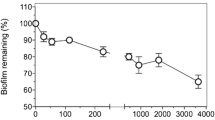
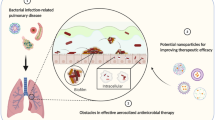
Abstract
Biofilm comprises a community of microorganisms which form on medical devices and can lead to various threatening infections. It is a major concern in various respiratory diseases like cystic fibrosis, chronic obstructive pulmonary disease, etc. The treatment strategies for such infections are difficult due to the resistance of the microflora existing in the biofilms against various antimicrobial agents, thus posing threats to the patient population. The present era witnesses the beginning of research to understand the biofilm physiology and the associated microfloral diversity by applying -omics approaches. There is very limited information about how the deposition of biofilm on the respiratory devices and lung itself affects the drug delivered, the delivery system, and other implications. The present mini review summarizes the basic introduction to the biofilms and its avoidance using various drug delivery systems with special emphasis on the respiratory diseases. Understanding the approaches, principles, and modes of drug delivery involved in preventing biofilm deposition will be of interest to both biological and formulation scientists, thereby opening avenues to explore the new vistas in biofilm research for identifying better treatments for pulmonary infectious diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: biofilms and its association with respiratory diseases
Biofilms comprise a community of microorganisms which are embedded in a group of exopolysaccharides, diverse proteins, and nucleic acids. Such potentially pathogenic bacterial biofilms have been shown to develop on the surfaces of medical devices, such as endotracheal tubes [1] and tracheostomy tubes [2], primarily depending on the manufacturing material of these tubes [3]. These biofilms can lead to life-threatening infections [4–11] and pose a major concern for the patients with chronic respiratory diseases, including cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) [12–16]. In cystic fibrosis patients, the biofilms have been demonstrated to be non-attached aggregates [17–19]. Notably, biofilms are also present on the lungs of patients with chronic pulmonary infections which are a major cause of morbidity and mortality [20].
The biofilm research in respiratory diseases is still not fully explored and needs immediate attention in order to maximize the drug effectiveness and treatment strategies. Most of the microflora which exists on the biofilms displays resistance to various antimicrobial agents which further increases threat to the patients with chronic pulmonary diseases. Moreover, the mechanisms contributing to this resistance are not illustrated, and it is largely phenotypic [9, 16, 21–27]. There are various factors and characteristics which affect the composition of microbial flora on the biofilm, some of which are attachment efficiency, cyclic stage, anti-effective hostile forces, physicochemical environment, mechanical factors such as shear forces, substratum, genotypic factors, nutrient sources, etc. [28].
The formation of biofilm on the treatment devices also exposes the patients to severe bacterial infections, thus worsening their disease condition [18]. Various approaches have been explored and investigated to encounter this problem such as surface modifications of the devices to alter bacterial adherence and incorporation of various antimicrobials onto the devices to prevent colonization of microflora [9]. Another approach includes use of electrical mode where the antimicrobials are released from the device surface so as to allow penetration of the antimicrobials through the biofilms [5, 29, 30].
Biofilm resistance: barrier to an effective drug delivery
There exists a difference of opinion regarding the efficacy of these approaches probably due to the biofilm resistance [31–34]. The phenomenon that has been attributed to this antimicrobial resistance includes the formation of stack of cells with various aqueous channels over and around the biofilm that acts as a penetration barrier resulting in impermeability [35]. The biofilm usually is coated with a polymer called the glycocalyx which is anionic in nature [36]. So it is also speculated that the chemical interaction between the biofilm polymer and the antimicrobial agent results in the formation of a penetration barrier which ultimately leads to a very low degree of antimicrobial absorption and effects [37]. Another postulated mechanism is heavy production of inactivating enzymes such as β-lactamases by the microflora which accumulates within the glycocalyx, thereby protecting the underlying cells [36, 38, 39]. Some studies have also questioned the microbial efflux pump system, oxygen deprivation [40–42], and colonization of anaerobic flora as contributors to the biofilm’s unresponsiveness to the antibiotics; however, certainly it requires further investigations [43]. Various other factors include oxygen deprivation [44–47] and enhanced growth of highly antibiotic resistant Pseudomonas aeruginosa strains within the airway mucus [48–50] of cystic fibrosis patients.
Approaches to prevent biofilm formation
Currently, treatment of respiratory diseases involves various drug delivery systems. Among all, inhalers (metered dose and dry powder) [51–55] are one of the most important and commonly employed drug delivery devices, which also significantly increase the chances of biofilm formation. This may lead to clinical complications, longer duration of treatment, and reduced patient compliance. Thus, it is crucial to regularly check biofilm formation, i.e., avoiding its deposition and subsequently dealing with any complications and potential hazards arising once the biofilm has established. Prevention of colonization and microflora deposition on diagnostic and drug delivery appliances remains the primary concern, followed by developing strategies for increasing accumulation of antimicrobials at the biofilm surface and also enhancing drug penetration into the biofilm [5]. Notably, the material properties of the medical device play a crucial role in preventing the formation of biofilms (Fig. 1).
Several recent reviews have shown that bacterial biofilm-associated chronic sinusitis in cystic fibrosis (CF) patients is primarily caused by P. aeruginosa infections and there is a lack of available treatments for such condition where the disease pathology is accompanied by opportunistic infections. It was also shown that the challenges with providing a suitable treatment include (i) identification of a suitable antimicrobial compound; (ii) selection of a suitable drug delivery device; and (iii) optimizing formulation variables to achieve effective targeted drug delivery (sinuses and nasal cavity) [5, 18, 56, 57]. One of such investigations involved the preparation of a novel inhaled combination powder containing amorphous colistin and crystalline rifapentine with enhanced antimicrobial activities against planktonic cells and biofilm of P. aeruginosa for respiratory infections [58]. Another research study has demonstrated the potential of amphibian AMP esculentin [1–21] as a new antibiotic formulation to treat infections caused by P. aeruginosa in sepsis and pulmonary infections, primarily by disrupting biofilms [59]. Remarkably, the results have also shown that esculentin [1–21] indeed prolonged survival of animals in both sepsis and pulmonary infection models [59].
Recent advances in controlling the biofilms are not only restricted to the material modifications of the drug delivery device but also include ultrasound enhancement of the antimicrobial transport that has been demonstrated to be effective against various microorganisms existing in the biofilms including Escherichia coli, P. aeruginosa, and Staphylococcus epidermidis [60–63]. In addition, various photodynamic approaches are highlighted to disrupt biofilms by generating reactive oxygen species [64]. Photodynamic approaches are most commonly employed for the pathogens associated with the oral cavity and skin [63, 65].
Formulation and drug delivery approaches to combat biofilm
Jones and colleagues have extensively investigated the capacity of liposome drug delivery in preventing growth and deposition of various pathogens [66–71]. They have explored various mechanistic insights into how the interaction between the liposomes and bacterial biofilms can avoid biofilm generation. Liposomal drug delivery applications have also been shown to be effective for the intracellular infections particularly with the reticulo-endothelial system where various pathogens like Francisella tularensis and Streptococcus pneumoniae [72, 73] were targeted. One of the advances with liposomal drug delivery includes stealth liposomes which are employed to deliver antibiotics such as gentamicin and have been demonstrated effective in a rat model of Klebsiella pneumoniae [74]. Other novel drug delivery approaches include polymer-based antimicrobial drug delivery including nanoparticles [75], microspheres and microparticles [76], hydrogels, micelles, fibrous scaffolds, and thermo-reversible gels [77–80].
In order to have effective delivery of antibiotics to the lungs for various pulmonary diseases, aerosolized systems are the best and effective approach for treatment and prophylaxis of pulmonary infections [81, 82]. Bacterial resistance in patients with cystic fibrosis is overcome by using high endobronchial concentration of tobramycin [83, 84]. Also, the antibiotic can be aerosolized in mechanically ventilated patients [85].
To design an effective drug delivery for pulmonary diseases, various critical factors need to be considered like physicochemical and mechanical characteristics of drug, intrinsic device characteristics and its performance, etc. The application of high-frequency ultrasound is another merit to be included on the aerosol systems which can help in eradicating the biofilms and delivering the drug with targeted approach and maximum therapeutic efficacy [86].
One of the recent advances include an infection-responsive system where the antibiotic is released from the drug delivery system once it receives the signals from the occurring infection such as the release of mediators, cytokines, etc. This approach is suitable for both prophylactic and prolonged use of antibiotics which in turn can avoid the associated side effects. One such investigation involves the delivery of gentamicin which was prepared as a drug delivery using polyvinyl alcohol as a drug-polymer conjugate for the treatment of wound infection where the signals appear in the form of levels of thrombin-like activity. The drug delivery system releases the gentamicin when it is incubated with thrombin and leucine aminopeptidase together, but not with any of the components alone. This system was found suitable and effective against Staphylococcus aureus in an animal model of infection [87]. Khun et al. investigated various conventional and new antifungal agents (triazoles, amphotericin B lipid (AMB) formulations, and echinocandins) for their antifungal activity against Candida albicans and C. parapsilosis biofilms which were grown on a bioprosthetic model where they demonstrated that Candida biofilms show unique susceptibilities to echinocandins and AMB lipid formulations [88]. Also, formulations for managing bacterial infections, comprising taurolidine in the form of gels, liquid, thixotropic gels, colloidal mixtures, dispensal suspensions, injectable polymers, or a microparticle, were prepared and were found effective for localized bacterial infections [89].
Various mathematical models and approaches have been established to understand the physiology of biofilms [90–94] and to investigate various novel drugs, antibiotics, antimicrobials, or biocides [95–100]. Khassehkhan and Eberl [101] guided the development of a robust mathematical modeling study that served as a platform for future models and numerical experiments to help in understanding the effect of probiotic cultures on the pathogenic films. Cipolla and colleagues [102] have shown the development of ciprofloxacin liposomal formulations (Lipoquin and Pulmaquin) which are in clinical trial for the treatment of lung infections. These liposomal formulations are believed to improve tolerability, increase patient compliance by reducing the dosing frequency, enhance penetration of biofilms, and promote treatment of intracellular infections [102]. Likewise, Halwani et al. [103] co-encapsulated gallium with gentamicin in liposomal formulation and demonstrated the combination to be more efficacious than the antibiotic alone, in eradicating antibiotic-resistant P. aeruginosa isolated from a growing planktonic or biofilm community [103].
Alhajlan et al. [104] have demonstrated the efficacy and safety of liposomal formulations containing clarithromycin with different surface charges against clinical isolates of P. aeruginosa from the lungs of cystic fibrosis patients [104, 105].
To develop a patient-compliant, effective drug delivery and treatment strategy to combat the bacterial colonization on the biofilms in the respiratory tract, various approaches like nebulization can be employed where the active moiety is aerosolized by inhalation to the respiratory tract [18]. Various compounds which are under investigation include antimicrobials [43, 106–112], silver efflux inhibitors, and certain enzymes [109, 113, 114]. These compounds mechanistically act on the extracellular polymeric substances (EPSs)/glycocalyx and various other structural components of the biofilms. This can be achieved by preparing the formulation in both aqueous solutions which can easily absorb and penetrate through the biofilm or in the form of various polymeric drug delivery systems [115] like nanoparticles and nanospheres [116–118], liposomes [119–122], microspheres [123, 124], hydrogels [125–127], micelles [128, 129], fibrous scaffolds [130–132], thermo-reversible gels [133, 134], etc., which can infiltrate through the cracks and consequently release the therapeutically active moiety over the passage of time [135, 136] (Fig. 2). Thomas et al. developed a new approach in the form of a dry aerosol where a blend of dispersion compound disintegrates bacterial colonies. One of such formulations includes ciprofloxacin HCl and glutamic acid as dispersion compound with l-leucine as an excipient. Live/dead assay along with confocal microscopy confirmed higher efficacy of the drug delivery system in eliminating biofilms in vitro in comparison to traditional antibiotic treatments [137].
Also, Sans-Serramitjana et al. prepared and evaluated nanocapsules containing colistin sulfate. The nanoencapsulated charged colistins have shown higher antimicrobial efficacy against biofilms comprising P. aeruginosa clinical isolates from CF patients as compared to the pure drug, colistin sulfate [104, 105]. Cheow et al. [138] demonstrated that inhaled formulations of levofloxacin-loaded polymeric nanoparticles have better antibacterial efficacy against the E. coli biofilm, thus likely to be a better treatment for respiratory infections with higher patient compliance and therapeutic activity [138]. Similarly, Loo et al. [139] presented that the combination of silver and curcumin nanoparticles possesses enhanced anti-biofilm activities against both P. aeruginosa and S. aureus [139].
One of the recent advancements to target the multidrug-resistant bacterial infections includes phage therapy, where the liposomal entrapment of phage has been shown to be highly effective in vitro as well as in vivo by overcoming the hurdles related to the clinical use of phage [140].
There are various lipid-based antibiotic delivery systems under experimental investigation which include drugs like ticarcillin [141], tobramycin [142], gentamicin [143], amikacin [122], ciprofloxacin [144], moxifloxacin [145], polymyxin B [146] colistin [147], vancomycin [148], clindamycin [149], and ceftaroline [149]. To overcome the challenges associated with oral antibiotic therapy in biofilm-related chronic pulmonary infections, especially in CF, several drugs are being assessed as potential candidates for inhalational antibiotic therapy which are currently in clinical trials, which include vancomycin and levofloxacin solution, vancomycin powder, liposomal amikacin [12], etc. These recent advances in optimizing the mode of antibiotic administration would certainly enhance the targeted and efficient delivery of antibiotics at high pulmonary concentrations necessary for disrupting complex biofilms.
Apart from the chemical moieties and drugs, the biofilms can also be targeted using natural compounds. Verkaik et al. have demonstrated comparable in vitro efficacy of various natural antimicrobials and chitosan-based formulations (toothpaste) with chlorhexidine against the bacterial flora comprising biofilms [150]. Such advances can further improve and strengthen the understanding of biofilm testing and designing of an ideal drug delivery system [151].
The choice and selection of the therapeutic moiety are very crucial as sometimes the active drug acts as both preventing the formation of biofilms and showing its therapeutic effectiveness in the treatment of disease pathology. However, in certain cases, a blend of active drug and a compound inhibiting the formation of biofilm can be employed which can be aerosolized simultaneously, exhibiting their application as an effective drug delivery system [86]. Recent developments indicate the application of high-frequency ultrasound as an effective addition to the aerosol systems, which would indeed improve targeted therapeutic effects.
Conclusions and prospects
Biofilm is a major concern in various respiratory diseases as well as associated medical devices and can cause life-threatening infections. The treatment strategies have various limitations that can be attributed to the antimicrobial resistance of the microflora comprising the biofilms, as well as the permeability of antimicrobials across the reasonably efficient barrier formed by biofilm. This leads to an unmet need of various advances, both in terms of treatment and prophylaxis, which can be utilized for the prevention of biofilm deposition and its associated effects. Preventive approaches to disrupt biofilms include development and validation of drugs that could induce/enhance pathways of biofilm self-destruction, such as energy limitation of microflora through inhibitors of oxidative phosphorylation. However, the major limitation is the lack of in-depth knowledge in the mechanisms involved in biofilm formation and persistence, which restricts the translation of the currently available technology into a clinically effective drug delivery system that can significantly overcome the complications due to biofilms in respiratory diseases. Various novel drug delivery systems such as nanoparticles, liposomes, niosomes, implantable matrices, fibrous scaffolds, micelles thermoreversible gels, etc., can be successfully and safely employed to target the biofilms. Also, recent discoveries like the infection-responsive system and application of high-frequency ultrasound with various aerosolized systems further open the horizons to develop in understanding advanced respiratory drug delivery systems with effective measures to overcome biofilm-associated problems. These novel approaches of drug delivery may open new vistas in the pulmonary clinics ensuring improved clinical outcome.
References
Adair CG, Gorman SP, Feron BM, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25(10):1072–6.
Solomon DH, Wobb J, Buttaro BA, Truant A, Soliman AM. Characterization of bacterial biofilms on tracheostomy tubes. Laryngoscope. 2009;119(8):1633–8.
Loo CY, Lee WH, Young PM, Cavaliere R, Whitchurch CB, Rohanizadeh R. Implications and emerging control strategies for ventilator-associated infections. Expert Rev Anti-Infect Ther. 2015;13(3):379–93.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108.
Smith AW. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev. 2005;57(10):1539–50.
Proal A. Understanding biofilms. Bacteriality—Exploring Chron Dis. 2008;26
Blenkinsopp S, Costerton J. Understanding bacterial biofilms. Trends Biotechnol. 1991;9(1):138–43.
Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002; 8(9).
Wilson M, Devine D. Medical implications of biofilms. Cambridge University Press; 2003.
Ghannoum MA, O’Toole GA. Microbial biofilms Vol 229. Washington, DC: ASM Press; 2004.
Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Ann Rev Microbiol. 2003;57(1):677–701.
Klinger-Strobel M, Lautenschläger C, Fischer D, et al. Aspects of pulmonary drug delivery strategies for infections in cystic fibrosis–where do we stand? Expert Opin Drug Deliv. 2015;12(8):1351–74.
Moran A, Annuk H. Recent advances in understanding biofilms of mucosae. Rev Environ Sci Biotechnol. 2003;2(2–4):121–40.
Gomes-Filho IS, Passos JS, da Cruz SS. Respiratory disease and the role of oral bacteria. J Oral Microbiol. 2010;2
Loera-Muro A, Ramírez-Castillo FY, Avelar-González FJ, Guerrero-Barrera AL. Porcine respiratory disease complex and biofilms. J Bacteriol Parasitol. 2015;6(6):1.
Boisvert A-A, Cheng MP, Sheppard DC, Nguyen D. Microbial biofilms in pulmonary and critical care diseases. Ann Am Thorac Soc. 2016;13(9):1615–23.
Bjarnsholt T, Jensen PO, Fiandaca MJ, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44(6):547–58.
Kłodzińska SN, Priemel PA, Rades T, Mørck NH. Inhalable antimicrobials for treatment of bacterial biofilm-associated sinusitis in cystic fibrosis patients: challenges and drug delivery approaches. Int J Mol Sci. 2016;17(10):1688.
Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg E. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407(6805):762–4.
Hassett DJ, Borchers MT, Panos RJ. Chronic obstructive pulmonary disease (COPD): evaluation from clinical, immunological and bacterial pathogenesis perspectives. J Microbiol (Seoul, Korea). 2014;52(3):211–26.
Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 2003;5(13):1213–9.
Kobayashi H. Airway biofilms: implications for pathogenesis and therapy of respiratory tract infections. Treat Respir Med. 2005;4(4):241–53.
Liu Y-CC, Post JC. Biofilms in pediatric respiratory and related infections. Curr Allergy Asthma Rep. 2009;9(6):449–55.
Marushko YV, Hyshchak T. Biofilm the formation in respiratory diseases. Influence of ambroxol on airway biofilms (literature review). CHILDS. Health. 2016;2(70):88–94.
Puig C, Domenech A, Garmendia J, et al. Increased biofilm formation by nontypeable Haemophilus influenzae isolates from patients with invasive disease or otitis media versus strains recovered from cases of respiratory infections. Appl Environ Microbiol. 2014;80(22):7088–95.
Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2(2):114–22.
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–32.
Aparna MS, Yadav S. Biofilms: microbes and disease. Braz J Infect Dis. 2008;12(6):526–30.
Del Pozo JL, Rouse M, Patel R. Bioelectric effect and bacterial biofilms. A systematic review. Int J Artif Organs. 2008;31(9):786.
Costerton JW, Ellis B, Lam K, Johnson F, Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother. 1994;38(12):2803–9.
Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45(4):999–1007.
Mah T-FC, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–9.
Gilbert P, Hodgson A, Brown M. Influence of the environment on the properties of microorganisms grown in association with surfaces. Microbiological quality assurance: a guide towards relevance and reproducibility of inocula. Boca Raton, FL: CRC Press Inc; 1995. p. 61–82.
Stewart PS. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob Agents Chemother. 1996;40(11):2517–22.
Nichols W. Biofilms, antibiotics and penetration. Rev Med Microbiol. 1991;2:177–81.
Sutherland IW. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol. 2001;9(5):222–7.
Cao B, Christophersen L, Kolpen M, et al. Diffusion retardation by binding of tobramycin in an alginate biofilm model. PLoS One. 2016;11(4):e0153616.
Giwercman B, Jensen E, Høiby N, Kharazmi A, Costerton J. Induction of beta-lactamase production in Pseudomonas aeruginosa biofilm. Antimicrob Agents Chemother. 1991;35(5):1008–10.
Ciofu O, Tolker-Nielsen T, Jensen PO, Wang H, Hoiby N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv Drug Deliv Rev. 2015;85:7–23.
Walters 3rd MC, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47(1):317–23.
Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48(7):2659–64.
Kolpen M, Mousavi N, Sams T, et al. Reinforcement of the bactericidal effect of ciprofloxacin on Pseudomonas aeruginosa biofilm by hyperbaric oxygen treatment. Int J Antimicrob Agents. 2016;47(2):163–7.
De Kievit TR, Parkins MD, Gillis RJ, et al. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2001;45(6):1761–70.
Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109(3):317–25.
Kolpen M, Hansen CR, Bjarnsholt T, et al. Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax. 2010;65(1):57–62.
Kolpen M, Bjarnsholt T, Moser C, et al. Nitric oxide production by polymorphonuclear leucocytes in infected cystic fibrosis sputum consumes oxygen. Clin Exp Immunol. 2014;177(1):310–9.
Cowley ES, Kopf SH, LaRiviere A, Ziebis W, Newman DK. Pediatric cystic fibrosis sputum can be chemically dynamic, anoxic, and extremely reduced due to hydrogen sulfide formation. mBio. 2015; 6(4).
Hassett DJ, Cuppoletti J, Trapnell B, et al. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv Drug Deliv Rev. 2002;54(11):1425–43.
Høiby N. Recent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. BMC medicine. 2011;9(1):1.
Kolpen M, Kuhl M, Bjarnsholt T, et al. Nitrous oxide production in sputum from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. PLoS One. 2014;9(1):e84353.
Byron PR. Drug delivery devices. Proc Am Thorac Soc. 2004;1(4):321–8.
Son Y-J, Longest PW, Hindle M. Aerosolization characteristics of dry powder inhaler formulations for the excipient enhanced growth (EEG) application: effect of spray drying process conditions on aerosol performance. Int J Pharm. 2013;443(1):137–45.
Timsina M, Martin G, Marriott C, Ganderton D, Yianneskis M. Drug delivery to the respiratory tract using dry powder inhalers. Int J Pharm. 1994;101(1–2):1–13.
Newman S, Busse W. Evolution of dry powder inhaler design, formulation, and performance. Respir Med. 2002;96(5):293–304.
Dua K, Hansbro NG, Foster PS, et al. Drug Deliv. and Transl. Res. (2016). doi:10.1007/s13346-016-0343-6.
Prat C, Lacoma A. Bacteria in the respiratory tract-how to treat? Or do not treat? Int J Infect Dis. 2016;51:113–22.
Blasi F, Page C, Rossolini GM, et al. The effect of N-acetylcysteine on biofilms: implications for the treatment of respiratory tract infections. Respir Med. 2016;117:190–7.
Zhou QT, Sun SP, Chan JG, et al. Novel inhaled combination powder containing amorphous colistin and crystalline rifapentine with enhanced antimicrobial activities against planktonic cells and biofilm of Pseudomonas aeruginosa for respiratory infections. Mol Pharm. 2015;12(8):2594–603.
Luca V, Stringaro A, Colone M, Pini A, Mangoni ML. Esculentin(1-21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cell Mol Life Sci. 2013;70(15):2773–86.
Rediske AM, Roeder BL, Nelson JL, et al. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob Agents Chemother. 2000;44(3):771–2.
Carmen J, Roeder B, Nelson J, et al. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. J Biomater Appl. 2004;18(4):237–45.
Pitt WG, Ross SA. Ultrasound increases the rate of bacterial cell growth. Biotechnol Prog. 2003;19(3):1038–44.
Carmen JC, Nelson JL, Beckstead BL, et al. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J Infect Chemother. 2004;10(4):193–9.
de Melo W, Avci P, de Oliveira M, et al. Photodynamic inactivation of biofilm: taking a lightly colored approach to stubborn infection. Expert Rev Anti-Infect Ther. 2013;11(7):669–93.
O’Neill JF, Hope CK, Wilson M. Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue. Lasers Surg Med. 2002;31(2):86–90.
Jones MN, Song Y-H, Kaszuba M, Reboiras MD. The interaction of phospholipid liposomes with bacteria and their use in the delivery of bactericides. Journal of drug targeting. 2009.
Kim H-J, Gias ELM, Jones MN. The adsorption of cationic liposomes to Staphylococcus aureus biofilms. Colloids Surf A Physicochem Eng Asp. 1999;149(1):561–70.
Catuogno C, Jones MN. The antibacterial properties of solid supported liposomes on Streptococcus oralis biofilms. Int J Pharm. 2003;257(1):125–40.
Ahmed K, Muiruri PW, Jones GH, Scott MJ, Jones MN. The effect of grafted poly (ethylene glycol) on the electrophoretic properties of phospholipid liposomes and their adsorption to bacterial biofilms. Colloids Surf A Physicochem Eng Asp. 2001;194(1):287–96.
Robinson AM, Bannister M, Creeth JE, Jones MN. The interaction of phospholipid liposomes with mixed bacterial biofilms and their use in the delivery of bactericide. Colloids Surf A Physicochem Eng Asp. 2001;186(1):43–53.
Hill KJ, Kaszuba M, Creeth JE, Jones MN. Reactive liposomes encapsulating a glucose oxidase-peroxidase system with antibacterial activity. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1997;1326(1):37–46.
Wong JP, Yang H, Blasetti KL, Schnell G, Conley J, Schofield LN. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J Control Release. 2003;92(3):265–73.
Ellbogen MH, Olsen KM, Gentry-Nielsen MJ, Preheim LC. Efficacy of liposome-encapsulated ciprofloxacin compared with ciprofloxacin and ceftriaxone in a rat model of pneumococcal pneumonia. J Antimicrob Chemother. 2003;51(1):83–91.
Bakker-Woudenberg IA, Schiffelers RM, ten Kate MT, et al. Targeting of antibiotics in bacterial infections using pegylated long-circulating liposomes. J Liposome Res. 2000;10(4):513–21.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–96.
Freiberg S, Zhu X. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282(1):1–18.
Yenice I, Çalış S, Kaş H, Özalp M, Ekizoğlu M, Hıncal A. Biodegradable implantable teicoplanin beads for the treatment of bone infections. Int J Pharm. 2002;242(1):271–5.
Gursel I, Yagmurlu F, Korkusuz F, Hasirci V. In vitro antibiotic release from poly (3-hydroxybutyrate-co-3-hydroxyvalerate) rods. J Microencapsul. 2002;19(2):153–64.
Schlapp M, Friess W. Collagen/PLGA microparticle composites for local controlled delivery of gentamicin. J Pharm Sci. 2003;92(11):2145–51.
Kelly H, Deasy P, Ziaka E, Claffey N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int J Pharm. 2004;274(1):167–83.
Riordan OTG. Inhaled antimicrobial therapy: from cystic fibrosis to the flu. Respir Care. 2000;45(7):836–45.
Klepser ME. Role of nebulized antibiotics for the treatment of respiratory infections. Curr Opin Infect Dis. 2004;17(2):109–12.
Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340(1):23–30.
Smith A. Inhaled antibiotic therapy: what drug? What dose? What regimen? What formulation? J Cyst Fibros. 2002;1:189–93.
Smaldone GC. Aerosolized antibiotics in mechanically ventilated patients. Respir Care. 2004;49(6):635–9.
Diot P, Dequin P, Rivoire B, et al. Aerosols and anti-infectious agents. J Aerosol Med. 2001;14(1):55–64.
Tanihara M, Suzuki Y, Nishimura Y, Suzuki K, Kakimaru Y, Fukunishi Y. A novel microbial infection-responsive drug release system. J Pharm Sci. 1999;88(5):510–4.
Kuhn D, George T, Chandra J, Mukherjee P, Ghannoum M. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46(6):1773–80.
Polaschegg H-D. Taurolidine formulations and delivery: therapeutic treatments and antimicrobial protection against bacterial biofilm formation. Google Patents; 2005.
Alpkvist E. Modelling and simulation of heterogeneous biofilm growth using a continuum approach. Univ.; 2005.
Klapper I, Dockery J. Finger formation in biofilm layers. SIAM J Appl Math. 2002;62(3):853–69.
Eberl HJ, Parker DF, Van Loosdrecht M. A new deterministic spatio-temporal continuum model for biofilm development. Comput Math Methods Med. 2001;3(3):161–75.
Picioreanu C, van Loosdrecht M, Heijnen J. Multidimensional modeling of biofilm structure. Delft University of Technology, Faculty of. Applied Sciences; 1999.
Eberl H. Mathematical modeling of biofilms. Vol 18: IWA publishing; 2006.
Anguige K, King J, Ward J. Modelling antibiotic-and anti-quorum sensing treatment of a spatially-structured Pseudomonas aeruginosa population. J Math Biol. 2005;51(5):557–94.
Cogan N, Cortez R, Fauci L. Modeling physiological resistance in bacterial biofilms. Bull Math Biol. 2005;67(4):831–53.
Dodds MG, Grobe KJ, Stewart PS. Modeling biofilm antimicrobial resistance. Biotechnol Bioeng. 2000;68(4):456–65.
Hunt S, Hamilton M, Stewart P. A 3D model of antimicrobial action on biofilms. Water Sci Technol. 2005;52(7):143–8.
Efendiev M, Demaret L, Lasser R, Eberl H. Analysis and simulation of a meso-scale model of diffusive resistance of bacterial biofilms to penetration of antibiotics. Adv. Math. Sci. Appl. 2008; 18(2).
Roberts ME, Stewart PS. Modeling antibiotic tolerance in biofilms by accounting for nutrient limitation. Antimicrob Agents Chemother. 2004;48(1):48–52.
Khassehkhan H, Eberl HJ. Modeling and simulation of a bacterial biofilm that is controlled by pH and protonated lactic acids. Computational and Mathematical Methods in Medicine. 2008; 9(1).
Cipolla D, Blanchard J, Gonda I. Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics. 2016;8(1):6.
Halwani M, Yebio B, Suntres Z, Alipour M, Azghani A, Omri A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J Antimicrob Chemother. 2008;62(6):1291–7.
Alhajlan M, Alhariri M, Omri A. Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors. Antimicrob Agents Chemother. 2013;57(6):2694–704.
Sans-Serramitjana E, Fusté E, Martínez-Garriga B, et al. Killing effect of nanoencapsulated colistin sulfate on Pseudomonas aeruginosa from cystic fibrosis patients. Journal of Cystic Fibrosis. 2015.
Sun J, Deng Z, Yan A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun. 2014;453(2):254–67.
Tegos PG, Haynes M, Jacob Strouse J, et al. Microbial efflux pump inhibition: tactics and strategies. Curr Pharm Des. 2011;17(13):1291–302.
Park S-C, Park Y, Hahm K-S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci. 2011;12(9):5971–92.
Wu H, Moser C, Wang H-Z, Høiby N, Song Z-J. Strategies for combating bacterial biofilm infections. Int J Oral Sci. 2015;7(1):1–7.
Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272(6):541–61.
Blackledge MS, Worthington RJ, Melander C. Biologically inspired strategies for combating bacterial biofilms. Curr Opin Pharmacol. 2013;13(5):699–706.
Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003;112(10):1466–77.
Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes. Int J Artif Organs. 2009;32(9):545–54.
Sulemankhil I, Ganopolsky JG, Dieni CA, Dan AF, Jones ML, Prakash S. Prevention and treatment of virulent bacterial biofilms with an enzymatic nitric oxide-releasing dressing. Antimicrob Agents Chemother. 2012;56(12):6095–103.
Markowska K, Grudniak AM, Wolska KI. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol. 2013;60(4):523–30.
Habimana O, Steenkeste K, Fontaine-Aupart M-P, Bellon-Fontaine M-N, Kulakauskas S, Briandet R. Diffusion of nanoparticles in biofilms is altered by bacterial cell wall hydrophobicity. Appl Environ Microbiol. 2011;77(1):367–8.
Messiaen A-S, Forier K, Nelis H, Braeckmans K, Coenye T. Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS One. 2013;8(11):e79220.
Merchant Z, Buckton G, Taylor MGK, et al. A new era of pulmonary delivery of nano-antimicrobial therapeutics to treat chronic pulmonary infections. Curr Pharm Des. 2016;22(17):2577–98.
Jones MN. Use of liposomes to deliver bactericides to bacterial biofilms. Methods Enzymol. 2005;391:211–28.
Dong D, Thomas N, Thierry B, Vreugde S, Prestidge CA, Wormald P-J. Distribution and inhibition of liposomes on Staphylococcus aureus and Pseudomonas aeruginosa biofilm. PLoS One. 2015;10(6):e0131806.
Rukavina Z, Vanić Ž. Current trends in development of liposomes for targeting bacterial biofilms. Pharmaceutics. 2016;8(2):18.
Meers P, Neville M, Malinin V, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61(4):859–68.
Långmark J, Storey MV, Ashbolt NJ, Stenström T-A. Accumulation and fate of microorganisms and microspheres in biofilms formed in a pilot-scale water distribution system. Appl Environ Microbiol. 2005;71(2):706–12.
Song X, Yaskell T, Klepac-Ceraj V, Lynch MC, Soukos NS. Antimicrobial action of minocycline microspheres versus 810-nm diode laser on human dental plaque microcosm biofilms. J Periodontol. 2014;85(2):335–42.
Finnegan S, Percival SL. Clinical and antibiofilm efficacy of antimicrobial hydrogels. Adv Wound Care. 2015;4(7):398–406.
Lee AL, Ng VW, Wang W, Hedrick JL, Yang YY. Block copolymer mixtures as antimicrobial hydrogels for biofilm eradication. Biomaterials. 2013;34(38):10278–86.
Hellriegel J, Günther S, Kampen I, Albero AB, Kwade A. A biomimetic gellan-based hydrogel as a physicochemical biofilm model. Journal of Biomaterials and. Nanobiotechnology. 2014; 2014.
Chen F, Rice KC, Liu X-M, Reinhardt RA, Bayles KW, Wang D. Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm Res. 2010;27(11):2356–64.
Liu Y, Busscher HJ, Zhao B, et al. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 2016;10(4):4779–89.
Albuquerque MT, Ryan SJ, Münchow EA, et al. Antimicrobial effects of novel triple antibiotic paste–mimic scaffolds on Actinomyces naeslundii biofilm. J Endod. 2015;41(8):1337–43.
Cao X, Zhao L, Song Z-B, Zhang X-Z, Qin J-Q. The influence of the alignment of electrospun fibrous scaffolds on the biological behavior of RSC96 cells. J Biomater Tissue Eng. 2014;4(6):488–91.
Romero D, Vlamakis H, Losick R, Kolter R. Functional analysis of the accessory protein TapA in Bacillus subtilis amyloid fiber assembly. J Bacteriol. 2014;196(8):1505–13.
Gilbert P, Jones M, Allison D, Heys S, Maira T, Wood P. The use of poloxamer hydrogels for the assessment of biofilm susceptibility towards biocide treatments. J Appl Microbiol. 1998;85(6):985–90.
Zhang X, Lee S, Liu Y, et al. Anion-activated, thermoreversible gelation system for the capture, release, and visual monitoring of CO2. Scientific reports. 2014; 4.
Ré A, Ferreira M, Freitas O, Aires C. Local antibiotic delivery in periodontitis: drug release and its effect on supragingival biofilms. Biofouling. 2016;32(9):1061–6.
Ramanathan K. Prevention and treatment of biofilms by hybrid-and nanotechnologies. Int J Nanomedicine. 2013;8:2809–19.
Thomas EBS, J Fiegel. Development of a dry powder aerosol for the dispersion and eradication of respiratory biofilms. Proceedings of the American Thoracic Society, AIChE Annual Meeting. 2008.
Cheow WS, Chang MW, Hadinoto K. Antibacterial efficacy of inhalable levofloxacin-loaded polymeric nanoparticles against E. coli biofilm cells: the effect of antibiotic release profile. Pharm Res. 2010;27(8):1597–609.
Loo C-Y, Rohanizadeh R, Young PM, et al. Combination of silver nanoparticles and curcumin nanoparticles for enhanced anti-biofilm activities. J Agric Food Chem. 2015;64(12):2513–22.
Singla S, Harjai K, Katare OP, Chhibber S. Encapsulation of bacteriophage in liposome accentuates its entry in to macrophage and shields it from neutralizing antibodies. PLoS One. 2016;11(4):e0153777.
Lagacé J, Dubreuil M, Montplaisir S. Liposome-encapsulated antibiotics: preparation, drug release and antimicrobial activity against Pseudomonas aeruginosa. J Microencapsul. 1991;8(1):53–61.
Pilcer G, Sebti T, Amighi K. Formulation and characterization of lipid-coated tobramycin particles for dry powder inhalation. Pharm Res. 2006;23(5):931–40.
Rukholm G, Mugabe C, Azghani AO, Omri A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: a time–kill study. Int J Antimicrob Agents. 2006;27(3):247–52.
Sweeney LG, Wang Z, Loebenberg R, Wong JP, Lange CF, Finlay WH. Spray-freeze-dried liposomal ciprofloxacin powder for inhaled aerosol drug delivery. Int J Pharm. 2005;305(1):180–5.
Ventura CA, Tommasini S, Crupi E, et al. Chitosan microspheres for intrapulmonary administration of moxifloxacin: interaction with biomembrane models and in vitro permeation studies. Eur J Pharm Biopharm. 2008;68(2):235–44.
McAllister S, Alpar H, Brown M. Antimicrobial properties of liposomal polymyxin B. J Antimicrob Chemother. 1999;43(2):203–10.
Wallace SJ, Li J, Nation RL, Prankerd RJ, Boyd BJ. Interaction of colistin and colistin methanesulfonate with liposomes: colloidal aspects and implications for formulation. J Pharm Sci. 2012;101(9):3347–59.
Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6(5):4279–87.
Shah SR, Henslee AM, Spicer PP, et al. Effects of antibiotic physicochemical properties on their release kinetics from biodegradable polymer microparticles. Pharm Res. 2014;31(12):3379–89.
Verkaik MJ, Busscher HJ, Jager D, Slomp AM, Abbas F, van der Mei HC. Efficacy of natural antimicrobials in toothpaste formulations against oral biofilms in vitro. J Dent. 2011;39(3):218–24.
Khassehkhan H, Eberl HJ. Modeling and simulation of a bacterial biofilm that is controlled by pH and protonated lactic acids. Comput Math Methods Med. 2008;9(1):47–67.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dua, K., Shukla, S.D., Tekade, R.K. et al. Whether a novel drug delivery system can overcome the problem of biofilms in respiratory diseases?. Drug Deliv. and Transl. Res. 7, 179–187 (2017). https://doi.org/10.1007/s13346-016-0349-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0349-0