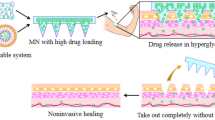
Abstract
Diabetes is one of the most serious chronic diseases today. Patients with diabetes need frequent insulin injections or blood sampling to monitor blood glucose levels. The microneedles are a painless transdermal drug delivery system, which has great advantages in achieving self-management. There have been a lot of researches on microneedles used in diabetes treatment. Microneedle-based treatment of diabetes has also changed from a simple and reliable system to a complex and efficient system. This review introduces microfluidic, glucose response, and other contents based on microneedles, and some challenges in the development of microneedles.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes is one of the fastest growing diseases in the twenty-first century. The amount of adults living with diabetes, aged 20–79 years old, has increased from 151 million in 2000 to 463 million in 2019, which is tripled in two decades. In addition, there are 1.1 million juvenile diabetic patients. International Diabetes Federation (IDF) estimates that the global annual diabetes-related medical expenditure is USD 760 billion and be probable to reach USD 825 billion by 2030 [1]. The Sustainable Development Goals has listed diabetes as one of the four main non-communicable diseases in 2015. Subsequently, the member states of Universal Health Coverage commit to develop national diabetes plans before 2030 and allocate corresponding technical and financial resources for diabetes. However, inequalities in high-quality medical care are still serious across the world, particularly in developing countries, where people cannot access to enough drug resources because of insufficient funding. Fortunately, the emergence and gradual maturity of polymer microneedles (MNs) may provide a more economical therapy of diabetes worldwide.
MN patch is a type of transdermal delivery technology under rapidly progress, which has been used for cosmetic [2, 3], vaccine [4, 5], diagnostic [6], or therapeutic applications including diabetes treatment. The needles of patch are usually in the range of 300–900 μm in height, 200–500 μm in base width, and 5–10 μm in tip radius. From the first MN patch was invented by Gerstel and Place in 1976 [7], the materials for manufacturing microneedles have expanded from silicon, glass, and metal to polymer materials during decades. These facile yet effective devices can break through the barrier of stratum corneum, thereby transporting drugs into the body through temporary microchannels. It is precisely because of this principle that transdermal delivery of insulin is possible [8]. Commonly, passive permeation requires the molecular weight of the drug is less than 500–600 Da [9], the log p-value is between 1 and 3 [10], and it is amphipathic. However, the molecular weight of insulin, as a protein hormone, exceeds 5800 Da. It is inefficient or impossible for most transdermal techniques to deliver insulin. For the same reason, oral administration also faces the challenge of passing through the intestinal mucosa. In addition, there are more difficulties including digestive system hydrolysis, enzymatic hydrolysis, and gastric acid environment. The suffering of the elderly in swallowing and the children’s resistance to tablets are additional issues that need to be considered.
Since the therapeutic availability of insulin was firstly proved in 1921 [11], the administration of injection has been almost the only recommended treatment for type 1 diabetes for a century. Nevertheless, there are many disadvantages of injections that need to be resolved. The premise of controlling blood glucose level within the normal range is frequent supplementation of exogenous insulin. However, the pain caused by needles always be a problem and frequent injections exacerbate it. Adipose tissue atrophy or hyperplasia [12], nerve damage, skin infections, etc., may also occur at the injection site [13]. Moreover, it is a great trouble for diabetics to meet the low temperature conditions required for the preservation and transportation of the insulin solution. Carrying with refrigerated devices and exposing abdomen for injections also cause tremendous psychological pressure on diabetics.
The emergence of microneedles not only solves the above problems, simpler waste disposal [14], lower material costs, and milder storage conditions [15] may also be the advantages of certain types of microneedles. Additionally, microneedle therapy does not require professional medical training, finger pressure, or the assistance of a micro-release device can complete treatment easily [16]. According to the instructions, to optimize the care of diabetics of the Chronic Care Model, the drug delivery system should support self-management [17]. These all mean that microneedles have huge economic advantages and broad application prospects in the treatment of diabetes [18].
The effectiveness of microneedles to deliver insulin has been proved by more and more studies [19,20,21,22]. Nowadays, microneedles for the treatment of diabetes also have been transitioned from the stage of proving effectiveness to the construction of a more controlled and efficient system. This review mainly introduces two different upgrading routes of microneedles. One is to develop an integrated microneedle system by introducing components with various physical functions, and the other is to directly control drug delivery based on chemical reactions of the MNs materials.
Integrated microneedles systems
Microneedles have developed a variety of microneedle forms such as solid microneedles, coated microneedles, hollow microneedles, dissolvable microneedles, and swelling microneedles. No matter what kinds of microneedles, they all need to meet two basic points: (1) to deliver adequate drug dose and (2) to deliver the required dose accurately. To enable microneedles satisfy these requirements, researchers naturally integrate various components with microneedles to form a complex and powerful system.
Integrated microneedles systems for drug delivery
In 2003, McAllister et al. [23] delivered insulin through HMNs successfully for the first time. After 30 min of infusion under a pressure of less than 14 psi, the blood glucose level of diabetic rats decreased steadily, which was 70% lower than that of rats 5 h ago.
Subsequently, the Prausnitz team [24] prepared an array consist of 16 microneedles for insulin delivery. Within 4 h of insulin injection, blood glucose in the mice dropped to 47% of the previous value and remained stable during the following 4 h. 29G hypodermic needle, which is the positive control group, was used to inject 50 mU and 500 mU insulin solution. The results showed that the plasma insulin content in the microneedle group was only 1.4 mU, while the outcome was similar to that of the 50 mU subcutaneous injection group. This order of magnitude difference resulted from the hollow microneedle delivering insulin near Capillary Loops, resulting in a stronger pharmacodynamic response.
Although hollow microneedles have such advantages, they cannot complete drug delivery as a single structure. In order to achieve the basic drug delivery capabilities, reservoirs, micro pumps, and microfluidic structures combined with hollow microneedles, these integrated systems achieved the ability to penetrate the skin surface and accurately deliver the prescribed amount of drug.
Microneedles integrated with micro-pumps
For people with type 1 diabetes, different diets often mean different doses of insulin. The ideal microneedles can deliver variable doses of drug with desired requirements.
Due to the advantages of extremely low operating frequency, fast response speed, and high reliability, piezoelectric (PZT) pumps have attracted widespread attention. In addition, an insulin PZT pump with a maximum flow rate of 5.74 ml/min under low driving voltage conditions was reported by Liu et al. [25]. Ma et al. [26] used hollow microneedle combined with PZT pump to deliver insulin solution as early as in 2006. However, the entire system simply connected the PZT pump and the hollow microneedle, which is not enough to compete with commercial insulin pumps.
However, follow-up researchers proposed a further design. As shown in Fig. 1, Meshkinfam et al. [27] designed a proof-concept of a drug delivery device assembled with a microneedle and a micropump. The thickness of the entire device is only 2.35 mm, but it can complete a series of processes including driving of the drug solution and transdermal delivery. They simulated drug release of the integrated device, and achieved the programmed output of insulin solution. Before this, Rao et al. [28] and Haldkar et al. [29] also modified the pedestal of the hollow microneedle and directly connected to the PZT pump.
In theory, the microneedles integrated with micropumps can deliver drug solution with an accuracy of microliters, which is of great significance for the precise and controllable delivery of hollow microneedles. Table 1 lists some designs that combine hollow microneedles with PZT pumps. The designs have gradually evolved from rough and simple to more tiny “Systems-on-A-Chip.” Although miniaturization should not be the only goal of the microneedle system, the miniature system allows diabetic patients to try more diverse forms of sports than traditional insulin pumps, and its small size and stylish appearance will greatly reduce the psychological stress of patients.
Because the “Systems-on-A-Chip” requires various types of materials and involves multiple processing steps, it has great difficulties in manufacturing. Therefore, many studies only simulate and optimize the piezoelectric properties and microneedle structure.
Microneedles integrated with microfluidic
Yeung et al. [30] prepared hollow microneedles with microfluidic structures based on 3D printing technology in 2019. And the use of different fluorescent dye solutions on skin verified good drug transdermal regulation and delivery capabilities of the devices. Prior to this, there have been many articles showing the great application potential of microfluidic structures in regulating the delivery of microneedles.
Microfluidic is a system that uses microtubes (tens to hundreds of microns in size) to process or manipulate tiny fluids (nanolitre to alitre in volume). It is an effective technology to achieve rapid transport and mix small volume of fluids by adjusting the reaction conditions, such as flow rate, concentration, etc. [31]. Microfluidic has been used to adjust the synthesis of some specific physical and chemical properties of nanoparticles, such as particle size, dispersion, and other parameters [32].
With the development of co-dosing therapy, more and more patients will receive multiple treatments at the same time. The combination of microfluidic and microneedles satisfies this need well [33]. For diabetic patients, microneedles open the skin channel painlessly, and additional microfluidics can simultaneously control the infusion of basal insulin and rapid-acting insulin, which better mimics the secretion curve of physiological signals. This integrated system can flexibly combine various biological agents, avoiding complex formulation development [34].
Microfluidic not only plays an important role in drug delivery of microneedles, but also plays a unique role in microneedle-based glucose monitoring systems. In 2019, Takeuchi et al. [35] integrated microfluidics on porous microneedles to detect blood sugar. The introduction of microfluidics enables an extremely small amount of tissue fluid to fill the whole structure and achieve continuous flow. The result of the PBS flow test verified the capacity of the device to extract fluid and enough temporal resolution. This may be another solution to the problem that microneedles are difficult to extract enough interstitial fluid in a short time.
Integrated microneedles systems for monitoring blood glucose levels
The blood glucose level is the most important valve to guide the therapy of diabetes. IDF recommends patients to assess glucose levels prior to meals, at bedtime, prior to exercises, and other lots of situations [45]. In fact, the use of microneedles for blood glucose monitoring has also been extensively studied.
Invernale et al. [46] designed a sensor composed of a platinum-plated stainless steel microneedle array. GOx enzyme was immobilized on the surface of the microneedles to convert blood glucose signals. The blood glucose level change could be converted into a corresponding current at a low voltage.
As shown in Fig. 2, Li et al.[47] prepared a mesoporous microneedle (MMN) using poly (glycidyl methacrylate) and polyethylene glycol (PEG) materials. The insulin delivery area consists of MMN combined and another microneedle sputtered with Au. The delivery area could control insulin delivery by regulating the iontophoretic current and durations. In the detection area, three electrode systems were printed on the back of mesoporous microneedle for glucose detection, and the working electrode is modified by GOx. Between these two areas is a control area printed on a flexible PCB board that integrates ion electrophoresis control, signal processing, and feedback. Such a fully integrated closed-loop system based on the microneedles realized monitors and treats diabetes in situ.
The schematic diagram of integrated closed-loop system based on mesoporous microneedles-iontophoresis [47]. a) Schematic and b) illustration of integrated MN system. c) The electrical field distribution and insulin concentration profile of the device. The effect of d) iontophoretic duration, e) electric field, f) porosity on delivery. g) Schematic and h) image of integrated MN system. i) Quantification of insulin released from device. j) Photograph of integrated device application. k) Blood glucose level, l) normoglycemia and minimum blood glucose and m) plasma insulin concentrations of diabetic rats during the experiments
Heifler et al. [48] also prepared a microneedle system for monitoring and treatment of diabetes. They replaced the three-electrode system with silicon nanowire field-effect transistors, which avoided the interference of chemical impurities oxidizing on the electrode surface. Anthraquinone was grafted on the surface of the electrode, which can react with hydrogen peroxide produced by glucose, resulting in changes in electric current, and realized the monitoring of blood glucose level. From there, they assembled a micropump on the microneedle to deliver the glucose solution and the results showed that there was an immediate jump in the sensor signal.
Integrated microneedle system based on 3D printing technology
For microneedles made of inorganic materials such as silicon [49,50,51], glass [23], ceramics [52], and metals [53], the preparation process is complicated and the cost is high. The early selection of silicon for preparing microneedles was often due to its excellent biocompatibility and mechanical properties. However, it can cause inflammation and infection when tips broke in the body. Moreover, it is difficult to integrate more components which made of different material, and it is inevitable to use high temperature, high voltage, or welding processes to assemble [54, 55]. Therefore, polymer materials for applications in bio-science, especially low-cost and disposable devices, have attracted great interest.
Back in 2015, Strambini et al. [56] adopted the standard processing technology of silicon micromachining to fabricate a hollow MN with an additional reservoir. However, these processing techniques not only suffer from the same complexity mentioned above, but also have limitations on the geometry and aspect ratio of MNs. Using the high-resolution TPP 3D printing technique, Moussi et al. [43] prepared a microneedle with a reservoir in one step. This method avoids the use of adhesives, and greatly reduces the total amount of work required for various components, and there is no need to worry about uncontrollable leakage of the drug solution. Regardless of the material cost and safety of the microneedles, 3D printing technology has huge technical advantages.
3D printing has become a viable option for manufacturing microneedles [57], and different methods such as fused deposition modeling (FDM), stereolithography (SLA), and two-photon polymerization (TPP) have been continuously developed. Despite the success of preparing multiple components in one step through 3D printing technology, there are still many challenges to integrate more components with microneedles in the future. There are a lot of uncertainties about whether 3D printing technique can complete the task. The various polymer materials of 3D printing could not meet all performance requirements, and there is also insufficient data on material safety. Therefore, microneedle integrated device from material preparation to the technique selection still has a lot of space for development. The high cost of 3D printing for mass production of microneedles is an additional problem. Therefore, there is still a lot of work to in materials, fabrication methods, structural design, and other aspects of preparing such a microneedle integrated device.
Structures of iontophoresis assisted solid microneedle system for insulin delivery [58]. a) Schematic illustration of a smartphone-powered transdermal drug delivery system. b) Schematic representation of the drug delivery mechanism of the device
Solid microneedles are theoretically defined as un-dissolvable microneedles that do not directly deliver drugs, and an additional step is required for drug administration. Solid microneedles are the first form of microneedles used for insulin delivery. However, a large number of subsequent studies on solid microneedles also found that they are difficult to deliver drugs quantitatively, while accurate dosage is an important requirement to ensure an effective and safe treatment. As shown in Fig. 3, Yang et al. [58] designed an integrated system based on solid microneedles for delivering insulin solution in 2020. The solid microneedle is pressed to open the skin barrier, and then the insulin solution loaded in the sponge enters the body. In particular, insulin was encapsulated in positive charge vesicles and the dosage was regulated by applying different current intensities. This is an important inspiration for the development of integrated systems for solid microneedles.
Table 1 lists the integration of hollow microneedles, micropumps, and microfluidic components. In fact, these are all types of MEMS. The integration of various MEMS components allows the microneedle system to retain the digital capability. With the development and progress of MEMS technology, the blood glucose monitoring system and the drug delivery system can also be integrated to realize the digital and intelligent treatment of diabetes [59].
Currently, the microneedle-based blood glucose sensor [35, 46, 60] and drug delivery system are still separately developed, the integrated microneedles are merely “semi-integrated” systems. There is still a long way to achieve the “closed loop” therapy of diabetes on a single microneedle patch. Nevertheless, the integrated microneedles achieve effective and precise control of drug delivery by introducing various structures. This system is easier to achieve data conversion and can digitally record and process the blood glucose signals and dosage of drug, which has broad prospects for future diabetes treatment [61].
Improvement of materials to optimize MN systems
Instead of a hybrid microneedle system that integrates various components to control drug delivery, other groups are committed to harness advances in materials science to regulate the release of insulin. To put it more vividly, the integrated microneedles stack up “physics value” to control insulin delivery, while this form of microneedles attempt to stack up “magic value.” As listed in Table 2, the researchers prepared microneedles by synthesizing various materials that can respond to changes in blood glucose levels to achieve “smart” drug delivery. To a certain extent, the microneedles for insulin delivery are advanced, compared with integrated microneedles for blood glucose sensors and drug delivery devices that are still being studied separately. These microneedles give up the conversion of electrical signals and physical functions, and directly uses the chemical reaction of the materials in the blood glucose environment to regulate the release of insulin [62].
Glucose-response materials used in microneedles
Although we discussed only some applications of “smart” microneedles below, it is worth mentioning that these works are benefited from the advancement of material research. Glucose-response microneedles are based on research on the properties of glucose reactive gels and various materials.
The polymer materials used to prepare responsive microneedles consists of two critical components, one that reacts with glucose and the other that changes in response to these chemical environments. In recent years, researchers have adopted glucose oxidase (GOx), phenylboronic acid (PBA) as glucose-sensitive factors, combined with the dissolution, swelling, and degradation of chemical materials, to design microneedle-based drug delivery systems to achieve a controlled release of drugs with glucose concentration.
Glucose-response microneedles based on GOx
Glucose oxidase can catalyze the oxidation of glucose to gluconic acid while consuming oxygen to generate hydrogen peroxide [63]. This reaction is the most classic in the glucose response system [64, 65]. Utilizing the gluconic acid and H2O2 generated by GOx, lots of materials could dissolve or swell and release insulin [66].
Hu et al. [67] synthesized a diblock copolymer (mPEG-b-polyserine) using the amine-initiated ring-opening polymerization of the N-carboxy-α-amino acid anhydride of serine. 4-(Hydroxymethyl) phenylboronic acid pentanol ester (PBE) was coupled to the pendant hydroxyl group of the serine residue through a carbonic acid bond. The diblock copolymer mPEG-BP (Ser-PBE) was self-assembled into polymer vesicles (PVs) by solvent evaporation method. Then insulin and GOx enzyme were encapsulated subsequently. As the microneedles dissolve into the body, the drug-loaded particles are unable to release insulin. In the presence of H2O2, PVs will lose its PBE side chain and become water-soluble again, leading to the disassemble of PVs and release of preloaded insulin [68, 69]. In vivo experiments proved that the blood glucose of mice treated with MN[I] (only loaded with insulin) and MN[PVs] (loaded with particles) dropped rapidly to about 90 mg/dL within 1 h. However, the blood glucose levels of MN[I] group rise rapidly within the next hour. The blood glucose of the MN[PVs] group remained in the normal range (< 200 mg/dL) for about 5 h, and then the blood glucose level gradually increased. This “smart” microneedle showed glucose-mediated decomposition in vivo and in vitro, and rapidly released encapsulated insulin under hyperglycemic conditions. It is important that when the blood sugar level reaches a normal blood sugar state, the insulin release rate decreases, which can avoid the risk of hypoglycemia.
Later, the toxicity of H2O2 produced in the catalytic turnover cycle was recognized and it was also found that H2O2 could inactivate GOx. Therefore, these problems be alleviated by introducing hydrogen peroxide scavengers, such as catalase (CAT) into the system. Since CAT can convert H2O2 to H2O and O2, CAT and GOx are often co-immobilized with GOx in different systems. Utilizing the same principle that GOx generates H2O2 under hyperglycemia, Wang et al. [70] modified insulin with 4-nitrophenyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxborane-2yl) benzyl carbonate, the modified insulin subsequently immobilized to the PVA matrix. It should be noted that the cross-linking agent of this step is H2O2-labile cross-linking agent. The GOx encapsulated in the gel is also fixed on the PVA matrix through the stable covalent bond which generated by free radical polymerization. Therefore, GOx will not be released during the entire cycle. Finally, the outer layer of the microneedle is covered with CAT to prevent H2O2 from leaking into the body. The article reported that the cross-linked gel can quickly release insulin after being triggered by H2O2 generated by GOx. In vivo experiments also showed that microneedles could effectively regulate blood glucose levels (BGLs), and CAT coating has significant prospects in eliminating inflammation caused by H2O2. However, it must be pointed out that CAT itself may be inactivated by H2O2, and H2O2 scavengers are additional required [71].
Glucose-response microneedles based on PBA
With the increasing adoption of GOx, many problems have also been exposed. In addition to reacting with glucose to produce toxic hydrogen peroxide, it is easy to inactivate as an enzyme and is difficult to use for a long time. Lorand and Edwards [80] discovered the reversible equilibria between PBA and polyols for the first time. Since then, PBA is anchored to different materials for the detection and purification of carbohydrates [81]. PBA is also often synthesized in materials to make them react with glucose.
Chen et al. [73] prepared semi-IPN hydrogel by catalytic polymerization of ammonium persulfate (APS)/tetramethylenediamine (TEMED). The source of the glucose response is the equilibrium of phenylboric acid between the uncharged and anionically charged boronate. The change of counterionic osmotic pressure was transformed into the structural changes of gel materials, and forming a dehydration layer on the surface of the microneedles, which controlled the release of insulin in the matrix. Unlike lots of microneedles that use nanoparticles to carry drugs, most of the drugs in them are released in a short period of time. However, this microneedle system provides rapid and sustained blood glucose control and ensures the stability of insulin in water for more than 2 months.
Wang et al. [72] used changes in polymer morphology resulting from the PBA reaction to open or close channels for drug-loaded particles. Mesoporous silica nanoparticles have been widely studied [82] as therapeutic drug nanocarriers due to their good biocompatibility [83], facile surface functionalization, and high loading capacity [84], and they have a high degree of stability under physiological conditions. In the article, mesoporous silica nanoparticles were used as the drug carriage, and glucose-sensitive poly(3-acrylamidephenylboronic acid) (PAPBA) polymer was employed as the gatekeeper. When the microneedles are inserted into the skin, the PAPBA chain becomes a tangled structure at normal BGLs, and inhibiting the drug from passing through nanopores. In the hyperglycemic state, the polymer chain adopts an extended conformation and the nanopores are opened. The results showed that the device has good glucose response ability, high drug loading efficiency, and low toxicity, which can provide an effective strategy of drug delivery for diabetic patients.
In addition, this responsive microneedle can be loaded with insulin and glucagon at the same time. Microneedle patches could control the blood glucose level within a reasonable range by regulating the release of insulin or glucagon. Wang et al. [85] prepared a hybrid microneedle patch, in which one-fourth of the patch containing insulin and three-fourths area containing glucagon. By adjusting the proportion of 3-(acrylamido) phenylboronic acid and 2-Aminoethyl methacrylate hydrochloride in these two regions, the positive charge of insulin region was weakened under hyperglycemia and negatively charged insulin was released. The increased electronegativity causes the gel network in the glucagon region to contract, preventing the release of glucagon. In hypoglycemia, this regulating effect is the opposite.
To improve the drug capacities of microneedles
It is a common challenge for lots of types of MNs that how to achieve adequate dosage. The insulin dosage required by diabetic patients depends on many factors, such as disease severity, age, weight, and diet. From 5 IU (International Unit), a day for infants to more than 80 IU a day for overweight adolescent [40]. For dissolvable microneedles (DMNs) of general size, several or more patches are administered to meet treatment requirement every day, even if the drug in the microneedles is thought to be fully delivered into the bodies. It should be noted that frequent dosing is the least desirable problem for diabetics.
Chen et al. [86] found that Bacille Calmette-Guerin (BCG) vaccine coated onto microneedles is stable at 25 °C for only 7 days. To improve stability of the drug, freeze-dried powder of BCG vaccine was directly encapsulated into vesicles of microneedles to reduce the reconstruction damage of the freeze-dried powder during microneedle preparation. The microneedles prepared by this method can maintain drug activity for up to 2 months. In 2019, Kim et al. [87] used powder form of finasteride in microneedles, combining with the use of solubilizers, solved the problem of lipophilic drug delivery successfully.
On this basis, Kim et al. [88] began to use the same method to deliver freeze-dried powder of insulin in microneedles again. They found that the loading capacity of DMNs is a general problem in insulin delivery. In the process of mixing insulin with the polymer matrix, the drug may lose its pharmaceutical activity when exposed to a wide range of stresses. Due to the limitation of the solubility of insulin in the viscous polymer solution, it is difficult to achieve high-dose transdermal delivery within the reasonable scale of microneedles. The freeze-dried powder means a more concentrated form of medicine, and at the same time eliminates the dissolution and degradation of insulin during the preparation process, effectively increasing the drug loading of the microneedle active drug. Even if DMNs were prepared with the saturated concentration of insulin (20 mg/ mL), the dosage of this way was about 2.5 times higher than that of DMNs. Caffarel-Salvador et al. [16] were also aware of the influence of the polymer solution on the drug loading capacity and therefore also adopted powder form in microneedles to achieve high dosage. They used a 10 × 10 array, 1000 μm long microneedle and estimated the maximum dose of the microneedle to be 2.2 mg per patch, which means that the dose of insulin per patch can reach approximately 57 IU.
Although this form of MNs has greater capacity and better stability, it is difficult to apply to the treatment of diabetes. Diabetes is a chronic disease that requires steady and continuous administration. However, it is difficult for the microneedles loaded with drug powder to meet these requirements, and there is a lack of data to prove the accuracy of the drug loading and the controllable degree of release. Therefore, there are still many challenges in treating diabetes systematically using this kind of microneedles.
Prospects
The drug dosage is greatly limited by the MNs volume, especially for the kind of response microneedles, where insulin can only exist in the tiny space of the microneedles. However, expanding the height and bottom diameter of the microneedle to improve the capacity will inevitably aggravate the pain of use. Although reducing the pH of the system could increase the concentration of the insulin solution, the active pharmaceutical ingredients are more probable to be inactivated in the microneedles prepared from the acidic solution. The acidic microneedles not only cause extra pain, but also raise extra safety concerns. The integrated microneedle systems are obviously not yet mature, and most of devices can only be regarded as “semi-integrated” systems, since MN-based drug delivery and blood glucose monitors are still two separate parts.
However, both have their own advantages. The responsive microneedles achieve the release of drug along with BGLs, enabling “closed loop” characteristics to the systems, and the insulin in the polymer matrix does not need to be stored in cold storage. The integrated system based on hollow microneedles can provide almost infinite drug and extremely precise accuracy of drug delivery. Moreover, the doses and blood glucose values recorded by the systems can be presented digitally in the future.
Although a commercial insulin pump is a relatively mature product, microneedle system could further reduce the pain of patients. In some cases, such as poor skin hygiene and long-term implantation, traditional insulin pump needles can cause itching, redness and even infection. Donnelly et al. [89] had compared microneedle with hypodermic needles in vitro, and the results showed that microneedle could significantly reduce the level of microbial infiltration. This means that the microneedle system has a higher safety profile. Last but not least, the efforts at small sizes of microneedle system will allow diabetics a greater amplitude of motion, reducing the psychological stress of wearing a insulin pump, and so on.
In summary, the microneedle-based systems for diabetes become more mature, but there are still many problems that need to be resolved, and finally an effective, safe, and autonomous system can be realized.
References
International Diabetes Federation. IDF Diabetes Atlas Ninth Edition. 2019. Achieved from https://diabetesatlas.org/en/resources/.
Han S-K, Lee S-J, Ha H-Y. Skin moisturizing effects of a microneedle patch containing hyaluronic acid and lonicerae flos. Processes. 2021;9(2):321.
Jang M, Baek S, Kang G, Yang H, Kim S, Jung H. Dissolving microneedle with high molecular weight hyaluronic acid to improve skin wrinkles, dermal density and elasticity. Int J Cosmet Sci. 2020;42(3):302–9.
Kim MC, Kim KH, Lee JW, Lee YN, Choi HJ, Jung YJ, Kim YJ, Compans RW, Prausnitz MR, Kang SM. Co-delivery of M2e virus-like particles with influenza split vaccine to the skin using microneedles enhances the efficacy of cross protection. Pharmaceutics. 2019;11(4):188.
Chen YH, Lai KY, Chiu YH, Wu YW, Shiau AL, Chen MC. Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination. Acta Biomater. 2019;97:230–8.
Zheng M, Wang Z, Chang H, Wang L, Chew SWT, Lio DCS, Cui M, Liu L, Tee BCK, Xu C. Osmosis-powered hydrogel microneedles for microliters of skin interstitial fluid extraction within minutes. Adv Healthcare Mater. 2020;9(10):1901683.
Gerstel MS, Place VA. Drug delivery device, United States Patent, NO. 3964482.
Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD. Insulin delivery systems combined with microneedle technology. Adv Drug Delivery Rev. 2018;127:119–37.
Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9.
Singh S, Singh J. Transdermal drug delivery by passive diffusion and iontophoresis: a review. Med Res Rev. 1993;13(5):569–621.
Bliss M. The history of insulin. Diabetes Care. 1993;16(Suppl. 3):4–7.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S98–110.
Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–17.
Donnelly RF, Singh TRR, Woolfson AD. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Delivery. 2010;17(4):187–207.
Richter-Johnson J, Kumar P, Choonara YE, du Toit LC, Pillay V. Therapeutic applications and pharmacoeconomics of microneedle technology. Expert Rev Pharmacoecon Outcomes Res. 2018;18(4):359–69.
Caffarel-Salvador E, Kim S, Soares V, Tian RY, Stern SR, Minahan D, Yona R, Lu X, Zakaria FR, Collins J, Wainer J, Wong J, McManus R, Tamang S, McDonnell S, Ishida K, Hayward A, Liu X, Hubalek F, Fels J, Vegge A, Frederiksen MR, Rahbek U, Yoshitake T, Fujimoto J, Roxhed N, Langer R, Traverso G. A microneedle platform for buccal macromolecule delivery. Sci Adv. 2021;7(4):eabe2620.
American Diabetes Association. Standards of medical care in diabetes-2020 abridged for primary care providers. Clinical diabetes: a publication of the American Diabetes Association. 2020;38(Suppl. 1):10–38.
Zong QD, Guo RR, Dong NJ, Ling GX, Zhang P. Design and development of insulin microneedles for diabetes treatment. Drug Delivery Transl Res. 2021.
Zhu M, Liu Y, Jiang F, Cao J, Kundu SC, Lu S. Combined silk fibroin microneedles for insulin delivery. ACS Biomater Sci Eng. 2020;6(6):3422–9.
Vora LK, Courtenay AJ, Tekko IA, Larraneta E, Donnelly RF. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int J Biol Macromol. 2020;146:290–8.
Ito Y, Yamazaki T, Sugioka N, Takada K. Self-dissolving micropile array tips for percutaneous administration of insulin. J Mater Sci: Mater Med. 2010;21(2):835–41.
Chen BZ, Ashfaq M, Zhu DD, Zhang XP, Guo XD. Controlled delivery of insulin using rapidly separating microneedles fabricated from genipin-crosslinked gelatin. Macromol Rapid Commun. 2018;39(20):1800075.
McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100(24):13755–60.
Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909–15.
Liu GJ, Yang ZG, Liu JF, Li XB, Wang H, Zhao T, Yang XL. A low cost, high performance insulin delivery system based on PZT actuation. Microsyst Technol. 2014;20(12):2287–94.
Ma B, Liu S, Gan Z, Liu G, Cai X, Zhang H, Yang Z. A PZT insulin pump integrated with a silicon microneedle array for transdermal drug delivery. Microfluid Nanofluid. 2006;2(5):417–23.
Meshkinfam F, Rizvi G. A MEMS based drug delivery device with integrated micro-needle array-design and simulation. J Biomech Eng. 2021;143(8):081010.
Rao KS, Sateesh J, Guha K, Baishnab KL, Ashok P, Sravani KG. Design and analysis of MEMS based piezoelectric micro pump integrated with micro needle. Microsyst Technol. 2020;26(10):3153–9.
Haldkar RK, Gupta VK, Sheorey T. Modeling and flow analysis of piezoelectric based micropump with various shapes of microneedle. J Mech Sci Technol. 2017;31(6):2933–41.
Yeung C, Chen S, King B, Lin H, King K, Akhtar F, Diaz G, Wang B, Zhu J, Sun W, Khademhosseini A, Emaminejad S. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics. 2019;13(6):064125.
DeMello J, DeMello A. Microscale reactors: Nanoscale products. Lab Chip. 2004;4(2):11N-N15.
Karnik R, Gu F, Basto P, Cannizzaro C, Dean L, Kyei-Manu W, Langer R, Farokhzad OC. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008;8(9):2906–12.
Khanna P, Strom JA, Malone JI, Bhansali S. Microneedle-based automated therapy for diabetes mellitus. J Diabetes Sci Technol. 2008;2(6):1122–9.
Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–40.
Takeuchi K, Takama N, Kim B, Sharma K, Paul O, Ruther P. Microfluidic chip to interface porous microneedles for ISF collection. Biomed Microdevices. 2019;21(1):28.
Li CG, Joung HA, Noh H, Song MB, Kim MG, Jung H. One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab Chip. 2015;15(16):3286–92.
Ashraf MW, Tayyaba S, Nisar A, Afzulpurkar N. Fabrication and analysis of hollow microneedles and polymeric piezoelectric valveless micropump for transdermal drug-delivery system. IET Commun. 2012;6(18):3248–56.
Nordquist L, Roxhed N, Griss P, Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration. Pharm Res. 2007;24(7):1381–8.
Strambini LM, Longo A, Diligenti A, Barillaro G. A minimally invasive microchip for transdermal injection/sampling applications. Lab Chip. 2012;12(18):3370–9.
Mishra R, Maiti TK, Bhattacharyya TK. Feasibility studies on nafion membrane actuated micropump integrated with hollow microneedles for insulin delivery device. J Microelectromech Syst. 2019;28(6):987–96.
Mishra R, Pramanick B, Maiti TK, Bhatracharyya TK. Glassy carbon microneedles-new transdermal drug delivery device derived from a scalable C-MEMS process. Microsyst Nanoeng. 2018;4:38.
Economidou SN, Uddin MJ, Marques MJ, Douroumis D, Sow WT, Li H, Reid A, Windmill JFC, Podoleanu A. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Addit Manuf. 2021;38:101815.
Moussi K, Bukhamsin A, Hidalgo T, Kosel J. Biocompatible 3D printed microneedles for transdermal, intradermal, and percutaneous applications. Adv Eng Mater. 2020;22(2):1901358.
Trautmann A, Roth GL, Nujiqi B, Walther T, Hellmann R. Towards a versatile point-of-care system combining femtosecond laser generated microfluidic channels and direct laser written microneedle arrays. Microsyst Nanoeng. 2019;5:6.
American Diabetes Association. 7. Diabetes technology: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S77–88.
Invernale MA, Tang BC, York RL, Le L, Hou DY, Anderson DG. Microneedle electrodes toward an amperometric glucosesensing smart patch. Adv Healthcare Mater. 2014;3(3):338–42.
Li X, Huang X, Mo J, Wang H, Huang Q, Yang C, Zhang T, Chen H-J, Hang T, Liu F, Jiang L, Wu Q, Li H, Hu N, Xie X. A fully integrated closed-loop system based on mesoporous microneedles-iontophoresis for diabetes treatment. Adv Sci. 2021;8(16).
Heifler O, Borberg E, Harpak N, Zverzhinetsky M, Krivitsky V, Gabriel I, Fourman V, Sherman D, Patolsky F. Clinic-on-a-needle array toward future minimally invasive wearable artificial pancreas applications. ACS Nano. 2021;15(7):12019–33.
Kim H, Theogarajan LS, Pennathur S. A repeatable and scalable fabrication method for sharp, hollow silicon microneedles. J Micromech Microeng. 2018;28(3):035007.
Gardeniers H, Luttge R, Berenschot EJW, de Boer MJ, Yeshurun SY, Hefetz M, Van’t Oever R, van den Berg A. Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Syst. 2003;12(6):855–62.
Stoeber B, Liepmann D. Arrays of hollow out-of-plane microneedles for drug delivery. J Microelectromech Syst. 2005;14(3):472–9.
Ovsianikov A, Chichkov B, Mente P, Monteiro-Riviere NA, Doraiswamy A, Narayan RJ. Two photon polymerization of polymer-ceramic hybrid materials for transdermal drug delivery. Int J Appl Ceram Technol. 2007;4(1):22–9.
Laurent PE, Bourhy H, Fantino M, Alchas P, Mikszta JA. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine. 2010;28(36):5850–6.
Velten T, Ruf HH, Barrow D, Aspragathos N, Lazarou P, Jung E, Malek CK, Richter M, Kruckow J. Packaging of bio-MEMS: Strategies, technologies, and applications. IEEE Trans Adv Packag. 2005;28(4):533–46.
Liu Y, Eng PF, Guy OJ, Roberts K, Ashraf H, Knight N. Advanced deep reactive-ion etching technology for hollow microneedles for transdermal blood sampling and drug delivery. IET Nanobiotechnol. 2013;7(2):59–62.
Strambini LM, Longo A, Scarano S, Prescimone T, Palchetti I, Minunni M, Giannessi D, Barillaro G. Self-powered microneedle-based biosensors for pain-free high-accuracy measurement of glycaemia in interstitial fluid. Biosens Bioelectron. 2015;66:162–8.
Zhu J, Zhou X, Libanori A, Sun W. Microneedle-based bioassays. Nanoscale Adv. 2020;2(10):4295–304.
Yang J, Li Y, Ye R, Zheng Y, Li X, Chen Y, Xie X, Jiang L. Smartphone-powered iontophoresis-microneedle array patch for controlled transdermal delivery. Microsyst Nanoeng. 2020;6(1).
Grayson ACR, Shawgo RS, Johnson AM, Flynn NT, Li YW, Cima MJ, Langer R. A BioMEMS review: MEMS technology for physiologically integrated devices. Proc IEEE. 2004;92(1):6–21.
Jina A, Tierney MJ, Tamada JA, McGill S, Desai S, Chua B, Chang A, Christiansen M. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J Diabetes Sci Technol. 2014;8(3):483–7.
Nakanishi N, Yamamoto H, Tsuchiya K, Uetsuji Y, Nakamachi E, Development of wearable medical device for bio-MEMS. In: Nicolau DV, editor., BioMEMS and Nanotechnology II. Brisbane; 2006. p. 60390P.
Yu J, Qian C, Zhang Y, Cui Z, Zhu Y, Shen Q, Ligler FS, Buse JB, Gu Z. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17(2):733–9.
Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase — an overview. Biotechnol Adv. 2009;27(4):489–501.
Wu Q, Wang L, Yu HJ, Wang JJ, Chen ZF. Organization of glucose-responsive systems and their properties. Chem Rev. 2011;111(12):7855–75.
Steiner M-S, Duerkop A, Wolfbeis OS. Optical methods for sensing glucose. Chem Soc Rev. 2011;40(9):4805–39.
Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43(10):3595–629.
Hu X, Yu J, Qian C, Lu Y, Kahkoska AR, Xie Z, Jing X, Buse JB, Gu Z. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017;11(1):613–20.
Joshi-Barr S, Lux CD, Mahmoud E, Almutairi A. Exploiting oxidative microenvironments in the body as triggers for drug delivery systems. Antioxid Redox Signal. 2014;21(5):730–54.
Lux CdG, Joshi-Barr S, Trung N, Mahmoud E, Schopf E, Fomina N, Almutairi A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J Am Chem Soc. 2012;134(38):15758–64.
Wang J, Ye Y, Yu J, Kahkoska AR, Zhang X, Wang C, Sun W, Corder RD, Chen Z, Khan SA, Buse JB, Gu Z. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12(3):2466–73.
Gordijo CR, Shuhendler AJ, Wu XY. Glucose-responsive bioinorganic nanohybrid membrane for self-regulated insulin release. Adv Funct Mater. 2010;20(9):1404–12.
Wang Y, Cheng S, Hu W, Lin X, Cao C, Zou S, Tong Z, Jiang G, Kong X. Polymer-grafted hollow mesoporous silica nanoparticles integrated with microneedle patches for glucose-responsive drug delivery. Front Mater Sci. 2021;15(1):98–112.
Chen S, Matsumoto H, Moro-oka Y, Tanaka M, Miyahara Y, Suganami T, Matsumoto A. Microneedle-array patch fabricated with enzyme-free polymeric components capable of on-demand insulin delivery. Adv Funct Mater. 2019;29(7):1807369.
Zhang Y, Wang J, Yu J, Wen D, Kahkoska AR, Lu Y, Zhang X, Buse JB, Gu Z. Bioresponsive microneedles with a sheath structure for H2O2 and pH cascade-triggered insulin delivery. Small. 2018;14(14):1704181.
Xu B, Cao Q, Zhang Y, Yu W, Zhu J, Liu D, Jiang G. Microneedles integrated with ZnO quantum-dot-capped mesoporous bioactive glasses for glucose-mediated insulin delivery. ACS Biomater Sci Eng. 2018;4(7):2473–83.
Tong Z, Zhou J, Zhong J, Tang Q, Lei Z, Luo H, Ma P, Liu X. Glucose- and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats. ACS Appl Mater Interfaces. 2018;10(23):20014–24.
Xu B, Jiang G, Yu W, Liu D, Zhang Y, Zhou J, Sun S, Liu Y. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J Mater Chem B. 2017;5(41):8200–8.
Ye Y, Yu J, Wang C, Nguyen N-Y, Walker GM, Buse JB, Gu Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv Mater. 2016;28(16):3115–21.
Yu JC, Zhang YQ, Ye YQ, DiSanto R, Sun WJ, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112(27):8260–5.
Lorand JP, Edwards JO. Polyol complexes and structure of the benzeneboronate ion. J Org Chem. 1959;24(6):769–74.
Preinerstorfer B, Laemmerhofer M, Lindner W. Synthesis and application of novel phenylboronate affinity materials based on organic polymer particles for selective trapping of glycoproteins. J Sep Sci. 2009;32(10):1673–85.
Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine. 2015;11(2):313–27.
Croissant JG, Fatieiev Y, Khashab NM. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv Mater. 2017;29(9):1604634.
Croissant JG, Fatieiev Y, Almalik A, Khashab NM. Mesoporous silica and organosilica nanoparticles: physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv Healthcare Mater. 2018;7(4):1700831.
Wang Z, Wang J, Li H, Yu J, Chen G, Kahkoska AR, Wu V, Zeng Y, Wen D, Miedema JR, Buse JB, Gu Z. Dual self-regulated delivery of insulin and glucagon by a hybrid patch. Proc Natl Acad Sci USA. 2020;117(47):29512–7.
Chen F, Yan Q, Yu Y, Wu MX. BCG vaccine powder-laden and dissolvable microneedle arrays for lesion-free vaccination. J Controlled Release. 2017;255:36–44.
Kim S, Eum J, Yang H, Jung H. Transdermal finasteride delivery via powder-carrying microneedles with a diffusion enhancer to treat androgenetic alopecia. J Controlled Release. 2019;316:1–11.
Kim S, Yang H, Eum J, Ma Y, Lahiji SF, Jung H. Implantable powder-carrying microneedles for transdermal delivery of high-dose insulin with enhanced activity. Biomaterials. 2020;232:119733.
Donnelly RF, Singh TRR, Tunney MM, Morrow DIJ, McCarron PA, O’Mahony C, Woolfson AD. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26(11):2513–22.
Funding
This work was financially supported by the National Natural Science Foundation of China (51873015), the Joint Project of BRC-BC (Biomedical Translational Engineering Research Center of BUCT-CJFH) (XK2020-05, RZ2020-01), and the long-term subsidy mechanism from the Ministry of Finance and the Ministry of Education of PRC.
Author information
Authors and Affiliations
Contributions
Wen Xuan Li, Xiao Peng Zhang, Bo Zhi Chen, and Wen Min Fei: Writing — Original draft preparation. Yong Cui and Can Yang Zhang, Conceptualization. Xin Dong Guo: Conceptualization, Project administration.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from individual or guardian participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W.X., Zhang, X.P., Chen, B.Z. et al. An update on microneedle-based systems for diabetes. Drug Deliv. and Transl. Res. 12, 2275–2286 (2022). https://doi.org/10.1007/s13346-021-01113-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01113-2