Abstract
The Australian canola industry was established in the 1970s and has expanded since that time, particularly in the last two decades. This review addresses the changes in farming practices since the year 2000 and the epidemiological and management consequences for blackleg, caused by the fungus Leptosphaeria maculans, the main disease impacting Brassica napus production. To help understand the change in production practices, a survey of over 100 growers and agronomists was conducted. Modern management practices include increased crop residue retention, frequency of canola in crop sequences, number of resistance genes in cultivars, use of hybrids and fungicides as well as earlier sowing and flowering times. While some of the changes identified in the survey and in this review increase the risk and severity of disease, including new symptoms like upper canopy infection, others provide novel strategies for control. Keeping these changes in mind, a set of research priorities towards long term sustainable management of blackleg disease are identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Integrated disease/pest management is a widely-adopted philosophy in agriculture that is used to maximise plant yields by a reduction in microbial diseases or animal pests through the implementation of multiple mitigation strategies. The concept was developed in the 1960s by entomologists and later extended to microbial diseases (Jacobsen 1997; Cook 2000). However, these management strategies are not static, in that the release of new technologies in farming systems, plant cultivars or chemicals can require revisions on management. However, the impact of such changes on disease management are rarely assessed.
This paper focuses on the management of Leptosphaeria maculans management in Australia, although blackleg disease is a major threat to canola (Brassica napus, oilseed rape) production worldwide, except in China (Fitt et al. 2006; Zhang et al. 2014). Blackleg disease results in 10–20% yield losses annually in Canada and the United Kingdom, with up to 90% yield loss caused by epidemics in Australia (West et al. 2001; Fitt et al. 2006; Sprague et al. 2006a; Zhang and Fernando 2018). Blackleg disease is stubble-borne, with sexual reproduction on infected crop residue remaining at the end of the growing season after harvest. The sexual spores are released following rainfall events and land on the cotyledons or leaves of seedlings, germinate and then invade the plant via wounds or stomata (Fig. 1). The fungus then causes necrotic lesions on the leaves and cotyledons before entering a biotrophic phase to allow growth down the petiole and into the stem (Hammond and Lewis 1987). At maturity, the fungus then switches back to a necrotrophic lifestyle and causes blackening of the stem, cankering of plants and, in extreme situations, killing the plant (Hammond et al. 1985).
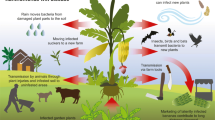
Impact of changing farming systems on the life cycle of Leptosphaeria maculans and control strategies to minimise the impact of blackleg disease. a Sowing and flowering times have moved forward significantly in Australia from 2000 compared to 2018, resulting in changes in exposure of spores released from crop residues. b The changes in farming practices have led to changes in disease epidemiology of L. maculans. Earlier sowing results in seedlings spending less time exposed to spores in the vulnerable growth stage. Since it is seedling infection that leads to crown canker, earlier sowing times will potentially reduce the severity of crown canker. In contrast, earlier flowering times have resulted in the flowers, pods and branches of plants now being exposed to spores, leading to the development of symptoms termed upper canopy infection (UCI). Highlighted in red are control strategies known to minimise different aspects of disease, as well as the disease drivers leading to increased disease pressure. MG, major gene resistance; QR, quantitative resistance
In the past 20 years, there have been both scientific and technological advances that have impacted the Australian canola industry to improve production (Fig. 2). Shifts in rainfall patterns have been a major driver for the implementation of many of these changes. The reliability of the autumn ‘break’, the rains on which winter grain crops are sown in southern Australia, has declined particularly during the Millennium drought (1998–2010), but is a trend that is likely to continue (Verdon-Kidd et al. 2014). Adequate moisture at sowing is critical for the successful establishment of small-seeded crops such as canola. There has also been significant reductions in growing season rainfall across the winter cropping regions of Australia since 1970 (www.bom.gov.au/state-of-the-climate/, accessed 13 May 2020). In this review we describe and then investigate via a survey of growers and agronomists, changes in practices associated with canola production in Australia and the related impact on management of L. maculans.
Summary of significant events in Australian canola production including the introduction new technologies, as well as changes in farming practices and cultivar variety. Decreased production in the early 2000s reflects the Millennium drought. R, resistance; CF, Clearfield®; RR, Roundup Ready®; UCI, upper canopy infection; CA, cropping area; conv, conventional; tri, triazine tolerant; GM, genetically modified
Survey to assess perceptions about changing farm practices
In addition to assessing published literature and government reports, we conducted a survey to determine the extent of change to identify: (i) how blackleg disease management strategies used by industry have changed over time; and (ii) areas of blackleg research to deliver updated management advice relevant to current farming practices.
A survey consisting of 30 questions was conducted using the online survey platform, Survey Monkey (www.surveymonkey.com.au), designed to determine which factors in canola and blackleg management have changed between 2000 and 2018. Respondents were asked general questions including whether they are a farmer or agronomist/advisor, the postcode of the location of their own or their client’s primary property, size of that primary property and long-term average annual growing season rainfall (April through October), which was used to categorise responses into low (<250 mm rainfall), medium (250–400 mm) or high (>400 mm) rainfall regions. For most of the questions, the respondents were asked to answer each in relation to historic on-farm practices (the year 2000 was selected as a guide to the timeframe) and current practices (2018). A total of 112 respondents, representing all canola-growing regions of Australia, participated in the survey. Of the 112, 74 respondents were advisors who provide advice to multiple canola growers within their regions.
The responses from survey respondents are ordered categorical data, e.g. 0–10%, 11–20%, 21–30%, >30% farm sown with canola. That is, the data are not continuous, but are classes arrayed in order and therefore the appropriate analyses are ordinal regression (Agresti 2010). These are related to linear regression and analysis of variance and other generalised linear models in that a linear model is written consisting of explanatory factors (or covariates) and error terms. However, conversion to a categorical (cf. continuous) response is made via the cumulative link model, using a logit link. This is akin to fitting models of the probability of exceeding a given threshold between successive classes, e.g. the probability of 11–20% rather than 0–10% canola. For each question, a series of models were fitted with a set of covariates: State of Australia, Year (2000 or 2018) and growing season rainfall (centered on median). Random effects of respondent ensured the analyses were paired in all cases. The following cumulative log odds mixed effect models were fitted with combinations of three covariates which were compared using Akaike’s Information Criteria and Likelihood Ratios; significance was determined through Chi-square tests. The models compared included a null model (just respondent), Year, Year + State, Year + Rainfall, Year + Rainfall + State. Two models were fitted with interactions with Year: Year + Rainfall + Year × Rainfall and; Year + Rainfall + Year × State. Rainfall and State are somewhat confounded, so we did not fit a model for all three terms plus interactions with Year. Analyses were run with the package “ordinal” (Christensen 2019) in the R software environment (Team 2019). For a summary of the survey questions and the statistical analysis see Supplementary File 1.
Intensification of production
Canola is one of the major oilseed crops worldwide. In 2000/2001, global production was 36.7 Mt, harvested from an area of 23.9 Mha (Gunstone 2004), and by 2017/2018 the area had doubled to 74.0 Mt from an area of 31.3 Mha (Van de Wouw and Howlett 2020). Globally yields have increased from 1.5 t/ha to 2.4 t/ha over the past 20 years (Van de Wouw and Howlett 2020; Fischer et al. 2014). In Australia specifically, canola production has also doubled in the past 20 years (Fig. 2), increasing from 1.8 Mt in 2000/2001 to 3.8 Mt in 2017/2018, with a peak of 4.4 Mt in 2016/2017; however, yields have fluctuated seasonally (Gunstone 2004; Van de Wouw and Howlett 2020). Whilst increased canola production globally has been driven by both increased area and improved yields, in Australia the increase has mainly been driven by an increase in area (Fig. 2). The increase in area sown to canola means that canola is now the third largest crop in Australia, worth AU$2.1 billion in 2017–18 (www.abs.gov.au), and has spread into lower rainfall regions rather than the typical high rainfall areas (Zhang et al. 2016a). Grower survey data also reflect the intensification of canola with respondents reporting a statistically significant increase in area sown to canola, from <10% of the farm area in 2000 to >30% in 2018 (Fig. 3a).
One potential factor driving canola intensity in Australia in the past 20 years is the increased diversity in commercially available cultivars in terms of herbicide resistance. According to canola cultivar lists published in the official blackleg rating guides produced by the Grains Research and Development Corporation (www.grdc.com.au/GRDC-FS-blacklegManagementGuide), over the past two decades the number of cultivars commercially available has almost doubled from 38 in 2001 to 60 in 2020, with large changes in available herbicide resistance (Fig. 2). The proportion of conventional cultivars (those lacking specific herbicide resistance) dropped from 50% to 5% of the available cultivars between 2001 and 2020. This large change was initially driven by the introduction of the non-genetically manipulated Clearfield® and triazine-tolerant varieties, and then by the introduction of the Round-up Ready® technology which provides new weed management options; by 2010, 27% of cultivars were harbouring this technology (Fig. 2). In 2020, the second-generation Round-up Ready® technology - termed TruFlex® - was released, which extends the glyphosate application windows for farmers providing extended opportunities for controlling weeds. In addition, herbicide stacks are also available within the same cultivars: Clearfield® and triazine-tolerance; Round-up Ready® and triazine-tolerance; TruFlex® and Clearfield®; and TruFlex® and triazinetolerance. The GM-herbicide technologies now represent almost 40% of the commercially available cultivars and are likely to increase due to the extended opportunities for weed control.
Other advances in cultivar diversity include hybrid canola varieties, winter dual-purpose varieties and high stability oil varieties. Hybrid canola varieties are generally considered more vigorous and weed competitive and have been shown to out-yield open-pollinated cultivars by up to 20% in both Australia and Canada, depending on rainfall and growing season length (Zhang et al. 2016a; Brandt et al. 2007). Consequently, hybrid cultivars have increased from 2% of the commercially available cultivars in Australia in 2001 to 88% in 2020 (Fig. 2). Despite the majority of commercially available cultivars being hybrids, according to the respondents in our grower survey, 43% grew open pollinated cultivars in 2018 whilst 57% grew hybrids. The popularity of the open pollinated cultivars probably reflects the high cost of hybrid seed (~$30/kg) and the ability to retain seed from the open pollinators (Zhang et al. 2016a).
The release of winter varieties underpinned the widespread and rapid adoption of canola for dual-purpose production in higher-rainfall environments in mixed farming enterprises (crop and livestock) with increases in whole farm productivity of up to $100 per ha (Kirkegaard et al. 2016a; Sprague et al. 2015; Bell et al. 2015). Dual-purpose refers to the grazing of established crops by livestock during vegetative growth in late autumn and winter, with the subsequent recovery of the crop to produce grain after the removal of animals, often with minimal impact on grain yield compared to an ungrazed crop (Kirkegaard et al. 2012). The availability of winter cultivars allows early sowing opportunities to capture maximum forage production as crops remain vegetative until after winter (Lilley et al. 2015). As all canola cultivars can be grazed and are a high-quality source of feed, dual-purpose production is being adopted across all rainfall zones.
As canola stubble is the inoculum source for the following season, reduced crop rotation coupled with increased canola production results in higher inoculum loads the following year. Tighter canola rotations in Canada and Germany decreased yield potentials and increased blackleg disease severity (Harker et al. 2015; Hegewald et al. 2017). Recent studies in Australia suggest that blackleg could be a contributing factor to the large gaps between achieved and potential grain yield of canola. An analysis of farm yields between 1998 and 2015 found yields were between 42% and 62% of the potential maximum, adjusted for water availability, as determined using the canola simulation model Agricultural Production Systems sIMulator (APSIM; www.yieldgapaustralia.com.au/maps/) which considers soil, water and management practices (Holzworth et al. 2018).
Blackleg crown canker severity (internal infection as reported in Marcroft et al. (2012)) has been measured from cultivars sown at between 30 and 60 sites across the canola-growing regions of Australia each year between 2004 and 2019 (Fig. 1). Nationally, blackleg disease severity has increased from 16.5% average internal infection in 2004 up to a peak of 47.1% in 2010, before slowly declining to 32.2% in 2019. Interestingly, the national average disease severity appears to be decreasing while at the same time canola intensity is increasing, despite the prediction of higher disease due to inoculum load. Possible reasons for this discrepancy are presented in the following sections.
Stubble management and retention
The primary inoculum source for blackleg disease is ascospores produced by sexual reproduction on crop stubble between growing seasons. Cultural practices, such as crop rotation and isolation from infected stubble, are important to reduce the risk of blackleg disease through avoidance of ascospore inoculum. Factors such as tighter rotations and stubble retention have impacted on the efficacy blackleg management strategies.
In the early 2000s in Australia and Canada, a break of 2 or 3 years between canola crops was common practice, allowing stubble to breakdown and ascospore production to diminish (Salisbury et al. 1995; Gladders et al. 2006; Ghanbarnia et al. 2012). However, in Australia the vast majority of ascospores are released in the first year after the canola crop, and therefore tighter canola rotations are viable (Marcroft et al. 2004). Our survey results suggest that these findings may have boosted the confidence of growers to increase the frequency of canola, as rotations have significantly tightened from a break between canola crops of >3 years in 2000 to the more common practice of growing canola in alternate years in 2018 (Fig. 3b). The tighter rotations in Australia are consistent with trends in Canada whereby canola is grown in alternate years or with no break at all (canola on canola; Zhang and Fernando 2018). Further to this, tightening of rotations to a wheat-canola-wheat system has also partially been driven by limited options for alternative break crops for specific soil types, such as pulses on acid soils.
Research in Australia and Europe showed that isolation of canola crops by >500 m from the previous year’s stubble is sufficient to avoid the highest ascospore concentrations and therefore minimise disease (Marcroft et al. 2004; Gossende et al. 2003). An isolateion strategy was easily achievable in the early 2000’s when canola was grown in wider rotations but with the tightening of rotations, physical separation is less relevant due to the difficulty of isolating the current crop from stubble. Furthermore, this situation is exacerbated by higher inoculum loads due to the overall increase in production area.
There have been significant changes to farming systems during the past two decades in stubble conservation, particularly with the wide uptake of soil conservation tillage practices. By 2016 no-till or zero-till strategies (practices whereby less than 30% of soil is disturbed) represented 75% of the cropping area in Australia, up from only 25% in 2001 (Umbers 2016; Fig. 2). These changes are reflected in the grower survey, whereby most respondents actively reduced stubble loads (by burning or cultivating) in 2000 in contrast to stubble being conserved in 2018 unless there was a specific reason (e.g. pests, disease or weeds), often resulting in stubble left standing (Fig. 3c, d). The changes in stubble conservation are consistent with minimum tillage practices. Machinery with GPS guided systems allows precision seeding of crops in the inter-row between stubble of the previous crop, much of which is left standing and intact after harvest, an innovation that was adopted in the mid-2000s in response to the increase in no-till practices (Fig. 2).
Sowing and flowering dates
Sowing and flowering times of canola crops in Australia have moved earlier which is supported by growers’ responses in our survey (Fig. 3e). In 2000, the median sowing date was early-May but by 2018 this had significantly shifted approximately 3 weeks earlier to mid-April. Similarly, targeted flowering times across all regions were an average of approximately 3 weeks earlier in 2018 compared to 2000. Comparison of responses by individuals were analysed for sowing and flowering date from 2000 to 2018, with 38% of respondents sowing 20 days earlier and 47% of respondents with crops flowering 20 days earlier (Fig. 3e).
Timely sowing and flowering is key to optimising canola yield potential. Kirkegaard et al. (2016b) reported declines in grain yield potential (6–6.5%), oil content (0.5–1.5%) and water-use efficiency (3.8–5.5%) for each week delay in sowing after early-April. Timely flowering can increase grain yield by up to 20% (J. Lilley pers. comm.). The critical period for yield development in canola occurs between 150 and 450 °C days after the start of flowering, with a peak at 350 growing degree days (Kirkegaard et al. 2018). During this phase, canola is unable to recover or compensate for reductions in grain and oil yield caused by stress events. In response to this, optimal start of flowering times using a crop simulation analysis have been identified at a regional level in Australia to avoid the impacts of abiotic stresses such as heat, frost and water stress (Lilley et al. 2019). However, the simulation analysis does not yet account for the effects of biotic stresses such as upper canopy blackleg infection, which are more likely to limit yield of earlier flowering crops (Sprague et al. 2018).
Earlier sowing has been facilitated by the development of improved germplasm and equipment. The introduction of herbicide-tolerant varieties allows in-crop control of weeds, which is a key rotational benefit of canola in predominantly cereal-based farming in Australia (Angus et al. 2015). Precision seeders and satellite-guided sowing with 2 cm accuracy have enabled establishment without cultivation into a seed bed. The previous practice of destroying canola stubble, normally by burning and then raking, leads to disturbing and drying the soil. However, the use of modern sowing equipment maintains the crop residue, minimising moisture loss through soil disturbance, with the seed placed next to the previous season’s residue. Additionally, hybrid cultivars with larger seed and more vigorous seedlings are better adapted to sowing in stubble conservation systems. Consequently, the combination of herbicide-tolerant cultivars, satellite-guided sowing equipment and hybrid seeds have resulted in farmers confidently sowing into dry soil that has full stubble retention. Therefore, crops are now often sown by calendar date rather than by soil moisture/weed status and, in some situations, sown dry prior to a rainfall event.
Rainfall is a major driver of maturation of L. maculans pseudothecia on stubble and ascospore release (McGee and Emmett 1977; Marcroft et al. 2003). Infection of canola seedlings prior to the 6th leaf stage leads to the development of severe crown cankers (Marcroft et al. 2005). Crops sown in early May remain in this vulnerable growth stage during peak ascospore release in autumn and winter (Fig. 1a) leading to severe crown canker. However, crops sown in early April grow rapidly through the vulnerable stage and likely avoid prolonged exposure to ascospore release, and therefore develop less severe crown cankering. These suggestions are consistent with the national disease severity data, whereby blackleg crown canker severity has declined since 2010 (Fig. 2).
Upper canopy infection
While the timing of flowering is not important for development of crown canker, earlier flowering is associated with increased severity of blackleg disease symptoms on plants post-stem elongation, collectively termed ‘upper canopy infection’ (Sprague et al. 2018; Fig. 1b). Upper canopy infection (UCI) refers to the development of lesions on the upper stems, branches, flowers, peduncles and siliques of canola. Yield-limiting symptoms were first detected in Australia in 2010 with L. maculans identified as the causal agent. Since then, the incidence and prevalence of UCI has increased. In field experiments, reductions in grain yield of up to 30% have been associated with UCI, as estimated by a comparison of crops with complete blackleg control by multiple fungicide applications with an untreated crop (Sprague et al. 2017). In Australia, peak ascospore release from stubble in contact with the ground usually occurs in the winter months from June until August (McGee and Emmett 1977), therefore the earlier flowering time exposes the reproductive parts of the plant to ascospores and increased frequency of cool, wet conditions that favor infection (Fig. 1).
Stubble conservation driven partly by satellite-guided sowing equipment, may also contribute to the emergence of UCI as stubble remains standing and persists for longer because only the root section is in contact with the ground. Compared to stubble knocked down and in contact with the ground, standing stubble is drier with delayed maturation and release of ascospores (McCredden et al. 2018), which may add to the prevalence of upper canopy infection.
Genetic diversity in canola germplasm
blackleg disease is managed through a three-pronged approach: genetic resistance, cultural practices, and fungicides (Van de Wouw et al. 2016; Zhang and Fernando 2018; Fitt et al. 2006). In Australia, genetic resistance is the main control strategy for growers, with two types of resistance: quantitative (minor-gene) and qualitative (major-gene). Quantitative resistance, often considered partial resistance, is thought to be conferred by multiple genes, each contributing a small part to the overall resistance phenotype (Delourme et al. 2006; Stuthmann et al. 2007). The genetics of this quantitative resistance are still poorly understood and only a small number of quantitative trait loci have been identified (Huang et al. 2019; Larkan et al. 2016; Raman et al. 2012, 2016, 2018, 2020). In contrast, qualitative or major gene resistance is considered a complete resistance and is controlled in a gene-for-gene manner, whereby for each resistance gene in the plant there is a corresponding avirulence gene in the pathogen (Flor 1955; Balesdent et al. 2005). For the host side of the Brassica spp. - L. maculans interaction, a total of 15 major resistance genes have been mapped and two cloned, whilst on the pathogen side 13 avirulence genes have been mapped and 11 have been cloned from 8 loci (Fudal et al. 2007; Gout et al. 2006; Balesdent et al. 2013; Ghanbarnia et al. 2012, 2015, 2018; Parlange et al. 2009; Plissonneau et al. 2016, 2017; Van de Wouw et al. 2009, 2014; Larkan et al. 2013, 2015; Delourme et al. 2004, 2006; Petit-Houdent et al. 2019). Major gene resistance, when effective, protects all parts of the plant (Elliott et al. 2016), however this resistance can be rendered ineffective due to changes in the fungal population.
Resistance breakdown has occurred in Europe (Rouxel et al. 2003; Brun et al. 2010), Canada (Zhang et al. 2016b), and most dramatically in Australia (Sprague et al. 2006b), whereby resistance has been overcome in less than 3 years and caused >90% yield loss. As such, these major resistance genes require management to prevent resistance breakdown. In Australia, Canada and France, all commercially released cultivars are characterised for their complement of known major genes for blackleg resistance (Marcroft et al. 2012; Van de Wouw and Howlett 2020; Zhang and Fernando 2018). This information is used by growers and advisors to rotate cultivars with different resistance genes (Van de Wouw and Howlett 2020). However, the success of resistance gene rotations for disease control is underpinned by knowledge on the structure of the local pathogen population where the genes are deployed and the effect of genes individually and in combination, many of which are complex. For example, L. maculans has multiple avirulence genes (AvrLm1-R3, AvrLm4–7 and AvrLm5–9) that interact with more than one host resistance gene (Parlange et al. 2009; Ghanbarnia et al. 2018; Larkan et al. 2013). In addition, some avirulence genes in L. maculans are linked and can be subject to selection even in the absence of the corresponding resistance gene (Van de Wouw et al. 2010). Comparisons between experiments on the epidemiological and evolutionary responses to resistance genes are confounded by multiple factors, such as the genetic background of host lines, which can be further complicated by the possible presence of quantitative blackleg resistance (Brun et al. 2010). Other factors include the unknown structure of the starting pathogen population, lack of markers for avirulence genes - which interact with the resistance genes - and the inability to rapidly screen whole populations for avirulence frequency profiles, which would enable detection of avirulence genes at low frequencies.
In experimental and agricultural systems, L. maculans has overcome major gene resistance in as little as 3 years (Sprague et al. 2006a; Rouxel et al. 2003; Brun et al. 2010). However, deployment of resistance genes in space and time can be an effective tool to reduce disease severity and manipulate evolution of pathogen populations (Bousset et al. 2018; Marcroft et al. 2012). In experiments conducted in the field to replicate combinations of spatial and temporal deployment of host resistance genes and dispersal of pathogen populations, the strategic deployment of resistance genes reduced blackleg severity and frequency of virulent isolates, even when resistance genes have been overcome (Bousset et al. 2018). Furthermore, Bousset et al. (2018) reported selection for isolates virulent towards multiple resistance genes in cultivars with the corresponding set of (stacked) resistance genes, but selection against these isolates in cultivars lacking the corresponding genes. Therefore, for rotation of cultivars to be used to manipulate fungal populations, cultivars with fewer resistance genes provide the most sustainable strategy.
The diversity of major resistance genes present in Australian cultivars has increased over the past 20 years (Fig. 2). Genetic diversity within Australian cultivars released prior to the year 2000 has been traced to 11 ancestral lines, many of which harboured Rlm4 and/or Rlm3 (Cowling 2007); this was reflected in our survey, whereby >70% of cultivars grown by respondents in 2000 contained resistance genes Rlm4 and/or Rlm3. Since that time, seven additional resistance genes have been introduced into Australian varieties: Rlm1, Rlm5, Rlm6, Rlm7, RlmS, LepR1 and LepR3 (Fig. 2). Whilst this increase in genetic diversity provides an opportunity to develop strategies to rotate genes to control blackleg, 73% of the cultivars grown in 2020 contain two or more resistance genes, with Rlm1 and/or Rlm4 present in all cultivars.
Fungicides for crop protection
Fungicides are an important tool for controlling blackleg in Australia. In the early 2000s, fungicides applied to seed and fertilizer at sowing became commercially available to provide significant control for blackleg disease (Khangura and Barbetti 2002, 2004; Marcroft and Potter 2008). Since 2011, several foliar fungicides have been commercialised for in-crop blackleg control (Van de Wouw et al. 2016; Fig. 2). Prior to the release of these foliar fungicides, all blackleg management decisions were made prior to or at sowing i.e. cultivar, paddock selection, and seed- or fertilizer-amended fungicides.
Since 2000, there has been a significant increase in fungicides applied at sowing (seed and/or fertilizer) to control blackleg in Australia (Fig. 3f). In 2000, 52% of respondents used at least one fungicide applied to seed (fluquinconazole) and/or fertilizer (flutriafol), 28% did not use any fungicide and 20% applied fungicides only when necessary. In 2018, 95% of respondents always used at least one of these fungicides, with the remainder using a fungicide if necessary. Of the respondents that always used a fungicide, those using only a seed dressing declined from 40% to 22% and the use of both fungicides increased from 2% to 55%. The significant increase in fungicide use was consistent across rainfall zones and states. As mentioned earlier, crown canker severity may be lower in earlier sown crops, which grow rapidly in warmer conditions and avoid ascospore showers during vulnerable stages of plant development. Therefore, fungicide application at sowing may not be warranted in some seasons or regions with blackleg controlled by strategic applications of foliar fungicides instead. The level of foliar fungicide use was captured in the survey for 2018. Of the respondents 49% applied a foliar fungicide if they perceived it was required dependent on seasonal conditions, the level of cultivar resistance and disease pressure, with the remainder evenly split between always (20%) and never (23%) applying a foliar fungicide.
Blackleg upper canopy infection on plants has become more prevalent in Australia since 2011 (Sprague et al. 2018). Research indicates that foliar fungicides applied during early lowering to control Sclerotinia stem rot (caused by Sclerotinia sclerotiorum) is also effective against L. maculans (Sprague et al. 2017). There are currently no registered products for control of blackleg upper canopy infection, however, some products registered for S. sclerotiorum control are effective against L. maculans. The majority of survey respondents (61%) considered fungicide application to control upper canopy infection if one was available, likely a reflection of the increase in prevalence and potential for high yield losses due to upper canopy infection (Sprague et al. 2018). Respondents were asked whether blackleg upper canopy infection was a consideration in the decision to apply fungicides for Sclerotinia stem rot. Of the 61 respondents that answered, the majority considered upper canopy infection only if conditions were suitable (52%), some always applied a fungicide specifically for upper canopy infection (14%) and the remainder never considered fungicide control for upper canopy infection (33%). The expansion of fungicide availability for blackleg and Sclerotinia stem rot control could result in up to five fungicide applications within a single season (Fig. 1).
Reliance on fungicides for blackleg control is unique to Australia, with little use in Canada and Europe due to inconsistent yield responses (Peng et al. 2020). The increased availability of fungicides and decreasing prices is driving a push towards a reliance on chemicals and genetic resistance, rather than cultural practices for blackleg control. As such, the three-pronged approach has become two, with reliance on the efficacy of these fungicides to minimise blackleg disease. Until recently, all fungicides to control blackleg in Australia were demethylation inhibitors of the ergosterol synthesis pathway (DMI class), resulting in strong selection pressure for the evolution of resistance. Resistance to DMI fungicides in L. maculans has been identified in Australia, the first report worldwide (Van de Wouw et al. 2017). Resistance is conferred by multiple mechanisms, such as insertions of fragments of transposable elements into the promoter region of the Erg11 gene (also known as Cyp51 in other fungal species; Yang et al. 2020). Fungicide resistance has been detected in approximately 18% of L. maculans populations screened from stubble samples across Australia, but the proportion of resistant isolates within these populations and the on-farm implications of fungicide efficacy remains unknown (Van de Wouw et al. 2017; Yang et al. 2020).
More recently, antifungals in the succinate dehydrogenase inhibitor (SDHI) class became available for blackleg control, either as single modes or a mixture with DMI’s, for application to seed or in-crop during vegetative growth, with more effective control than DMI’s (Marcroft, unpublished data). The SDHI fungicides are at medium-high risk for the development of fungicide resistance as they act on a single enzyme site (www.frac.info accessed 21 May 2020). Reduced sensitivity to SDHI’s are reported in field populations of other fungi, such as Botrytis cinerea (e.g. Veloukas et al. 2011), Zymoseptoria tritici (e.g. Dooley et al. 2016) and Alternaria species (e.g. Avenot et al. 2009), with mutations providing cross-resistance to varying degrees between different SDHI’s. Current Australian stewardship guidelines for SDHI’s in canola recommend a limit of two applications during vegetative growth for blackleg and two applications during the flowering period for Sclerotinia stem rot (www.croplife.org.au, accessed 21 May 2020) despite the fact that L. maculans populations will be exposed to sprays applied during flowering.
With the heavy reliance on fungicides to maintain current canola intensity and the potential for up to five fungicide applications within a growing season, strategic use of fungicides to minimise the risk of fungicide resistance is essential. This might include avoiding an application at sowing if sown early and therefore likely to avoid spore release, or the rotation of fungicides with different actives or mixtures to reduce selection pressure. However, most commercially purchased seed in Australia is sold pre-treated with a fungicide, therefore growers may not have the choice to skip a seedling fungicide application.
Research priorities for long-term management of blackleg
From this review of the changes in farming practices, we have identified areas of research that are most likely required to support Australian canola production now and into the future to limit blackleg disease. These include:
-
(i)
Developing a framework to implement successful rotation of major resistance genes, to which breeders and industry can then respond with a suitable suite of cultivars and deployment strategies
While rotation of resistance genes has been shown to minimize blackleg disease (Marcroft et al. 2012; Bousset et al. 2018), there is limited information on appropriate implementation strategies to maximise the benefits for disease control and limit evolution of the pathogen. Recent studies have indicated that not all resistance genes can be rotated in the same sequence (Van de Wouw and Howlett 2020) and that stacking of resistance genes can undermine rotation (Bousset et al. 2018). The frequency of virulence alleles in a population needed to overcome resistance is unknown. Understanding these gaps in knowledge is essential to develop and implement effective and sustainable use of major gene resistance.
-
(ii)
Understanding the impact of earlier sowing times on exposure to spore showers and subsequent development of crown canker
As identified in the survey, there has been a shift to earlier sowing dates. Earlier sowing potentially allows seedlings to germinate and grow quickly through the yulnerable stage of development that leads to crown cankers (Marcroft et al. 2005). Therefore, earlier sowing may potentially reduce the need for fungicides at the seedling or 4–10 leaf stages. Further investigation is required to enable recommendations to industry on the use of fungicide application at sowing.
-
(iii)
Screening for genetic resistance (major gene and quantitative) to upper canopy infection
The prevalence of upper canopy infection is has increased rapidly due to changes in flowering times and potentially exacerbated by changes in stubble management. Preliminary data suggest that effective major gene resistance can control upper canopy infection (Sprague et al. 2018), whilst the contribution of quantitative resistance is unknown. The development of a phenotyping method to screen upper canopy infection is required to allow selection of resistant gemplasm.
-
(iv)
Assessment of novel strategies to reduce inoculum carryover on stubble in minimum tillage systems and under predicted increases in canola production and (v) identifying the epidemiology of stubble retention such as timing and quantity of ascospore release
Preliminary data suggest that the maintenance of stubble in the farming system has significantly changed the patterns of ascospore release, potentially leading to higher inoculum levels later in the growing season and in the following season. Further research is needed to determine the full extent of this change e.g. is ascospore release greater in the second year when standing stubble is knocked down, therefore potentially increasing disease risk? These potential changes in disease epidemiology are not specific to blackleg disease but are relevant to other stubble borne disease.
-
(v)
Monitoring and predicting the evolution of fungicide resistance
Increased fungicide use increases the risk of the evolution of fungicide resistance. Fungicide resistance has already been detected to the DMI fungicides (Van de Wouw and Howlett 2012; Yang et al. 2020), but the levels of this resistance in the field and the implication for their efficacy in the field is still being investigated. Monitoring for resistance towards the new fungicide classes as well as the development of fungicide resistant management strategies should be a priority as these fungicides gain momentum.
-
(vi)
Developing genetic tools for high throughput analysis of blackleg populations to improve understanding of evolutionary drivers for resistance genes and fungicides.
Analysis of genes involved in virulence and fungicide resistance has shown that the genes are found in specific AT-rich repetitive regions of the fungal genome (Rouxel et al. 2011). As a consequence of the gene location, these genes are readily mutated due to the movement of transposable elements. Understanding genetic structures and identification of these mobile genetic elements may be useful in understanding which populations are at risk of evolving virulence/fungicide resistance and allow the development of molecular markers for tracking such changes in population.
Conclusions
There has been a rapid shift in farm management practices in Australia as growers adapt to changes in climate and technology, availability of improved genetics, and advances in weed and pest control. The intensification of production, conservation tillage practices, and earlier sowing and flowering times have significant implications for the epidemiology and integrated management of blackleg disease (summarised in Fig. 1b).
Although this review is of the changing farming systems in Australia, the findings are of international significance given the global importance of blackleg and consumer expectation to improve sustainability of canola production, particularly in regard to chemical pest control. In this context, many of the identified research foci are of broader relevance to all canola-producing regions of the world, particularly the areas of resistance gene management and characterisation of pathogen populations.
References
Agresti A (2010) Analysis of ordinal categorical data, vol 656. Wiley, New York
Angus JF, Kirkegaard JA, Hunt JR, Ryan MH, Ohlander L, Peoples MB (2015) Break crops and rotations for wheat. Crop Pasture Sci 66:523–552
Avenot HF, Sellam A, Michailides TJ (2009) Characterisation of mutations in the membrane-anchored AaSDHC and AaSDHD of succinate dehydrogenase from Alternaria alternata isolates conferring field resistance to the fungicide boscalid. Plant Pathol 58:1134–1143
Balesdent MH, Barbetti MJ, Li H, Sivasithamparam K, Gout L, Rouxel T (2005) Analysis of Leptosphaeria maculans race structure in a worldwide collection of isolates. Phytopathology 95(9):1061–1071
Balesdent MH, Fudal I, Ollivier B, Bally P, Grandaubert J, Eber F, Chevre AM, Leflon M, Rouxel T (2013) The dispensable chromosome of Leptosphaeria maculans shelters an effector gene conferring avirulence towards Brassica rapa. New Phytol 198(3):887–898
Bell LW, Dove H, McDonald SE, Kirkegaard JA (2015) Integrating dual-purpose wheat and canola into high rainfall livestock systems in South-Eastern Australia. 3. An extrapolation to whole-farm grazing potential, productivity and profitability. Crop Pasture Sci 66(4):390–398
Bousset L, Sprague SJ, Thrall PH, Barrett LG (2018) Spatio-temporal connectivity and host resistance influence evolutionary and epidemiological dynamics of the canola pathogen Leptosphaeria maculans. Evol Appl 11:1354–1370
Brandt SA, Malhi SS, Ulrich D, Lafond GP, Kutcher AM (2007) Seedling rate, fertilizer level and disease management effects on hybrid versus open pollinated canola (Brassica napus L.). Can J Plant Sci 87:255–266
Brun H, Chevre AM, Fitt BD, Powers S, Besnard AL, Ermel M, Huteau V, Marquer B, Eber F, Renard M, Andrivon D (2010) Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytol 185(1):285–299
Christensen RHB (2019) Ordinal—regression models for ordinal data. vol 2019.12-10. R package
Cook R (2000) Advances in plant health management in the twentieth century. Annu Rev Phytopathol 38:95–116
Cowling WA (2007) Genetic diversity in Australian canola and implications for crop breeding for changing future environments. Field Crop Res 104:103–111
Delourme R, Pilet-Nayel ML, Archipiano M, Horvais R, Tanguy X, Rouxel T, Brun H, Renard M, Balesdent MH (2004) A cluster of major specific resistance genes to Leptosphaeria maculans in Brassica napus. Phytopathology 94:578–583
Delourme R, Chevre AM, Brun H, Rouxel T, Balesdent MH, Dias JS, Salisbury P, Renard M, Rimmer SR (2006) Major gene and polygenic resistance to Leptosphaeria maculans in oilseed rape (Brassica napus). Eur J Plant Pathol 114(1):41–52
Dooley H, Shaw MW, Mehenni-Ciz J, Spink J, Kildea S (2016) Detection of Zymoseptoria tritici SDHI-insensitive field isolates carrying the SdhC-H152R and SdhD-R47W substitutions. Pest Manag Sci 72:2203–2207
Elliott VL, Marcroft SJ, Howlett BJ, Van de Wouw AP (2016) Gene-for-gene resistance is expressed in cotyledons, leaves and pods in the Leptosphaeria maculans-Brassica napus pathosystem but not during stem colonisation. Plant Breed 135:200–207
Fischer T, Byerlee D, Edmeades G (2014) Crop yields and global food security: will yield increase continue to feed the world? Paper presented at the ACIAR Monographs, Canberra
Fitt BDL, Brun H, Barbetti MJ, Rimmer SR (2006) World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur J Plant Pathol 114(1):3–15
Flor HH (1955) Host-parasite interactions in flax rust - its genetic and other implications. Phytopathology 45(12):680–685
Fudal I, Ross S, Gout L, Blaise F, Kuhn ML, Eckert MR, Cattolico L, Bernard-Samain S, Balesdent MH, Rouxel T (2007) Heterochromatin-like regions as ecological niches for avirulence genes in the Leptosphaeria maculans genome: map-based cloning of AvrLm6. Mol Plant-Microbe Interact 20(4):459–470
Ghanbarnia K, Lydiate DJ, Rimmer SR, Li G, Kutcher R, Larkan NJ, McVetty PBE, Fernando DWG (2012) Genetic mapping of the Leptosphaeria maculans avirulence gene corresponding to the LepR1 resistance gene of Brassica napus. Theor Appl Genet 124:505–513
Ghanbarnia K, Fudal I, Larkan NJ, Links MG, Balesdent M, Profotova B, Fernando DWG, Rouxel T, Borhan H (2015) Rapid identification of the Leptosphaeria maculans avirulence gene AvrLm2 using an intraspecific comparative genomics approach. Mol Plant Pathol 16:699–709
Ghanbarnia K, Ma L, Larkan NJ, Haddadi P, Fernando DWG, Borhan MH (2018) Leptosphaeria maculans AvrLm9: a new player in the game of hide and seek with AvrLm4-7. Mol Plant Pathol 19(7):1754–1764
Gladders P, Evans N, Marcroft S, Pinochet X (2006) Dissemination of information about management strategies and changes in farming practices for the exploitation of resistance to Leptosphaeria maculans (phoma stem canker) in oilseed rape cultivars. Eur J Plant Pathol 114:117–126
Gossende S, Penaud A, Aubertot JN, Schneider O, Pinochet X (2003) Evolution of soil surface oilseed rape residues and their ability to produce spores of Leptosphaeria maculans: preliminary results. In: 11th International Rapeseed Congress, Copenhagen, Denmark
Gout L, Fudal I, Kuhn ML, Blaise F, Eckert M, Cattolico L, Balesdent MH, Rouxel T (2006) Lost in the middle of nowhere: the AvrLm1 avirulence gene of the Dothideomycete Leptosphaeria maculans. Mol Microbiol 60(1):67–80
Gunstone F (2004) Rapeseed and canola oil: production, processing, properties and use. Wiley-Blackwell, Oxford
Hammond KE, Lewis BG (1987) Differential responses of oilseed rape leaves to Leptosphaeria maculans. Trans Br Mycol Soc 88:329–333
Hammond KE, Lewis BG, Musa TM (1985) A systemic pathway in the infection of oilseed rape plants by Leptosphaeria maculans. Plant Pathol 34:557–565
Harker KN, O'Donovan JT, Turkington TK, Blackshaw RE, Lupwayi NZ, Smith EG, Johnson EN, Gan Y, Kutcher HR, Dosdall LM, Peng G (2015) Canola rotation frequency impacts canola yield and associated pest species. Can J Plant Sci 95:9–20
Hegewald H, Koblenz B, Wensch-Dorendorf M, Christen O (2017) Yield, yield formation, and blackleg disease of oilseed rape cultivated in high-intensity crop rotations. Arch Agron Soil Sci 63:1785–1799
Holzworth DP, Huth NI, Fainges J, Brown H, Zurcher E, Cichota R, Verrall S, Herrmann NI, Zheng B, Snow V (2018) APSIM next generation: overcoming challenges in modernising a farming systems model. Environ Model Softw 103:43–51
Huang Y, Paillard S, Kumar V, King G, Fitt B, Delourme R (2019) Oilseed rape (Brassica napus) resistance to growth of Leptosphaeria maculans in leaves of young plants contributes to quantitative resistance in stems of adult plants. PLoS One 14(9):e0222540
Jacobsen B (1997) Role of plant pathology in integrated pest management. Annu Rev Phytopathol 35:373–391
Khangura R, Barbetti M (2002) Efficacy of impact to manage blackleg (Leptosphaeria maculans) in canola. Aust J Agric Res 53:311–321
Khangura R, Barbetti M (2004) Time of sowing and fungicides affect blackleg (Leptosphaeria maculans) severity and yield in canola. Aust J Exp Agric 44:1205–1213
Kirkegaard JA, Sprague SJ, Hamblin P, Graham J, Lilley JM (2012) Refining crop and livestock management for dual-purpose canola (Brassica napus). Crop Pasture Sci 63:429–443
Kirkegaard JA, Lilley JM, Brill RD, Sprague SJ, Fettell NA, Pengilley GC (2016a) Re-evaluating sowing time of spring canola (Brassica napus L.) in South-Eastern Australia - how early is too early? Crop Pasture Sci 67:381–396
Kirkegaard JA, Lilley JM, Morrison MJ (2016b) Drivers of trends in Australian canola productivity andd future prospects. Crop Pasture Sci 67:i–ix
Kirkegaard JA, Lilley JM, Brill RD, Ware AH, Walela CK (2018) The critical period for yield and quality determination in canola (Brassica napus L.). Field Crop Res 222:180–188
Larkan NJ, Lydiate DJ, Parkin IA, Nelson MN, Epp DJ, Cowling WA, Rimmer SR, Borhan MH (2013) The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytol 197:595–605
Larkan NJ, Ma L, Borhan H (2015) The Brassica napus receptor-like protein RLM2 is encoded by a second allele of the LepR3/Rlm2 blackleg resistance locus. Plant Biotechnol J 13:983–992
Larkan NJ, Raman H, Lydiate DJ, Robinson SJ, Yu F, Barbulescu DM, Raman R, Luckett D, Burton W, Wratten N, Salisbury P, Rimmer R, Borhan MH (2016) Multi-environment QTL studies suggest a role for cysteine-rich protein kinase genes in quantitative resistance to blackleg disease in Brassica napus. BMC Plant Biol 16:183
Lilley JM, Bell LW, Kirkegaard JA (2015) Optimising grain yield and grazing potential of crops across Australia’s high-rainfall zone: a simulation analysis. 2. Canola. Crop Pasture Sci 66(4):349–364. https://doi.org/10.1071/CP14240
Lilley JM, Flohr BM, Whish JPM, Farre I, Kirkegaard JA (2019) Defining optimal sowing and flowering periods for canola in Australia. Field Crop Res 235:118–128
Marcroft SJ, Potter TD (2008) The fungicide fluquinconazole applied as a seed dressing to canola reduces Leptosphaeria maculans (blackleg) severity in South-Eastern Australia. Australas Plant Pathol 37:396–401
Marcroft SM, Sprague SJ, Pymer SJ, Salisbury P, Howlett BJ (2003) Factors affecting the production of inoculum of the blackleg fungus (Leptosphaeria maculans) in South-Eastern Australia. Aust J Exp Agric 43:1231–1236
Marcroft SJ, Sprague SJ, Pymer SJ, Salisbury PA, Howlett BJ (2004) Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in South-Eastern Australia. Aust J Exp Agric 44:601–606
Marcroft SJ, Sosnowski MR, Scott ES, Ramsey MD, Salisbury PA, Howlett BJ (2005) Brassica napus plants infected by Leptosphaeria maculans after the third to fifth leaf growth stage in South-Eastern Australia do not develop blackleg stem canker. Eur J Plant Pathol 112:289–292
Marcroft SJ, Van de Wouw AP, Salisbury PA, Potter TD, Howlett BJ (2012) Rotation of canola (Brassica napus) cultivars with different complements of blackleg resistance genes decreases disease severity. Plant Pathol 61:934–944
McCredden J, Cowley RB, Marcroft SJ, Van de Wouw AP (2018) Changes in farming practices impact on spore release patterns of the blackleg pathogen, Leptosphaeria maculans. Crop Pasture Sci 69:1–8
McGee C, Emmett RW (1977) Blackleg (Leptosphaeria maculans (Desm.) Ces. Et de not.) of rapeseed in Victoria: crop losses and factors which affect disease severity. Aust J Agric Res 28:47–51
Parlange F, Daverdin G, Fudal I, Kuhn ML, Balesdent MH, Blaise F, Grezes-Besset B, Rouxel T (2009) Leptosphaeria maculans avirulence gene AvrLm4-7 confers a dual recognition specificity by the Rlm4 and Rlm7 resistance genes of oilseed rape, and circumvents Rlm4-mediated recognition through a single amino acid change. Mol Microbiol 71(4):851–863
Peng G, Liu X, McLaren DL, McGregor L, Fengqun Y (2020) Seed treatment with the fungicide fluopyram limits cotyledon infection by Leptosphaeria maculans and reduces blackleg of canola. Can J Plant Pathol (in press)
Petit-Houdent Y, Degrave A, Meyer M, Blaise F, Ollivier B, Marais CL, Jauneau A, Audran C, Rivas S, Veneault-Fourrey C, Brun H, Rouxel T, Fudal I, Balesdent MH (2019) A two genes-for-one gene interaction between Leptosphearia maculans and Brassica napus. New Phytol 223:397–411. https://doi.org/10.1111/nph.15762
Plissonneau C, Daverdin G, Ollivier B, Blaise F, Degrave A, Fudal I, Rouxel T, Balesdent M (2016) A game of hide and seek between avirulence genes AvrLm4-7 and AvrLm3 in Leptosphaeria maculans. New Phytol 209(4):1613–1624
Plissonneau C, Rouxel T, Chevre AM, Van de Wouw AP, Balesdent MH (2017) One gene-one name: the AvrLmJ1 avirulence gene of Leptosphaeria maculans is AvrLm5. Mol Plant Pathol 19(4):1012–1016
Raman R, Taylor B, Marcroft S, Stiller J, Eckermann P, Coombes N, Rehman A, Lindbeck K, Luckett D, Wratten N, Batley J, Edwards D, Wang X, Raman H (2012) Molecular mapping of qualitative and quantitative loci for resistance to Leptosphaeria maculans causing blackleg disease in canola (Brassica napus L.). Theor Appl Genet 125(2):405–418
Raman H, Raman R, Coombes N, Song J, Diffey S, Klilian A, Lindbeck K, Barbulescu DM, Batley J, Edwards D, Salisbury PA, Marcroft SJ (2016) Genome-wide association study identified new loci for resistance to Leptosphaeria maculans in canola. Front Plant Sci 7:1513
Raman H, Raman R, Diffey S, Qiu Y, McVittie B, Barbulescu DM, Salisbury P, Marcroft S, Delourme R (2018) Stable quantitative resistance loci to blackleg disease in canola (Brassica napus L.) over continents. Frontiers in. Plant Sci 9:1622
Raman R, Diffey S, Barbulescu DM, Coombes N, Luckett D, Salisbury P, Cowley RB, Marcroft S, Raman H (2020) Genetic and physical mapping of loci for resistance to blackleg disease in canola (Brassica napus L.). Sci Rep 10:4416
Rouxel T, Penaud A, Pinochet X, Brun H, Gout L, Delourme R, Schmit J, Balesdent MH (2003) A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape. Eur J Plant Pathol 109(8):871–881
Rouxel T, Grandaubert J, Hane JK, Hoede C, van de Wouw AP, Couloux A, Dominguez V, Anthouard V, Bally P, Bourras S, Cozijnsen AJ, Ciuffetti LM, Degrave A, Dilmaghani A, Duret L, Fudal I, Goodwin SB, Gout L, Glaser N, Linglin J, Kema GH, Lapalu N, Lawrence CB, May K, Meyer M, Ollivier B, Poulain J, Schoch CL, Simon A, Spatafora JW, Stachowiak A, Turgeon BG, Tyler BM, Vincent D, Weissenbach J, Amselem J, Quesneville H, Oliver RP, Wincker P, Balesdent MH, Howlett BJ (2011) Effector diversification within compartments of the Leptosphaeria maculans genome affected by repeat-induced point mutations. Nat Commun 2:n202
Salisbury P, Ballinger DJ, Wratten N, Plummer KM, Howlett BJ (1995) Blackleg disease on oilseed Brassicas in Australia - a review. Aust J Exp Agric 35:665–674
Sprague SJ, Balesdent MH, Brun H, Hayden HL, Marcroft SJ, Pinochet X, Rouxel T, Howlett BJ (2006a) Major gene resistance in Brassica napus (oilseed rape) is overcome by changes in virulence of populations of Leptosphaeria maculans in France and Australia. Eur J Plant Pathol 114:33–44
Sprague SJ, Marcroft SJ, Hayden HL, Howlett BJ (2006b) Major gene resistance to blackleg in Brassica napus overcome within three years of commercial production in southeastern Australia. Plant Dis 90(2):190–198
Sprague SJ, Kirkegaard JA, Graham J, Bell L, Seymour M, Ryan M (2015) Forage and grain yield of diverse canola maturity types (Brassica napus) in the high rainfall zone of Australia. Crop Pasture Sci 66:260–274
Sprague SJ, Graham J, Brill R, McMaster C (2017) Early-flowering canola - what is the blackleg risk? Paper presented at the 18th Australian agronomy conference, Ballarat, Victoria, Australia
Sprague SJ, Marcroft SJ, Lindbeck KD, Ware AH, Khangura RK, Van de Wouw AP (2018) Detection, prevalence and severity of upper canopy infection on mature Brassica napus plants caused by Leptosphaeria maculans. Crop Pasture Sci 69:65–78
Stuthmann DD, Leonard JJ, Miller-Garvin J (2007) Breeding crops for durable resistance to disease. Adv Agron 95:319–367
Team RC (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Umbers A (2016) GRDC farm practices survey report 2016. Grains Research and Development Corporation, Kingston
Van de Wouw AP, Howlett BJ (2012) Estimating frequencies of virulent isolates in field populations of a plant pathogenic fungus, Leptosphaeria maculans, using high-throughput pyrosequencing. J Appl Microbiol 113:1145–1153
Van de Wouw AP, Howlett BJ (2020) Advances in understanding the Leptosphaeria maculans - Brassica pathosystem and their impact on disease management. Can J Plant Pathol 42(2):149–163
Van de Wouw AP, Marcroft SJ, Barbetti MJ, Hua L, Salisbury PA, Gout L, Rouxel T, Howlett BJ, Balesdent MH (2009) Dual control of avirulence in Leptosphaeria maculans towards a Brassica napus cultivar with 'sylvestris-derived' resistance suggests involvement of two resistance genes. Plant Pathol 58(2):305–313
Van de Wouw AP, Cozijnsen AJ, Hane JK, Brunner PC, McDonald BA, Oliver RP, Howlett BJ (2010) Evolution of linked avirulence effectors in Leptosphaeria maculans is affected by genomic environment and exposure to resistance genes in host plants. PLoS Pathog 6(11):e1001180
Van de Wouw AP, Lowe RGT, Elliott CE, Dubois DJ, Howlett BJ (2014) An avirulence gene, AvrLmJ1, from the blackleg fungus, Leptosphaeria maculans, confers avirulence to Brassica juncea cultivars. Mol Plant Pathol 15(5):523–530
Van de Wouw AP, Marcroft SJ, Howlett BJ (2016) Blackleg disease of canola in Australia. Crop Pasture Sci 67:273–282
Van de Wouw AP, Elliott VL, Chang S, López-Ruiz F, Marcroft SJ, Idnurm A (2017) Identification of isolates of the plant pathogen Leptosphaeria maculans with resistance to the triazole fungicide fluquinconazole using a novel in planta assay. PLoS One 12:e0188106
Veloukas T, Leroch M, Hahn M, Karaoglanidis GS (2011) Detection and molecular characterisation of boscalid-resistant Botrytis cinerea isolates from strawberry. Plant Dis 95:1302–1307
Verdon-Kidd DC, Kiem AS, Moran R (2014) Links between the big dry in Australia and hemishperic multi-decadal climate variability - implications for water resource management. Hydrol Earth Syst Sci 18:2235–2256
West JS, Kharbanda PD, Barbetti M, Fitt BDL (2001) Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol 50:10–27
Yang Y, Marcroft SJ, Forsyth LM, Zhao J, Ziqin L, Van de Wouw AP, Idnurm A (2020) Sterol demethylation inhibitor fungicide resistance in Leptosphaeria maculans is caused by modifications in the regulatory region of ERG11. Plant Dis 104:1280–1290
Zhang X, Fernando DWG (2018) Insights into fighting against blackleg disease of Brassica napus in Canada. Crop Pasture Sci 69(1):40–47
Zhang X, White RP, Demir E, Jedryczka M, Lange RM, Islam M, Li ZQ, Huang YJ, Hall AM, Zhou G, Wang Z, Cai X, Skelsey P, Fitt BDL (2014) Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathol 63:598–612
Zhang H, Berger JD, Seymour M, Brill R, Herrmann C, Quinlan R, Knell G (2016a) Relative yield and profit of Australian hybrid compared with open-pollinated canola is largely determined by growing-season rainfall. Crop Pasture Sci 67(4):323–331
Zhang X, Peng G, Kutcher HR, Balesdent MH, Delourme R, Fernando DWG (2016b) Breakdown of Rlm3 resistance in the Brassica napus-Leptosphaeria maculans pathosystem in western Canada. Eur J Plant Pathol 145:659–674
Acknowledgements
We thank the participants of the survey and those that promoted participation, and John Kirkegaard for comments on the manuscript. This work was supported by the Australian Grains Research and Development Corporation (project UM00051) and Australian Research Council (LP170100548). We thank Andrew Ware, Kurt Lindbeck, Ravjit Khangura, Andrew Wherrett, Andrea Hills, Jenny Davidson and Vicki Elliott for help with collection of disease severity data between 2004 and 2020.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
ESM 1
(PDF 1.20 MB)
Rights and permissions
About this article
Cite this article
Van de Wouw, A.P., Marcroft, S.J., Sprague, S.J. et al. Epidemiology and management of blackleg of canola in response to changing farming practices in Australia. Australasian Plant Pathol. 50, 137–149 (2021). https://doi.org/10.1007/s13313-020-00767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-020-00767-9