Abstract
Solid dispersion has emerged as a method of choice and has been extensively investigated to ascertain the in vivo improved performance of many drug formulations. It generally involves dispersion of drug in amorphous particles (clusters) or in crystalline particles. Comparatively, in the last decade, amorphous drug–polymer solid dispersion has evolved into a platform technology for delivering poorly water-soluble small molecules. However, the success of this technique in the pharmaceutical industry mainly relies on different drug–polymer attributes like physico-chemical stability, bioavailability and manufacturability. The present review showcases the efficacy of amorphous solid dispersion technique in the research and evolution of different drug formulations particularly for those with poor water soluble properties. Apart from the numerous mechanisms of action involved, a comprehensive summary of different key parameters required for the solubility enhancement and their translational efficacy to clinics is also emphasized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
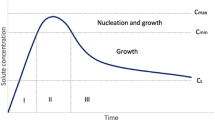
The destination of any drug delivery system is to achieve prompt action and to offer a therapeutic amount of the drug to the proper site in the body. Among different routes of drug delivery, oral ingestion is one of the most convenient and commonly employed routes. However, poor bioavailability (BA) is one of the major problems associated with this route of administration [1–4]. BA of orally administered drugs mainly depends on their solubility in the biological fluids and dissolution rate. The low solubility of these drugs in biological fluids results in their poor BA and restricted therapeutic efficiency. It is well known that a therapeutic drug with poor aqueous solubility or new chemical entity (NCE) will typically exhibit dissolution rate limited absorption, and a drug with poor membrane permeability will typically exhibit permeation rate limited absorption (Fig. 1) [5]. It is estimated that nearly 40 % of NCE currently being discovered are poorly water soluble [6]. Unfortunately, many of these potential drugs are abandoned in the early stages of development due to the solubility issues [7]. Therefore, in order to improve the BA of oral drugs, the research is now mainly restricted to two broader areas of pharmaceutical sciences, i.e. to improve the permeability of the poorly permeable drugs and to enhance the solubility and dissolution rate of poorly water-soluble drugs [8, 9].
Scientist have made it evident that any technique capable of generating a supersaturation state of drug in the gastro-intestinal (GI) fluid can be substantially helpful in raising the oral BA of that drug [10, 11]. Some of these techniques which can achieve supersaturation state are: solid dispersion (SD), microemulsion, solid solutions, eutectic mixtures, self-emulsifying drug delivery system (SEDD), liposome, micronization, polymeric form, polymorphs, pseudo-polymorphs (including solvates), complexation, hydrotropy, micelle formation, nano-suspensions, etc. [12–14]. From this varied list of available techniques, SD is considered as one of the most suitable tools to overcome the complex solubility associated issues of different drugs. The method involves dispersion of one or more active pharmaceutical ingredients (API) in an inert carrier or matrix in solid state. Earlier work have also documented that SD is not only one of the most successful methodology to improve dissolution and BA of poorly water soluble drugs but also is simple, economic, and advantageous [15, 16]. Thus, we aimed to present a short review briefly showcasing the different aspects of SD technique.
Solid dispersion (SD)
The term SD refers to “drug dispersed in a solid matrix” or a group of solid products consisting of at least two different components, generally, a hydrophilic matrix and a hydrophobic drug [5]. The matrix can be either crystalline or amorphous in nature. The drug can be dispersed molecularly, in amorphous particles (clusters) or in crystalline particles. It is one of the most successful and promising strategy which aims to enhance the solubility thereby increasing the oral BA of poorly water soluble NCE [8]. It was discovered as an innovative concept by Sekiguchi et al. in 1961. They studied SD of sulfathiazole using urea as a carrier and reported the enhanced solubility up to several folds, without deciphering the details of mechanistic involved [17]. Since from this discovery, the technique is widely explored for many NCE and achieved a breakthrough success.
Merits of amorphous SD [5, 11, 18–20]
-
1.
Particles with reduced particle size: Diminution in particle size leads to high surface area, resulting in an increased dissolution rate and consequently improved BA.
-
2.
Particles with improved wettability: SD offers high wettability which subsequently enhances the drug solubility. Carriers (e.g. urea) used in preparation of SD mediates wetting ability of the drug.
-
3.
Particles with higher porosity: SD porosity mainly depends on the nature of carriers used during preparation. SD using linear polymers produces larger and highly porous particles in comparison to reticular polymers which results in a higher dissolution rate and improved BA.
-
4.
Suitable for acidic, basic, neutral and zwitter ionic drugs
-
5.
Offers alternate pathways to improve BA
-
6.
Faster dissolution and absorption of drug, which may lead to quick onset of action: Solubility of drug candidate in aqueous media imparts faster dissolution profiles and absorption of drug results in immediate inception of action.
-
7.
Improve exposure (increase BA, more rapid onset, and decrease dose)
-
8.
Support toxicology studies and clinical tools
-
9.
Masking of unpleasant taste, smell of drugs and offers commercial product manufacturing
-
10.
Homogeneous distribution of a small amount of drug in solid state is possible.
-
11.
Allows dispensing of liquid (up to 10 %) or gaseous compounds in a solid dosage: SDs are more adequate to patients than liquid or gaseous systems as they give rise to solid oral dosage forms instead of liquid because solubilization products convert into solid powder state.
Limitations of amorphous SD [11, 21–24]
-
1.
Laborious and expensive methods of preparation at commercial level: Methodology and the technical requirement of individual method increase the expenditure of SD production at commercial level.
-
2.
Reproducibility of physicochemical characteristics varies with various techniques: Various strategies applies for production of SD, each method has their own characteristic product and lacking of reproducible results leads to variability in physicochemical characteristics of SD.
-
3.
Difficulty in incorporating the method into formulation of dosage forms: In spite of extensive proficiency, SD is not mostly exploited at commercial level. It may be due to throughout processing (mechanical stress) or storage (temperature and humidity stress) problems. The amorphous state possibly may cause crystallization.
-
4.
Recrystallization of SD: It is one of the major limitations of SD. In amorphous form, they exist thermodynamically unstable and have the propensity to transform to a more stable form under recrystallization.
-
5.
Stability of the drug and vehicle depends on solvent used: Variability of approaches desires individual set of conditions, carriers and solvent system, product results from such methods imparts specific storage conditions failing to which leads to stability issues.
The concept of SD is not a new emerging trend in the scientific community, but it turns out the breakthrough achievements; since, from 1960s various milestones and turning points in the field of solubility enhancement via SD technique. The historical milestones in the field of SD are mentioned below (Table 1) [5, 10, 11].
Recent trends of SD
Depending on the physical state of the carrier which is amorphous or crystalline, the SD can be divided into various categories. Figure 2 explores these categories as [36–40].
First generation
The first generation SD is basically crystalline in nature and comprised of urea [41] and sugars such as sorbitol and mannitol [42]. These carriers, especially sugars, have high melting point which is not favorable for preparing SD by melting method. Urea exhibits high solubility in water and many common organic solvents while sugars have poor solubility in most of organic solvents; therefore, sugars were less commonly used than other carriers [43–45].
Second generation
Second generation SD contains amorphous carriers, which are mostly polymers. Amorphous SD can be classified into amorphous solid solutions (glass solutions) and amorphous solid suspensions according to the physical state of the drug [46–48]. In amorphous solid solution, the drug and amorphous carrier are completely miscible to form molecularly homogenous mixture while amorphous solid suspension consists of two separate phases. In second generation SD, the drug lies in supersaturated state owing to forced solubilization in carrier. These systems may cause reduction of the drug particle size up to a molecular level which solubilize or co-dissolve the drug and offer superior wettability and dispersibility of the drug and finally produce amorphous forms of the drug and carriers.
Third generation
Although the amorphous SD can enhance drug release rate, the subsequent supersaturation state of drugs may cause drug precipitation and decrease the drug concentration both in vitro and in vivo, subsequently hamper the BA of drugs. This phenomenon is very common especially with sugar glass based SD [49]. The drug may also recrystallize from an amorphous state in the preparation process (cooling or solvent removal) and during storage [44, 50]. So, in the third generation SD, the surface active agents or self-emulsifiers are introduced as carriers or additives and showed significant improvement in overcoming the above problems such as precipitation and recrystallization [7, 10]. These systems possess improved dissolution rate as well as highest degree of bioavailability, and better stability due to inclusion of surface activity.
Fourth generation
The fourth generation SD offers controlled release pattern and known as controlled release solid dispersion (CRSD) containing poorly water-soluble drugs with a short biological half-life. CRSD of poorly water-soluble drugs enhances the solubility as well as extends the release in a controlled manner. In CRSD, the molecular dispersion of poorly water-soluble drugs in carriers will improve the drug solubility while water insoluble polymers or swellable polymers can be used to delay the drug release in the dissolution medium [11].
In the pharmaceuticals, a wide range of polymers are used for the preparation of amorphous SD which includes, hydroxypropylmethyl cellulose (HPMC), poly (vinyl pyrrolidone/vinyl acetate co polymer) (PVP), polyacrylates, hydroxypropylmethyl cellulose acetate/succinate (HPMC AS), and polymethacrylates which offers the promising results [51, 52].
Mechanism of drug release SD
There are two main pathways which offer drug release from immediate release SD: (i) drug-controlled release and (ii) carrier-controlled release. When SD is introduced in water, the carrier often absorbs water rapidly due to their hydrophilic property or dissolves in it and form carrier layer or gel layer [53, 54]. Figure 3 summarizes different methods utilized for solubility enhancement by SD. SD also has other advantages in improving the drug BA such as wettability, porosity enhancement, and polymorphic change. The fast dissolution or water absorption of carrier molecules surrounding drug particles can improve the drug wettability, especially when surfactants or emulsifiers are incorporated in SD. The improved wettability and dispersibility of the drug due to the surfactant action may reduce the interfacial tension between hydrophobic drug particle and aqueous solvent phase, cause an increase in effective surface area exposed to the dissolution medium. This also retards agglomeration or aggregation of the particles, which can slow down the dissolution [5, 11].
Current approaches for SD manufacturing
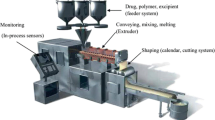
Various reported methods are utilized in preparation of SD. These methods deal with the challenge of mixing a matrix and a drug, preferably on a molecular level (Fig. 4) while matrix and the drug are generally poorly miscible. During many of the preparation techniques, de-mixing (partially or completely) and formation of different phases are observed. Phase separations like crystallization or formation of amorphous drug clusters are difficult to control and therefore unwanted [44, 48, 49].
Hot melt extrusion (HME)
HME is a simple and a single-step operation, previously confined to only food and plastic industries; but later on, Speiser and Huttenrauch were first to suggest that this process can be employed for the manufacturing of SD [55–57]. The process is carried out in a hot melt extruder. Its extreme ability to operate in a continuous fashion and without the need of organic solvent, gives an extra edge over other techniques. In this method, the drug and carrier are mixed, heated, melted, homogenized, and extruded in a form of tablets, rods, or pellets, or milled and blended with other excipients for different purposes [58–60]. The intense mixing and agitation forced by the rotating screw during the process cause disaggregation of drug particles in the molten polymer, resulting in a homogenous dispersion [61, 62]. This process involves the transformation of a solid mass of the particles into a viscous liquid or semisolid mass by heating and intense mixing. Examples of SD of drug prepared by HME technique include carbamazepine, nicardipine hydrochloride, 17β estradiol hemihydrates, theophylline, chlorpheniramine malate, 5-aminosalicylic acid, diclofenac sodium, diltiazem hydrochloride, etc. [63].
Hot melt encapsulation
In this method, the drug and carrier are melted together at a temperature above the eutectic point, which is the lowest possible melting point of the mixture. Then the melted mixture can be cooled or solidified using different techniques such as ice bath agitation [11], spreading as a thin layer on ferrite plate or stainless steel plate [64]. The resultant solid is then crushed, sieved, pulverized to reduce the particle size, or injection molded into dosage forms without undergoing milling. The advantage of this method is that it does not require any solvent.
Meltrex® and melt agglomeration
Meltrex® is one of the registered methods for preparing SD [65, 66]. It is the process which can easily combine both approaches simultaneously, i.e., amorphous embedding of drug and thus by modifying the release profile of drug up to zero order. Meltrex™ based on the HME principle is a patented SD manufacturing process. In Meltrex™ technology, the use of a special twin screw extruder and the presence of two independent hoppers allow conveying the extruded mass continuously throughout the extrusion channel and thus reduce the residence time of the drug (approximately 2 min) in the extruder as well as to avoid thermal stress to the drug and excipient [44, 48]. In general, the amorphous SD prepared by melting method are soft, sticky, and have poor flow properties and poor compressibility which hinder their applications in a large pharmaceutical scale of tableting. However, melt agglomeration method, in which the carrier acts as a meltable binder, is a feasible method to solve these problems. Melt agglomeration is processed in high shear mixers or rotary processor with the mixture prepared by three ways, i.e., adding the molten carrier containing the drug to the heated excipients [11, 39] adding the molten carrier to a heated mixture of the drug and excipients or heating a mixture of the drug, carrier, and excipients to a temperature within or above the melting range of the carrier. Some examples of SD prepared by Meltrex® technique include paracetamol, nifedipine, and furosemide. The Meltrex® technology provide various benefits like; fine-tuned pharmacokinetic profile up to zero-order kinetics, improved BA, circumvention of problems associated with polymorphism [47]. Thus, Meltrex® technology is a profitable strategic tool from drug rescue through prevention of generic erosion.
Solvent evaporation method (solvent method)
In the solvent evaporation method, SD is obtained after the evaporation of solvent from the solution containing a drug and carrier [67]. The method has solved some critical problems of the melting method like decomposition of drugs and carriers at high temperature. This is because the solvent removal in this method can be performed through alternate techniques such as freeze drying technique without heat. However, an important prerequisite of this method is the sufficient solubility of the drug and carrier in a solvent or co-solvent. The disadvantage of this method is that the residual solvent remaining after the evaporation process may cause toxicity and complete solvent removal is nearly impossible [68, 69].
Other disadvantages of the solvent method are its larger environmental consequences, the high cost of production due to the extra facilities required for solvent removal and protection against explosion as well as low scalability. For these reasons, HME is more favorable than solvent method to prepare SD [70].
Vacuum drying and rotary evaporation
Phase separation is a challenge that can arise during the solvent removal process. This is mainly due to the increased molecular mobility aroused during heating process which may subsequently result in phase separation. Thus, vacuum drying and rotary evaporation at a moderate temperature is used to avoid the risk of phase separation and to prevent the degradation of drugs and carriers at high temperature [71]. After the solvent evaporation process, the resultant SD may be stored in a vacuum desiccator for complete removal of residual solvent. Although these methods are easy to execute, but have rather lengthy procedures.
Spray drying
Spray drying is one of the oldest techniques for drying any material. It is a unit operation which has the capacity to transform a liquid, slurry, and a semisolid into a free flowing powder form [62, 67, 71]. This process is typically used in the production of coarse (up to 500 μm) for food, pharmaceutical, and industrial powders. It can likewise be utilized to set up micro-particulate powders for NCE, excipients, pulmonary and bio-therapeutic particle engineering, drying of crystalline APIs, and encapsulation [67]. It is an efficient technology for SD manufacturing because it permits extremely rapid solvent evaporation thereby resulting in fast transformation of an API-carrier solution to solid API-carrier particles. The size of the SD particles prepared by spray drying can be customized by modulating the droplet size via nozzle to meet the requirements for further processing or applications [72]. SD of drug prepared by spray drying technique includes: fenofibrate, etoricoxib, curcumin, ibuprofen, itraconazole, mefenamic acid, griseofulvin, cyclosporine, tolfenamic acid, tolbutamide, etc. [73].
Freeze drying
Freeze drying is an alternative method for drying SD. This method shows promise as a suitable technique for the incorporation of drugs into stabilizing matrices because the drug is subjected to minimal thermal stress during the SD formation. This method includes two steps: freezing and lyophilization [67, 17]. The freezing rate is very important to control phase separation. The basic process consists of immersing the API-carrier solution in liquid nitrogen until it is fully frozen which is then lyophilized [73]. An important advantage of this method is the minimized risk of phase separation; however, the main disadvantage is that most organic solvents have low freezing temperatures and do not stay frozen during sublimation [43, 57].
Cryogenic processing techniques
SD can also be obtained by cryogenic processing techniques including spray freeze drying (SFD) and ultra-rapid freezing (URF). These methods increase the freezing rate compared to freeze drying technique. SFD allows for a reduction in the primary particle size of drug particles without intense frictional or mechanical forces which can cause degradation of the API through thermal stress [9, 74]. SFD is a very promising drying technique in which the solution of the drug and polymer is sprayed into/on liquid nitrogen or cold dry air and the frozen droplets are subsequently lyophilized [5, 75].
Super-critical fluid technology (SCFT)
SCFT is a technique which is capable of producing fine drug particles and it is usually known for its valuable product quality, batch to batch reproducibility, and ease of fabrication [4]. In current scenario, the SCFT has a wide spread utility ranging from food processing to pharmaceutical applications [3], but current pharmaceutical applications include: drug extraction and analysis, drug particle and drug polymer engineering, pharmaceutical processing, and preparation of drug delivery system. SD prepared by this technique includes 5-fluorouracil, piroxicam, atorvastatin, ampicillin, oxeglitazar, felodipine, carbamazepine, artemisinin, etc. [5].
Electrospinning
Electrospinning is a technique where SD is formed from a polymeric solution through a millimeter-scale nozzle known as “Taylor cone.” This progression rivets the appliance of a strapping electrostatic field over a conductive capillary connecting to a reservoir containing a polymer solution or melt and a conductive collection screen [17, 74]. The application of an electric field by means of a high-voltage source grades in charge being persuade within the polymer solution, which causes the instigation of a jet. Upon rising the electrostatic field other than critical value, charge species accrue on the plane drop subvert the crescent form into a conical shape (Taylor’s cone). Ahead of the decisive value, a charged polymer jet is expelled from the tip of the cone. The expelled jet afterwards carried near collection screen via the electrostatic force. This technique has remarkable impending for the fabrication of controlling the release of biomedicine and nanofibers [76].
Microwaves irradiation
Microwave irradiation (0.3–300 GHz): in this technique, the drug and carrier, e.g., porous amorphous silicon dioxide, are unvaryingly assorted among solvent and then subjected to microwave reactor at 500 W for different episode of time between 5 and 15 min. It is reported that drug release tendency is higher in samples subjected to microwave irradiation for a longer period of time [77].
Characterization of SD
Characterization is an important phenomenon and primarily involves the quantitative analysis of different physicochemical properties. Majority of efforts to characterize and investigate the molecular arrangement in SD are mainly focused to differentiate between amorphous and crystalline material [10, 52, 60, 74]. The dissolution enhancement of poorly water-soluble drugs in SD can be performed by the standard dissolution methods. However, other properties of SD such as the physical states of drugs, the drug–carrier interaction and the physical and chemical stability of drugs should also be importantly evaluated. Physicochemical characterization of the SD essentially predicts the pharmaceutical applicability of SD. Estimation of these properties helps in understanding the solubility enhancement mechanisms of SD and physicochemical stability. Therefore, it is necessary to characterize the product for optimum results [78–80]. Figure 5 summaries various approaches used for the characterization of SD.
Technical issues related to SD
Despite of its advantages in improving the solubility profile of poorly water-soluble drugs, there is a need to overcome the problems arising during preparation and storage of SD (Table 2) [81–84].
Stability of SD
SDs exhibit high conformational entropy and lower molecular mobility. Molecular mobility is an input factor governing the stability of amorphous phases, because polymers improve the physical stability of amorphous drugs in SDs by increasing the glass transition temperature (Tg) of the miscible blend, in that way plummeting the molecular mobility at regular storage temperatures, or by intermingle explicitly through functional groups of the drugs. For a polymer to be effective in preventing crystallization, it must be comprised to be molecularly miscible among the drug at very high viscosity; below the Tg, there is enough mobility for an amorphous system to crystallize over pharmaceutically relevant time scales [79–81]. There is more deteriorating effect of moisture and temperature on SDs than on physical mixtures. For optimal stability of amorphous SDs, molecular mobility should be low as possible. Nevertheless, SD, moderately or fully amorphous, is thermodynamically unsteady. In SD restrain crystalline particles: these particles projects towards nuclei that can be the starting point for further crystallization [67]. Usually, SD is primed with water soluble low melting point synthetic polymers such as polyvinyl pyrrolidone, mannitol, or polyethylene glycol. These polymers demonstrate privileged results in drug dissolution augmentation, but the amount of these polymers required is relatively large, around 1:2 to 1:8 (drug to polymer) ratio. Stability studies for SD includes: humidity studies, isothermal calorimetry, DSC (Tg, temperature recrystallization), dynamic vapor sorption, and saturated solubility studies [52, 79].
Clinical applications of SD
SD technique with its wide array of therapeutic applications has broadly benefited mankind. Oral route is considered as the most convenient mode of administering therapeutic drugs against different diseases; however, drug degradation, incomplete absorption, and poor BA are some of the problems encountered by this approach. Recent advancement in the SD technology has gained attention in pharmaceutical industries due to improvement in the solubility of poorly water soluble drugs [4, 5, 9, 48]. Interestingly, Cai et al. improvised the clinical utility of SD methodology for selective brain targeting. They reported that gastrodin and borneol co-loaded sustained-release dispersions reduces the stomach irritation and improves the utility of borneol for oral brain-targeting [85]. SD has been reported to be a promising strategy for improving the BA of curcumin, a compound with known clinical benefits [86]. Moreover, SD formulation of paclitaxel, a chemotherapeutic drug with a pharmacokinetic booster, when administered along with ritonavir resulted in an increased BA of paclitaxel. This adjuvant therapeutic approach was reported to be clinically significant for developing low-dose metronomic chemotherapy of paclitaxel [87]. Similarly, SD formulation was also reported to improve the benefits of docetaxel, a potent anticancer drug broadly used for oral cancers [88]. In a phase I trial in patients with advanced solid malignancies, NanoCrystal dispersion formulation of 2-methoxyestradiol dispersion was reported to be well tolerated with improved BA [89]. Aboelwafa and Fahmy reported a SD based orodispersible formulation to overcome the solubility issues associated with Meloxicam (MLX) in healthy human subjects [90]. Along with time, the SD technology also evolved and remained not restricted to laboratory scale alone, but explored successfully on commercial platforms for developing numerous products with effective therapeutic efficacy. A comprehensive account of commercial products utilizing SD techniques has been presented in Table 3 [82, 83, 91].
Future perspectives of SD
SD can be briefly pronounced as a technology of importance not only due to its ability to enhance drug solubility to several folds but also for improving their BA and developing controlled release preparations. However, conventional techniques for SD possess certain practical limitations like total solvent removal in dispersions prepared by solvent method needs to be addressed [92–94]. The most frequent threats that infiltrate and attenuate the SD are its stability concern and incompatibility for industrial scale up [95–99]. Exploring the novel formulation approaches will certainly help to overcome these issues [100–102]. Recent advances on SD area can be explored as: (i) use of new carriers system, (ii) addition of co-additives such as super disintegrants, surfactants, and pH modifiers, and (iii) advancement in characterization methods and development of novel preparation. These issues are interrelated and will be continuously investigated in the coming time [103, 104].
Conclusion
SD is an emerging strategy which made a significant advancement in recent past with reference to its effectiveness by improving pharmacokinetics, bioavailability, and physical stability of the active pharmaceutical ingredient and new chemical entities. SD can improve their stability and performance by increasing drug-polymer solubility, amorphous fraction, particle wettability, and particle porosity. Moreover, new and optimized manufacturing techniques that are easily scalable are also coming out of academic and industrial research. However, physical and chemical stability of both the drug and the carrier in SD are major developmental issues. In view of this, selection of carrier, drug–carrier ratio, and understanding the drug release mechanism from matrix warrants careful attention. This review showcases the striking features of this technology which might be an effective alternative that has infinite potential. Moreover, this review also highlights the pioneer breakthroughs that helped to overcome the existing shortfalls in technological applications. Various impressive applications of SD in the field of pharmacy like ODT, MDT, matrix tablets, mucoadhesive microparticulate system, dry powder for reconstitution along with several marketed products have been catalogued. We strongly believe that under tightly controlled manufacturing processes, SD can be suitably adapted for commercial scale and various cost effective dosage form can be launched. SD is poised to open new avenues in research and therapy.
References
Baird J, Taylor L. Evaluation of amorphous SD properties using thermal analysis techniques. Adv Drug Deliv Rev. 2012;64:396–421.
Lipinski C, Lombardo F, Dominy B, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2012;46:3–26.
Mooter G. The use of amorphous SDs: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today Technol. 2011;9:79–85.
Kawabata Y, Wada K, Nakatani M, et al. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420:1–10.
Chau L, Chulhun P, Beom J. Current trends and future perspectives of SDs containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85:799–813.
Vasconcelos T, Sarmento B, Costa P. SDs as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12:1068–75.
Vasanthavada M, Tong W, Joshi Y, et al. Phase behaviour of amorphous molecular dispersions I: determination of the degree and mechanism of solid solubility. Pharm Res. 2004;21:1598–606.
Potta S, Minemi S, Nukala N, et al. Development of solid lipid nanoparticles for enhanced solubility of poorly soluble drugs. J Biomed Nanotechnol. 2010;6:634–40.
Rautio J, Kumpulainen H, Heimbach T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–70.
Pokharkar V, Mandpe L, Padamwar M, et al. Development, characterization and stabilization of amorphous form of a low Tg drug. Powder Technol. 2006;167:20–5.
Surampalli G, Sabbani P, Nanjwade K, et al. Amorphous SD method for improving oral bioavailability of poorly water-soluble drugs. J Pharm Res. 2013;6:476–80.
Rashid R, Kim D, Din F, Mustapha O, et al. Effect of hydroxypropylcellulose and Tween 80 on physicochemical properties and bioavailability of ezetimibe-loaded solid dispersion. Carbohydr Polym. 2015;130:26–31.
Tuong N, Ha-Lien P, Van Tran T, et al. Development of a modified-solid dispersion in an uncommon approach of melting method facilitating properties of a swellable polymer to enhance drug dissolution. Int J Pharm. 2015;484:228–34.
Onoue S, Terasawa N, Nakamura T, et al. Biopharmaceutical characterization of nanocrystalline SD of coenzyme Q10 prepared with cold wet-milling system. Eur J Pharm Sci. 2014;53:118–25.
Liu H, Ilevbare GA, Cherniawski BP, et al. Synthesis and structure–property evaluation of cellulose ω-carboxyesters for amorphous SDs. Carbohydr Polym. 2014;100:116–25.
Zhang Z, Chen Y, Deng J, et al. SD of berberine–phospholipid complex/TPGS 1000/SiO2: preparation, characterization and in vivo studies. Int J Pharm. 2014;465:306–16.
Onoue S, Nakamura T, Uchida A, Ogawa K, et al. Physicochemical and biopharmaceutical characterization of amorphous solid dispersion of nobiletin, a citrus polymethoxylated flavone, with improved hepatoprotective effects. Eur J Pharm Sci. 2013;49(4):453–60.
Ormes J, Zhang D, Chen A, et al. Design of experiments utilization to map the processing capabilities of a micro-spray dryer: particle design and throughput optimization in support of drug discovery. Pharm Dev Technol. 2013;18:121–9.
Martins R, Siqueira S, Freitas L. Spray congealing of pharmaceuticals: study on production of SDs using Box–Behnken design. Drying Technol. 2012;30:935–45.
Paudel G, Mooter D. Influence of solvent composition on the miscibility and physical stability of Naproxen/PVP K 25 SDs prepared by co-solvent spray-drying. Pharm Res. 2012;29:251–70.
Baret L, Voorpoles J, Kieken F. Powder for reconstitution. US. 2011;20110082161.
Kiser P, Gupta K. Linear order release polymer. US. 2011;2011004507.
Tiwari R, Janakiraman K, Agarwal R, et al. Solid dosage forms of HIV protease inhibitors. US. 2011;20110034489.
Besse J, Laurence B, Pournin J. Solid orodispersible and/or dispersible composition, without an excipient of known effect and its process of preparation. US. 2009;20090110725 .
Patel K, Pillai R. SD compositions. US. 2008;7423004.
Bedrosian C. Therapeutic methods. US. 2007;20070185150.
Terracol D, Duclos R. Process for the production of dry pharmaceutical forms and the thus obtained pharmaceutical compositions. US. 2000;6027747.
Fort J, Krill S, Law D, et al. SD pharmaceutical formulations. US. 1997;7364752.
Nakamichi K, Izumi S, Yasuura H. Method of manufacturing SD. US. 1995;5456923.
Nakano M, Uemura T, Morizane S, et al. Method of producing a SD of the sparingly water-soluble drug nilvadipine. US. 1994;5340591.
Baudier P, Boeck D, Fossion A. Novel galenic forms of verapamil, their preparation and medicines containing said novel galenic forms. US. 1989;4859469.
Riegelman S, Chiou W. Increasing the absorption rate of insoluble drugs. US. 1979;4151273.
Mayersohn M, Gibaldi M. New method of SD for increasing dissolution rates. J Pharm Sci. 1966;55:1323–24.
Tachibani T, Nakamura A. A method for preparing aqueous colloidal dispersion of organic materials by using water soluble polymers: dispersion of beta-carotene by polyvinyl pyrrolidone. Colloid Polym Sci. 1965;203:130–3.
Levy G. Effect of particle size on dissolution and gastrointestinal absorption rates of pharmaceuticals. Am J Pharm Sci. 1963;135:78–92.
Sekiguchi K, Obi N. Studies on absorption of eutectic mixtures. A comparison of the behavior of eutectic mixtures of sulphathiazole and that of ordinary sulphathiazole in man. Chem Pharm Bull. 1961;9:866–72.
Li B, Liu H, Amin M, et al. Enhancement of naringenin solution concentration by solid dispersion in cellulose derivative matrices. Cellulose. 2013;20(4):2137–49.
Satomi O, Suzuki H, Kojo Y, et al. Self-micellizing SD of cyclosporine A with improved dissolution and oral bioavailability. Eur J Pharm Sci. 2014;62:16–22.
Bhatnagar P, Dhote V, Mahajan SC, Mishra PK. Solid dispersion in pharmaceutical drug development: from basics to clinical applications. Curr Drug Deliv. 2014;11:155–71.
van Hoogevest P, Liu X, Fahr A. Drug delivery strategies for poorly water-soluble drugs: The industrial perspective. Expert Opin Drug Deliv. 2011;8:1481–500.
Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4:403–16.
Mooter G, Weuts I, Ridder T, et al. Evaluation of Inutec SP1 as a new carrier in the formulation of SDs for poorly soluble drugs. Int J Pharm. 2006;316:1–6.
Craig D. The mechanisms of drug release from SDs in water soluble polymers. Int J Pharm. 2002;231:131–44.
Leuner C, Dressman J. Improving drug solubility for oral delivery using SDs. Eur J Pharm Biopharm. 2000;50:47–60.
Sekiguchi K, Obi N. Studies on absorption of eutectic mixtures. Studies on Absorption of Eutectic Mixture. II. Absorption of Fused Conglomerates of Chloramphenicol and Urea in Rabbits. Chem Pharm Bull. 1964;12:134–44.
Van Drooge D, Hinrichs W, Visser M, et al. Characterization of the molecular distribution of drugs in glassy SDs at the nano-meter scale, using differential scanning calorimetry and gravimetric water vapour sorption techniques. Int J Pharm. 2006;310:220–9.
Zajc N, Obreza A, Bele M. Physical properties and dissolution behaviour of nifedipine/mannitol SDs prepared by hot melt method. Int J Pharm. 2005;291:51–8.
Tanaka N, Imai K, Okimoto K, et al. Development of novel sustained-release system, disintegration controlled matrix tablet (DCMT) with SD granules of nilvadipine (II): in vivo evaluation. J Control Release. 2006;112:51–6.
Urbanetz N. Stabilization of SDs of nimodipine and polyethylene glycol 2000. Eur J Pharm Sci. 2006;28:67–76.
Chauhan H, Hui-Gu C, Atef E. Correlating the behavior of polymers in solution as precipitation inhibitor to its amorphous stabilization ability in SDs. J Pharm Sci. 2013;102:1924–35.
Crowley M, Zhang F, Repka M, et al. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev Ind Pharm. 2007;33:909–26.
Vilhelmsen T, Eliasen H, Schaefer T. Effect of a melt agglomeration process on agglomerates containing SDs. Int J Pharm. 2005;303:132–42.
Lim H, Balakrishnan P, Hwang D, et al. Development of novel sibutramine base-loaded SD with gelatin and HPMC: physicochemical characterization and pharmacokinetics in beagle dogs. Int J Pharm. 2010;397:225–30.
Rodriguez J, Torre-Iglesias P, Vegas-Sánchez M, et al. Changed crystallinity of mebendazole SD: improved anthelmintic activity. Int J Pharm. 2011;403:23–8.
Bley H, Fussnegger B, Bodmeier R. Characterization and stability of SDs based on PEG/polymer blends. Int J Pharm. 2010;390:165–73.
Miyazaki T, Aso Y, Yoshioka S, et al. Differences in crystallization rate of nitrendipine enantiomers in amorphous SDs with HPMC and HPMCP. Int J Pharm. 2011;407:111–8.
Yunxia BI, Rahman A, David J. Solid dispersion of poorly soluble compounds comprising crospovidone and at least one water-soluble polymer. WO. 2013;2013040187 A.
Fort J, Krill S, Law D, et al. Solid dispersion pharmaceutical formulations. US. 2008;7364752 B1.
Ali W, Williams A, Rawlinson C. Stochiometrically governed molecular interactions in drug: poloxamer SDs. Int J Pharm. 2010;391:162–8.
Van Drooge D, Hinrichs W, Frijlink H. Anomalous dissolution behaviour of tablets prepared from sugar glass-based SDs. J Control Release. 2004;97:441–52.
Joshi H, Tejwani R, Davidovich M, et al. Bioavailability enhancement of a poorly water-soluble drug by SD in polyethylene glycol–polysorbate 80 mixture. Int J Pharm. 2004;269:251–8.
Maniruzzaman M, Boateng J, Bonnefille M, et al. Taste masking of paracetamol by hot-melt extrusion: an in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2012;80:433–42.
Saerens L, Dierickx L, Lenain B, et al. Raman spectroscopy for the in-line polymer–drug quantification and solid state characterization during a pharmaceutical hot-melt extrusion process. Eur J Pharm Biopharm. 2011;77:158–63.
Ghebremeskel A, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing SDs of poorly soluble API: selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int J Pharm. 2007;328:119–29.
Parve B, Teli B, Birajdar A. Solid dispersions: an overview on solubility enhancement of poorly water soluble drugs. Int J Pharm Bio Sci. 2014;5:7–25.
Martin N, Pichler A, Richter F et al. Solid dispersion comprising amorphous lorcaserin hydrochloride. WO. 2014;2014135545 A1.
Passerini N, Albertini B, González M, et al. Preparation and characterisation of ibuprofen–poloxamer 188 granules obtained by melt granulation. Eur J Pharm Sci. 2002;15:71–8.
Verhoeven E, Beer T, Schacht E, et al. Influence of polyethylene glycol/polyethylene oxide on the release characteristics of sustained-release ethylcellulose mini-matrices produced by hot-melt extrusion: in vitro and in vivo evaluations. Eur J Pharm Biopharm. 2009;72:463–70.
Szuts A, Láng P, Ambrus R, et al. Applicability of sucrose laurate as surfactant in SDs prepared by melt technology. Int J Pharm. 2011;410:107–10.
Won D, Kim M, Lee S, et al. Improved physicochemical characteristics of felodipine SD particles by supercritical anti-solvent precipitation process. Int J Pharm. 2005;301:199–208.
Cui F, Yang M, Jiang Y, et al. Design of sustained-release nitrendipine microspheres having SD structure by quasi-emulsion solvent diffusion method. J Control Release. 2003;91:375–84.
Mogalian E, Oliyai R, Stefanidis D, et al. Solid dispersion formulation of an antiviral compound. US. 2014;20140212487 A1.
Martins R, Siqueira S, Freitas L. Spray congealing of pharmaceuticals: study on production of SDs using Box–Behnken design. Drying Technol. 2012;30:935–45.
Rein Richard H. Cryogenic cooling. US. 1968;3416977 A.
Chauhan B, Shimpi S, Paradkar A. Preparation and evaluation of glibenclamide-polyglycolized glycerides SDs with silicon dioxide by spray drying technique. Eur J Pharm Sci. 2005;26:219–30.
Yu DG, Zhu LM, White K, et al. Electrospun nanofiber based drug delivery systems. Health. 2009;1:67–75.
Hu J, Johnston K, Williams R. Spray freezing into liquid (SFL) particle engineering technology to enhance dissolution of poorly water soluble drugs: organic solvent versus organic/aqueous co-solvent systems. Eur J Pharm Sci. 2003;20:295–303.
Purvis T, Mattucci M, Crisp M, et al. Rapidly dissolving repaglinide powders produced by the ultra-rapid freezing process. AAPS Pharm Sci Tech. 2007;8:52–60.
Mura P, Moyano J, Rodriguez M, et al. Characterization and dissolution properties of ketoprofen in binary and ternary SDs with polyethylene glycol and surfactants. Drug Dev Ind Pharm. 2005;31:425–34.
Bhattacharya S, Suryanarayanan R. Local mobility in amorphous pharmaceuticals—characterization and implications on stability. J Pharm Sci. 2009;98:2935–53.
Duddu S, Sokoloski T. Dielectric analysis in the characterization of amorphous pharmaceutical solids. 1. Molecular mobility in poly (vinyl pyrrolidone)-water systems in the glassy state. J Pharm Sci. 1995;84:773–6.
Goddeeris C, Willems T, Houthoofd K, et al. Dissolution enhancement of the anti-HIV drug UC 781 by formulation in a ternary SD with TPGS 1000 and Eudragit E100. Eur J Pharm Biopharm. 2008;70:861–8.
Ohara T, Kitamura S, Kitagawa T, et al. Dissolution mechanism of poorly water-soluble drug from extended release SD system with ethylcellulose and hydroxyl propyl methyl cellulose. Int J Pharm. 2005;302:95–102.
Iqbal Z, Babar A, Ashraf M. Controlled-release naproxen using micronized ethyl cellulose by wet-granulation and solid-dispersion method. Drug Dev Ind Pharm. 2002;28:129–34.
Cai Z, Lei X, Lin Z, et al. Preparation and evaluation of sustained-release solid dispersions co-loading gastrodin with born eolasan oral brain-targeting enhancer. Acta Pharm Sinica B. 2014;4:86–93.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60.
Moes J, Koolen S, Huitema A, et al. Development of an oral solid dispersion formulation for use in low-dose metronomic chemotherapy of paclitaxel. Eur J Pharm Biopharm. 2013;83:87–94.
Moes JJ, Koolen SL, Huitema AD, et al. Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001). Int J Pharm. 2011;420:244–50.
Tevaarwerk AJ, Holen KD, Alberti DB, et al. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin Cancer Res. 2009;15:1460–5.
Aboelwafa AA, Fahmy RH. A pilot human pharmacokinetic study and influence of formulation factors on orodispersible tablet incorporating meloxicam solid dispersion using factorial design. Pharm Dev Technol. 2012;17:1–14.
Kolasinac N, Kachrimanis K, Homsek M, et al. Solubility enhancement of desloratadine by SD in poloxamers. Int J Pharm. 2012;436:161–70.
Muhrer G, Meier U, Fusaro F, et al. Use of compressed gas precipitation to enhance the dissolution behaviour of a poorly water-soluble drug: generation of drug microparticles and drug–polymer SDs. Int J Pharm. 2006;308:69–83.
Tran T, Tran P, Lim J, et al. Physicochemical principles of controlled release SD containing a poorly water soluble drug. Ther Deliv. 2010;1:51–62.
Pouton C. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29:278–87.
Moschwitzer J. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453:142–56.
Yoshioka M, Hancock B, Zografi G. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J Pharm Sci. 1994;83:1700–5.
Vyazovkin S, Dranca I. Physical stability and relaxation of amorphous indomethacin. J Phys Chem B. 2005;109:18637–644.
Serajuddin A. SD of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88:1058–66.
Ahuja N, Katare O, Singh B. Studies on dissolution enhancement and mathematical modelling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur J Pharm Biopharm. 2007;65:26–38.
Ayenew Z, Paudel A, Van den Mooter A. Can compression induce demixing in amorphous SDs? A case study of naproxen–PVP K25. Eur J Pharm Biopharm. 2012;81:207–13.
Waard H, Hinrichs W, Visser M, et al. Unexpected differences in dissolution behaviour of tablets prepared from SDs with a surfactant physically mixed or incorporated. Int J Pharm. 2008;349:66–73.
Beg S, Swain S, Rizwan M, Irfanuddin M, et al. Bioavailability enhancement strategies: basics, formulation approaches and regulatory considerations. Curr Drug Deliv. 2011;8:691–702.
Vasanthavada M, Tong W, Joshi Y, et al. Phase behaviour of amorphous molecular dispersions II: role of hydrogen bonding in solid solubility and phase separation kinetics. Pharm Res. 2005;22:440–8.
Chiou W, Riegelman S. Preparation and dissolution characteristics of several fast-releases SDs of griseofulvin. J Pharm Sci. 1969;58:1505–510.
Conflict of interest
The authors state no conflict of interest and have received no payment in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, D.K., Dhote, V., Bhargava, A. et al. Amorphous solid dispersion technique for improved drug delivery: basics to clinical applications. Drug Deliv. and Transl. Res. 5, 552–565 (2015). https://doi.org/10.1007/s13346-015-0256-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-015-0256-9