses will therefore limit the growth of S. rubrovittatus. These results are consistent with those of several previous studies, which demonstrate that removing grass weeds around paddy fields reduces the occurrence of pecky rice bugs (Kikuchi and Kobayashi 2001; Niiyama and Itoyama 2006; Ono et al. 2007; Yokota and Suzuki 2008). However, our results also show that T. caelestialium can grow on poaceous plants, regardless of whether ears are present. As a result, the cutting of grass weeds may have a limited effect on the development of T. caelestialium because the leaves of the host plants will still be present. The percentage adult emergence of T. caelestialium was similar on leaves and spikelets. However, spikelets provide more nourishment than leaves, as indicated by the shorter period of nymphal development and longer length of adult forewings when nymphs were raised on the spikelets than when they were raised on the leaves of host plants. Forewing length is positively associated with survival time and the number of eggs in T. caelestialium adults (Shintani 2009). Therefore, the presence of grass ears will also promote T. caelestialium nymphal growth and adult fecundity.
The time of year (season) can influence the growth of host plants. Shintani (2009) found that the nutritional quality of host plants such as L. multiflorum and D. ciliaris exhibits seasonal variation, and that this results in reduced development of T. caelestialium nymphs during the summer. Therefore, further research is required to determine the effect of seasonal variation on the nutritional quality of other T. caelestialium and S. rubrovittatus host plants. In addition, differences between the developmental stages of host plants may affect nymphal growth. Acquisition of this knowledge may improve our understanding of how pecky rice bugs use host plants.
The plant preferences of phytophagous insects for oviposition are important. Because some insects exhibit asymmetry between plants preferred for oviposition and those suitable for nymphal development (Digweed 2006; Eben and López-Carretero 2008; Gratton and Welter 1998; Roininen and Tahvanainen 1989), the host plants used by T. caelestialium and S. rubrovittatus cannot be determined by measuring nymphal development alone. Furthermore, selection of oviposition sites by adults greatly limits the range of host plants used by immature stages that are less mobile (Bernays and Chapman 1994; Schoonhoven et al. 2005; Wennström et al. 2010). To date, field investigations have revealed that T. caelestialium oviposits both on the ear and leaf sheaths of poaceous plants, whereas S. rubrovittatus oviposits only on the ear (Nagasawa 2007). These observations correspond with the suitability of plant parts for nymphal growth that was demonstrated in the present study. Because S. rubrovittatus nymphs particularly depend on spikelets, oviposition on spikelets will ensure the presence of food for the offspring on the plants. It remains unclear whether host plant selection for oviposition corresponds with the suitability of food plants for the nymphal growth of these bugs.
The nymphs of both bugs failed to grow on either the leaves or spikelets of rice plants in the present study. Previous studies (Ishimoto 2008; Ishimoto and Sato 2006) have indicated that, when reared on rice plant seedlings, S. rubrovittatus was unable to grow and a low percentage of T. caelestialium emerged as adults. This is in accordance with our results and indicates that rice plants without ears have little value as food for these bugs. The nymphs of these bugs are reported to grow on hulled rice (Ishimoto 2008; Ishimoto and Sato 2006), In addition, Ishimoto (2007) reported that survival rates of T. caelestialium nymphs greatly depend on the presence of split hulls. This indicates that growth of the nymphs depends on opportunity to suck the contents of the rice grains from splits in the hulls. While T. caelestialium lay eggs on rice plants (Nagasawa et al. 2012), S. rubrovittatus adults lay few eggs on these plants (Ishimoto 2011; Nagasawa et al. 2012). This results in few S. rubrovittatus nymphs emerging on rice plants. Therefore, in paddy fields, emergence of S. rubrovittatus nymphs necessitates oviposition on plants other than rice (e.g., Poaceae or Cyperaceae plants). Removal of these weeds in paddy fields may be effective in preventing the occurrence of nymphs on rice plants. Although T. caelestialium is able to emerge on rice plants, it cannot grow on rice without split-hull paddies. Therefore, removal of suitable host plants around paddy fields may also be effective in preventing growth of T. caelestialium nymphs.
In conclusion, we demonstrate that the seasonal abundance of host plants affect the development of the pecky rice bugs T. caelestialium and S. rubrovittatus. We identified poaceous plants that enhance the growth of the rice bugs. Preventing the growth of these plants around rice paddies could help to suppress the occurrence of pecky rice. In order to apply this knowledge, we need to know how the abundance of these host plants, and their distance from paddy fields, is correlated with the occurrence of pecky rice. Our results may be important for developing effective management strategies to predict and control pecky rice bugs and improve rice production in Japan.
References
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman & Hall, New York
Digweed SC (2006) Oviposition preference and larval performance in the exotic birch-leafmining sawfly Profenusa thomsoni. Entomol Exp Appl 120:41–49
Douglas WA, Tullis EC (1950) Insects and fungi as causes of pecky rice. US Dep Agric Tech Bull 1015:1–20
Eben A, López-Carretero A (2008) Asymmetry of larval diet breadth and oviposition preference in Leptinotarsa undecimlineata. Entomol Exp Appl 128:27–33. doi:10.1111/j.1570-7458.2008.00696.x
Gratton C, Welter SC (1998) Oviposition preference and larval performance of Liriomyza helianthi (Diptera: Agromyzidae) on normal and novel host plants. Environ Entomol 27:926–935
Hachiya K (1999) Migration of rice leaf bug, Trigonotylus caelestialium (Kirkaldy) into rice fields and forecasting of the abundance. Plant Prot 53:268–272 (in Japanese)
Hayashi H (1986) Ecology and control of the sorghum plant bug (Stenotus rubrovittatus Matsumura) causing the pecky rice. Plant Prot 40:321–326 (in Japanese)
Hayashi H, Nakazawa K (1988) Studies on the bionomics and control of the sorghum plant bug, Stenotus rubrovittatus Matsumura (Hemiptera: Miridae) 1. Habitat and seasonal prevalence in Hiroshima Prefecture. Bull Hiroshima Agric Exp Stn 51:45–53 (in Japanese with English summary)
Higuchi H, Takahashi A (2000) Method of rearing the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae) with wheat seedlings. Proc Assoc Plant Prot Hokuriku 48:23–25 (in Japanese)
Ingram JW (1927) Insects injurious to the rice crop. US Dep Agric Farmers Bull 1543:1–16
Ishimoto M (2004) Seasonal prevalence of occurrence of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae) on paddy rice plants. Jpn J Appl Entomol Zool 48:79–85 (in Japanese with English summary)
Ishimoto M (2007) Effects of the ripening stage and the occurrence of split-hull paddy of rice plant on development of nymphs of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae). Jpn J Appl Entomol Zool 51:107–114. doi:10.1303/jjaez.2007.107 (in Japanese with English summary)
Ishimoto M (2008) Development of sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae) nymphs reared on hulled rice. Jpn J Appl Entomol Zool 52:139–141. doi:10.1303/jjaez.2008.139 (in Japanese with English summary)
Ishimoto M (2011) Oviposition of sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae) on rice plants. Jpn J Appl Entomol Zool 55:193–197. doi:10.1303/jjaez.2011.193 (in Japanese with English summary)
Ishimoto M, Sato H (2006) Effects of supplying grains as supplementary food on development of nymphs and fecundity of adults of rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae). Jpn J Appl Entomol Zool 50:305–310. doi:10.1303/jjaez.2006.305 (in Japanese with English summary)
Ito K (1978) Ecology of the stink bugs causing pecky rice. Rev Plant Prot Res 11:62–78
Kato S, Hasegawa H (1950) Calocoris rubrovittata Mats., a new pest of Sorghum sudanense. Oyo Kontyu 6:149 (in Japanese)
Kawasawa T, Kawamura M (1977) Kamemushi Hyakushu. Zenkoku Nouson Kyouiku Kyoukai, Tokyo (in Japanese)
Kikuchi A, Kobayashi T (2001) Effect of weed mowing on the abundance of Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae). Annu Rep Plant Prot North Japan 52:143–145 (in Japanese)
Kikuchi A, Kobayashi T (2004) Growth and oviposition of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae), on gramineous weeds and Italian rye grass. Annu Rep Plant Prot North Japan 55:149–154 (in Japanese)
Monma Y, Kikuchi A (2004) The development and preference of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae), for ground cover plants. Annu Rep Plant Prot North Japan 55:128–130 (in Japanese)
Nagasawa A (2007) Gramineous plants used as oviposition sites by pecky rice bugs, Trigonotylus caelestialium (Kirkaldy) and Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae). Proc Assoc Plant Prot Hokuriku 56:29–31 (in Japanese)
Nagasawa A, Higuchi H (2008) A method for rearing Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae) by using the spikelets of gramineous weeds as oviposition sites and wheat seedlings for rearing nymphs. Jpn J Appl Entomol Zool 52:1–6. doi:10.1303/jjaez.2008.1 (in Japanese with English summary)
Nagasawa A, Higuchi H (2010) Storage of stem cuttings of wheat seedlings under high-humidity conditions to improve larval emergence after oviposition by Stenotus rubrovittatus (Matsumura) (Heteroptera: Miridae). Jpn J Appl Entomol Zool 54:197–203. doi:101.1303/jjaez.2010.197 (in Japanese with English summary)
Nagasawa A, Takahashi A, Higuchi H (2012) Host plant use for oviposition by Trigonotylus caelestialium (Hemiptera: Miridae) and Stenotus rubrovittatus (Hemiptera: Miridae). Appl Entomol Zool. doi:10.1007/s13355-012-0123-9
Niiyama T, Itoyama K (2006) Control of rice leaf bug, Trigonotylus caelestialium (Heteroptera: Miridae) by application of herbicide to their source of immigration. Annu Rep Plant Prot North Japan 57:129–133 (in Japanese)
Ono T, Kashin J, Kidokoro T (2007) Control of sorghum plant bug, Stenotus rubrovittatus (Matsumura) (Hemiptera: Miridae), in rice paddy field by grass mowing in relation to migration source. Annu Rep Plant Prot North Japan 58:75–79 (in Japanese)
Osada T (1993) Illustrated grasses of Japan. Heibonsha, Tokyo (in Japanese and English)
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Roininen H, Tahvanainen J (1989) Host selection and larval performance of two willow-feeding sawflies. Ecology 70:129–136
Sato H, Ishimoto M, Yokoyama Y (2009) Seasonal prevalence of the sorghum plant bug, Stenotus rubrovittatus (Heteroptera: Miridae), in Niigata prefecture. Proc Assoc Plant Prot Hokuriku 58:7–12 (in Japanese)
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect–plant biology. Chapman & Hall, London
Shintani Y (2009) Effect of seasonal variation in host-plant quality on the rice leaf bug, Trigonotylus caelestialium. Entomol Exp Appl 133:128–135. doi:10.1111/j.1570-7458.2009.00915.x
Takita M (2005) Fluctuation in the number of males of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae), captured by synthetic sex pheromone traps placed in fields overgrown with weeds. Proc Assoc Plant Prot Hokuriku 54:19–22 (in Japanese)
Watanabe T, Higuchi H (2006) Recent occurrence and problem of rice bugs. Plant Prot 60:201–203 (in Japanese)
Watanabe K, Ishii S, Domon K (2002) Control of the rice leaf bug, Trigonotylus caelestialium (Kirkaldy) (Heteroptera: Miridae), by cultivating upland field. Annu Rep Plant Prot North Japan 53:168–172 (in Japanese)
Wennström A, Hjulström LN, Hjältén J, Julkunen-Tiitto R (2010) Mother really knows best: host choice of adult phytophagous insect females reflects a within-host variation in suitability as larval food. Chemoecology 20:35–42. doi:10.1007/s00049-010-0040-8
Yokota H, Suzuki T (2007) Effects of heading frequency of gramineous weeds on population density of Stenotus rubrovittatus in footpath between paddy fields. Annu Rep Plant Prot North Japan 58:88–91 (in Japanese)
Yokota H, Suzuki T (2008) Most effective timing of mowing on the population density of the nymphs of the overwintering generation of Stenotus rubrovittatus in the footpath between paddy fields. Annu Rep Plant Prot North Japan 59:116–119 (in Japanese)
Acknowledgments
We thank Dr. A. Takahashi of Hokuriku Research Center, NARO Agricultural Research Center, and Dr. M. Ishimoto of Niigata Agricultural Research Institute, Crop Research Center, for their valuable suggestions. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagasawa, A., Higuchi, H. Suitability of poaceous plants for nymphal growth of the pecky rice bugs Trigonotylus caelestialium and Stenotus rubrovittatus (Hemiptera: Miridae) in Niigata, Japan. Appl Entomol Zool 47, 421–427 (2012). https://doi.org/10.1007/s13355-012-0135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-012-0135-5