Abstract
Poplar fungi canker is a disease that constitutes a serious threat to the poplar plant, a model tree in forest research. The control of this disease by use of chemical fumigants is associated with unwanted side effects such as environmental pollution and drug resistance. However, poplar canker can be effectively controlled with fengycin, an environmentally friendly bio-surfactant of microbial origin. Fengycin homologues are a series of lipopeptides with variations in both the length and branching of the β-hydroxy fatty acid. This study was aimed at purifying and characterizing fengycin homologues produced by Bacillus subtilis from poplar wood bark. A total of 13 fengycin homologues were obtained through 70% ammonium sulfate precipitation, methanol extraction, ion exchange chromatography, gel filtration chromatography, and semi-preparative high-performance liquid chromatography (HPLC). Electro-spray ionization mass spectrometry (ESI-MS) and electro-spray ionization tandem mass spectrometry (ESI-MS/MS) were employed to elucidate the chemical structures of these compounds, and the structures were further characterized by amino acid analysis. Thirteen (13) of the active compounds were fengycin A or B homologues. All the 13 homologues exhibited antifungal activity against the indicator strain Botryosphaeria dothidea. This finding is considered significant, in that it is the first report on the production of so many fengycin homologues and isomers by Bacillus subtilis. Thus, B. subtilis from poplar wood bark is a rich source of environment-friendly, anti-microbial agents for control of poplar canker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poplar is a model tree in forest research (Bradshaw et al. 2000). However, the development of poplar plantation in China is hampered by poplar canker, a disease that causes local necrosis in poplar branches. Poplar canker comprises two types: poplar bacteria canker and poplar fungi canker (Ma et al. 2015). Poplar fungi canker is caused by some pathogenic fungi, such as Botryosphaeria dothidea (Jiang et al. 2015), and Cytospora chrysosperma (Jia et al. 2010). Although chemical treatments are useful in protecting poplar plants from the disease, overuse of chemical fungicides can cause environmental pollution and increased drug resistance. One promising and environmentally-friendly alternative method involves biological control, which uses microorganisms to kill or inhibit pathogenic organisms (Cazorla and Mercado-Blanco 2016).
Bio-surfactants are microbial metabolites with good emulsifying and surface activities (Coronel-León et al. 2015). Lipopeptides are the most important bio-surfactants in environmental applications (Ghojavand et al. 2011). Lipopeptides include iturin, surfactin and fengycin (Rautela et al. 2014; Ma et al. 2015). Antimicrobial lipopeptides and their homologues or isomers are a group of active peptides with similar structures or identical molecular weight (Pathak et al. 2014). Fengycin, one of the most important and promising lipopeptides, is produced by Bacillus species (Ongena et al. 2005). Fengycin is a promising antifungal agent against pathogenic fungi (Wang et al. 2004). It exists as a mixture of homologues or isoforms which differ in fatty acid (FA) chain lengths, and in the amino acid composition of the peptide ring (Vanittanakom et al. 1986). Fengycin homologues include fengycin A and fengycin B, which are characterized by the presence of Ala and Val residues, respectively, at position 6 of the peptide ring (Wang et al. 2004). Fengycin homologues possess strong antimicrobial properties, especially against filamentous pathogenic fungi (Williams et al. 2002).
In the present study, Botryosphaeria dothidea was used as the target fungus for evaluation of the bio-control potential of the antagonistic bacterium Bacillus subtilis.
Materials and methods
Microorganisms and culture conditions
Bacillus subtilis strain N6–34 with strong antifungal activity was isolated from healthy poplar bark in Ning Yang Gao-qiao logging station at Shandong Taian, China. The indicator strain Botryosphaeria dothidea was stored in the College of Forestry, Shandong Agricultural University. The isolated Bacillus subtilis strain N6–34 and the indicator strain were cultured separately on potato dextrose agar (PDA) plates at 30 °C and sub-cultured every 2 months.
Identification and phylogenetic analysis of the N6–34 strain
Morphological, physiological and biochemical characteristics were determined according to Bergey’s Manual of Determinative Bacteriology (9th Edition). The 16S rDNA gene was amplified by polymerase chain reaction (PCR) using the primer pair 5’-GCGCGTATGCAATACGAGCGGCA-3′ and 5’-GCGTCGCGACTTCGCAACCCA T-3′, and the amplified PCR product was sequenced as previously described (Jagoueix et al. 1994). The 16S rDNA gene sequences of some closely related strains were selected from the GenBank by the BLAST searching program, and a neighbor-joining phylogenetic tree was constructed using the software MEGA (version 6.0). The BLAST was performed using 16S rDNA database (https://www.ncbi.nlm.nih.gov/).
Extraction of crude antifungal substances from strain N6–34
A single colony of the Bacillus subtilis strain N6–34 was inoculated into a 250 mL Erlenmeyer flask containing 50-mL LB broth medium (5 g/L yeast extract, 10 g/L tryptone and 5 g/L NaCl); and cultured at 30 °C for 18 h in an agitator at 200 rpm. One milliliter of the 18 h-old seed liquid (2% inoculation volume) was inoculated into a 250-mL Erlenmeyer flask containing 50 mL fermentation medium [20.0 g glucose, 10.0 g corn flour, 20.0 g soybean powder, 0.5 g (NH4)2SO4, 1.5 g KH2PO4, 1.0 g CaCO3, and 1 L distilled water]. After culturing for 72 h, the fermentation broth was centrifuged at 6000×g for 20 min to remove the bacterial cells. The cell-free supernatant was subjected to 70% ammonium sulfate precipitation overnight at 4 °C, and the pellet recovered by centrifugation was freeze-dried. The dried crude antifungal pellet was extracted twice with anhydrous methanol for 30 min. The combined extract was concentrated using a rotary evaporator at 45 °C and dissolved in 70% methanol.
HPLC analysis of extract
Fractions with antifungal activity were obtained using a semi-preparative high-performance liquid chromatography (HPLC) procedure. After removing the acetonitrile in a rotary evaporator, the collected fractions were freeze-dried and subjected to Shandong Qibiao Testing to determine the amino acid composition (Yang et al. 2015). To obtain active substances at concentrations above 95%, only the peak points of the HPLC chromatogram were collected.
Isolation and purification of antifungal substances
The extracted crude antifungal substances were further purified using ion-exchange chromatography on a DEAE Sepharose Fast Flow column (4.0 × 30.0 cm) at a monitoring wavelength of 220 nm. The column was eluted with a linear NaCl concentration gradient from 0 to 0.5 M at a flow rate of 2 mL/min. Peaks with antifungal activity were collected and concentrated by gel filtration in a Sephadex G25 column (2.0 × 80.0 cm) eluted with distilled water at a flow rate of 1 mL/min. The peaks with antifungal activity were concentrated and purified by semi-preparative HPLC with a C18 column (Ultimate XB-C18, 21.2 × 250 mm, 5 μm, Welch) on Waters 2535 at a monitoring wavelength of 220 nm. The mobile phases were ultra-pure water containing 0.1% (v/v) trifluoroacetic acid and acetonitrile. The purification was carried out with a gradient elution at a flow rate of 5 mL/min, at a column temperature of 25 °C. The separated major peaks were collected manually and subsequently tested for antifungal activity against Botryosphaeria dothidea.
The antifungal activity test of the isolated compounds
Spore suspensions were harvested from 5 to 7-d old Botryosphaeria dothidea cultures with sterile deionized water containing 0.1% Tween 80. The spore suspensions were passed through 4 layers of sterile gauze to remove hyphal fragments and the number of spores was counted with a hemocytometer. The concentration of the spore suspensions was adjusted to 106 spores ml−1 with sterile deionized water and stored at 4 °C for the next step. A 200 μl of the above spore suspensions was spread on the surface of a PDA plate (9 cm diameter) with a sterile glass spreader. Wells (9 mm diameter) were formed in the PDA plates using a sterile cork borer, and the isolated fractions (100 μl) collected from semi-preparative HPLC were then added to the wells. The plates were incubated at 30 °C for 18 h, and the inhibition zones of the treated areas were measured by the cross method. The compounds with high antifungal activities (compounds 5, 6, 7, 8 and 9) were selected for use in synergistic effect tests of mixtures of compounds. All the compounds were mixed in equal ratio, and all samples were tested in triplicate.
Mass spectrometry
The HPLC fractions with antifungal activity were concentrated for ESI-MS and ESI-MS/MS analyses. The purity of the eluted fractions was determined by analytical HPLC (Agilent 1100, Agilent Technologies, USA). Concentrated fractions with purity higher than 95% were directed to the ion trap mass spectrometer through ESI sprayer (LTQ Orbitrap Elite, Thermo Fisher, USA). The MS spectra were recorded on positive ion mode within the mass range of 200–2000 m/z, and the molecular ions detected were further characterized by ESI–MS/MS using the same instrument. The conditions for ESI-MS/MS were as follows: ion source injection voltage at 35 kV, capillary temperature at 275 °C, helium as collision gas, high purity nitrogen gas as atomizing and drying gas at a flow rate of 35 arbitrary units, and detection method set as positive ion mode. The ESI–MS/MS data were analyzed using Data Analysis software v4.0.
Results
Identification of bacterial strain N6–34
The results showed that strain N6–34 cells were rod-shaped and non-motile. The cells ranged from 0.4 to 0.6 μm in width, and 1.5 to 2.0 μm in length. Details of their physiological and biochemical properties are summarized in Table 1.
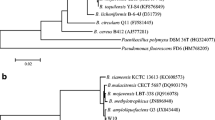
The phylogenetic tree of N6–34 constructed using 16S rDNA gene sequences (1478 base pairs) obtained by PCR is shown in Fig. 1. Strain N6–34 was closely related to Bacillus subtilis (GenBank accession number KM492822) with 99% homology. Based on these results, it was identified as Bacillus subtilis.
Antifungal substances from strain N6–34
Using semi-preparative HPLC, the crude antifungal substances were separated into 14 fractions as shown in Fig. 2. Each HPLC fraction was assayed for antifungal activity, and the results are shown in Fig. 3. All 14 fractions exhibited antifungal activity against Botryosphaeria dothidea except fraction 1 which showed weak antifungal activity.
Results from ESI-MS and ESI-MS/MS analysis
The mass spectra of HPLC fractions 2–14 showed a set of doubly-protonated molecular ions ([M + 2H]2+) of 732.42, 746.43, 746.44,753.44, 724.42, 738.44, 738.44, 739.42, 753.40, 746,42, 739.41, 745.45, and 745.45 m/z, respectively. The estimated molecular weight of fractions 2–14 were 1463, 1491, 1491, 1505, 1447, 1475, 1475, 1477, 1505, 1491, 1477, 1489, and 1489 Da, respectively. The doubly-protonated molecular ion peaks of all the active fractions were selected as precursor ions for further ESI-MS/MS analysis. The results showed that product ions of 966 and 1080 m/z appeared in the MS/MS spectra of precursor ions of 732.42, 724.42, 738.44, 738.44, 739.42, and 739.41 m/z (corresponding to fractions 2, 6, 7, 8, 9, and 12, respectively). The product ions of 994 and 1108 m/z appeared in the MS/MS spectra of precursor ions of 746.43, 746.44, 753.44, 753.40, 746.42, 745.45, and 745.45 m/z (corresponding to fractions 3, 4, 5, 10, 11, 13, and 14, respectively). Since peaks at 1080 and 966 m/z clearly indicate fengycin A, and peaks at 1108 and 994 m/z are indicative of fengycin B (Wang et al. 2007), fractions 2, 6, 7, 8, 9, and 12 were identified as fengycin A, while fractions 3, 4, 5, 10, 11, 13, and 14 were identified as fengycin B (partial data and structure of fengycin A and B are shown in Figs. 4 and 5, respectively).
The antifungal substances separated in fractions 2 to 14 were tentatively identified based on their product ions and estimated molecular weights (Table 2).
These substances consisted of two groups: the first group with molecular weights of 1463, 1477, 1491, and 1505 Da, differing by 14 Da (i.e. one –CH2– group); and the second group with molecular weights of 1447, 1475, and 1489 Da, differing by 14 or 28 Da (i.e. two –CH2– groups). The difference in molecular weight between similar members of each group (1477 and 1475 Da; 1491 and 1489 Da) is 2 Da, indicating that the first group has a saturated FA chain, while the second group has an unsaturated FA. Some fractions with different retention times in the HPLC chromatogram had the same precursor and product ions in ESI-MS/MS i.e. fractions 3, 4, and 11; fractions 7 and 8; fractions 9 and 12; and fractions 13 and 14. These results suggest the presence of isomeric substances with identical molecular weights but different side chain configurations.
Amino acid composition of isolated antifungal substances
The purity of the active substances in freeze-dried preparative HPLC fractions 5 and 6 was above 95% as determined by analytical HPLC. Results of amino acid analysis showed that fraction 5 contained Glu (Gln), Orn, Tyr, Thr, Val, Pro, and Ile in a molar ratio of about 3:1:2:1:1:1:1 (Fig. 6); and fraction 6 contained Glu (Gln), Orn, Tyr, Thr, Ala, Pro, and Ile (Fig. 7) in a molar ratio of about 3:1:2:1:1:1:1. Both fractions differed only in the substitution of Ala for Val.
Chemical structures of the antifungal substances
Fractions 5 and 6 were selected for elucidation of chemical structure using their corresponding ESI-MS/MS spectra (structures of the remaining fractions could be elucidated by the same method). Fraction 5 was fragmented into small ions by ESI-MS/MS. The ESI-MS/MS spectrum of the doubly-charged precursor ion at 753.44 m/z of fraction 5 is shown in Fig. 6. The b-type ions of fraction 5 were b1 (398.30 m/z), b2 (512.38 m/z), b3 (675.45 m/z), b4 (776.56 m/z), b5 (905.6 m/z), b6 (1004.67 m/z), b8 (1229.74 m/z), b9 (1392.80 m/z), and b10 (1505.81 m/z). Two y-type ions were y8 (994.51 m/z) and y9 (1108.59 m/z). The m/z difference (99.07) between b5 (905.6 m/z) and b6 (1004.67 m/z) corresponded to the molecular weight of Val, and the m/z difference (225.07) between b8 (1229.74 m/z) and b6 (1004.67 m/z) corresponded to the molecular weight of the dipeptide Pro-Gln. The m/z difference (113.01) between b9 (1392.80 m/z) and b10 (1505.81 m/z) corresponds to the molecular weight of Leu or Ile, which have identical molecular weights. The b-type ions at 398.30 m/z corresponded to FA-Glu, which suggests that the fatty acyl moiety has the elemental composition of C17H33O2. The set of product ions (1392.80, 1229.74, 1004,67, 905.6, 776.56, 675.45, 512.38, and 398.30) fragmented by the doubly charged precursor ion of 753.44 m/z suggest sequential loss of Ile/Leu, Tyr, Gln-Pro, Val, Glu, Thr, Tyr, and Orn from C-terminal of the parent ion. The other set of product b-type ions (1108.59 and 994.51) could be due to neutral losses of FA-Glu and FA-Glu-Orn from the N-terminal, respectively. The amino acid composition analysis of fractions 5 and 6 revealed that there was no Leu in these two compounds, indicating that the amino acid residue at position 10 was Ile in the compound in fraction 5. Based on the above results, the complete sequence of the compound in fraction 5 was deduced as FA-Glu-Orn-Tyr-Thr-Glu-Val-Pro-Gln-Tyr-Ile, and fraction 5 was identified as C17 fengycin B (Pathak et al. 2014).
The ESI-MS/MS spectrum and amino acid sequence deduction of fraction 6 are shown in Fig. 7. The b-type ions of fraction 6 were b1 (368.29 m/z), b2 (482.37 m/z), b3 (645.43 m/z), b4 (746.48 m/z), b5 (875.55 m/z), b6 (946.15 m/z), b8 (1171.72 m/z), and b9 (1334.75 m/z). The two y-type ions were y8 (966.48 m/z) and y9 (1080.56 m/z). The m/z difference (70.6) between b5 (875.55 m/z) and b6 (946.15 m/z) represents the molecular weight of Ala, and the m/z difference (225.07) between b6 (946.15 m/z) and b8 (1171.72 m/z) represents the molecular weight of the dipeptide Pro-Gln. The m/z difference (114.08) between y8 (966.48 m/z) and y9 (1080.56 m/z) represents the molecular weight of Orn. The b-type ions at 368.29 m/z correspond to FA-Glu, and suggest that the fatty acyl moiety has the elemental composition of C15H27O2. Based on these results, the complete amino acid sequence of the compound in fraction 6 was deduced as FA-Glu-Orn-Tyr-Thr-Glu-Ala-Pro-Gln-Tyr-Ile. Thus fraction 6 was identified as C15 fengycin A (containing one double bond in the FA chain). By the same method, the compounds in all the active fractions separated using HPLC were identified as either fengycin A or fengycin B homologues or isomers with C15 to C17.
The results of the synergistic effect on antifungal activity of the compounds are shown in Table 3. It can be seen that the antifungal activity obtained for a mixture of two or more compounds was always much higher than the antifungal activity obtained when a compound was tested alone.
Discussion
In this study, a new endophytic bacterial strain, N6–34 was isolated from healthy poplar bark and shown to produce fengycins. Strain N6–34 was identified as Bacillus subtilis by phenotypic and genetic analyses. Bacillus subtilis is a species capable of producing antifungal substances such as surfactin (Meng et al. 2016; Khyati et al. 2014), and iturin (Ayed et al. 2014). There are many previous reports on production of antifungal lipopeptide fengycins by Bacillus subtilis, but the number of fengycin homologues isolated was not as many as that reported in the present study. These include four fengycin homologues isolated by Chan et al. (2009), as well as six fengycin A homologues from C14 to C18, and four fengycin B homologues from C14 to C17 reported by Bie et al. (2009). However, the Bacillus subtilis strain N6–34 isolated in the present study produced 13 fengycin antifungal compounds in two different isomer categories. To the best of our knowledge, this is the first report of so many fengycin homologues and isomers produced by Bacillus subtilis. Compounds in the fengycin family, such as fengycin A and B, are lipopeptides with strong antifungal activities against some pathogenic fungi. These two fengycins differ only in the amino acid residue at position 6 of the peptide ring. Some new fengycins, such as fengycin C and fengycin S, were characterized recently (Lee et al. 2010; Villegas-Escobar et al. 2013). It has been reported that five fengycin A homologues from C14 to C18 and one fengycin B are produced by Bacillus subtilis strain M4 (Ongena et al. 2005). Interestingly, the authors identified the compound with molecular weight of 1491 Da as C18 fengycin A, whereas in the present study, the compound with molecular weight of 1491 Da was identified as C16 fengycin B. These different compounds with identical molecular weights are fengycin isomers with differences in side chain configuration. Considering that the molecular weight difference (28 Da) between Val and Ala corresponds to the molecular weight of two -CH2- groups, the two compounds with identical molecular weights may be correctly identified as belonging to different fengycin family groups. That is to say, the molecular weight of fengycin A homologues may be identical to that of some fengycin B homologues. In the present study, the compound with molecular weight of 1491 Da was clearly identified as C16 fengycin B by ESI-MS/MS combined with amino acid composition analysis.
ESI-MS/MS is a powerful tool for elucidating the chemical structures of peptides, but it fails to distinguish between Leu and Ile because of their identical molecular weights. Amino acid analysis is a convenient and simple method for confirming the amino acid residues and their molar ratios in peptides, and also for distinguishing between isomeric amino acids. Since the samples were hydrolysed with HCl prior to amino acid analysis, conversion of Gln to Glu was likely. Thus, this method cannot distinguish between these two amino acids. However, when amino acid analysis is combined with ESI-MS/MS, it can unambiguously determine the amino acid composition of a peptide. In this study, almost all the fengycins (including the isomers) were well separated. Neither ESI-MS nor ESI-MS/MS can determine the side chain configuration of different isomers, which may be elucidated by other methods such as nuclear magnetic resonance (NMR). However, NMR requires larger sample amounts which, when obtained, will allow testing of every fraction in the present work to identify the spatial structure of the side chain configurations.
In this study, 13 antifungal compounds were isolated at different concentrations. The concentrations of compounds 5, 7 and 10 were much higher than those of the other compounds. However, the antifungal activities of these compounds did not depend on their degree of abundance. Indeed, from results shown in Fig. 3, the antifungal activities were much higher in compounds 5, 6, 7, 8 and 9 than in the other compounds. A close examination of the results in Figs. 2 and 3 reveals that the compounds with longer FA chain length (compounds 5 and 9) and double bonds (compounds 6, 7 and 8) produced higher antifungal activities than compounds without these features. Although compounds 5 and 10 are isomers with the same molecular weight, the two compounds had different antifungal activities, due most probably to their differences in side chain configuration. A similar explanation is applicable to compounds 9 and 12.
Conclusion
An endophytic bacterium, designated as strain N6–34, was isolated from poplar wood bark and identified as Bacillus subtilis by phylogenetic and physiological characterization. The isolate was active against some pathogenic fungi. After purification using 70% ammonium sulfate precipitation, methanol extraction, anion exchange chromatography, gel filtration chromatography, and HPLC, 13 antifungal fractions were almost completely separated. The chemical structures of two representative antifungal substances from the isolate were elucidated by amino acid analysis, ESI-MS, and ESI-MS/MS. The antifungal substances consisted of two groups: one (6 fractions) corresponded to fengycin A homologues, while the other (7 fractions) corresponded to fengycin B homologues. The methodology used in this study cannot lead to the determination of the L- and D-isomeric forms. However, this problem can be resolved by the use of some other methodologies, such as capillary electrophoresis and HPLC with chiral columns.
References
Bie XM, Lu XU, Lu FX (2009) Identification of fengycin homologues from Bacillus subtilis with ESI MS/CID. J Microbiol Methods 79:272–278
Bradshaw H, Ceulemans R, Davis J, Stettler R (2000) Emerging model system in plant biology: poplar (Populus) as a model forest tree. J Plant Growth Regul 19:306–313
Cazorla FM, Mercado-Blanco J (2016) Biological control of tree and woody plant diseases: an impossible task? J BioControl 61:233–242
Chan YK, Savard ME, Reid LM (2009) Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat. BioControl 54:567–574
Coronel-León J, de Grau G, Grau-Campistany A, Farfan M, Rabanal F, Manresa A, Marqués AM (2015) Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: production, chemical characterization and properties. Ann Microbiol 65:2065–2078
Ding F, Deng XX, Hong N, Zhong Y, Wang GP, Yi GJ (2009) Phylogenetic analysis of the citrus Huanglongbing (HLB) bacterium based on the sequences of 16S rDNA and 16S/23S rDNA intergenic regions among isolates in China. Eur J Plant Pathol 124:495–503
Ghojavand H, Vahabzadeh F, Azizmohseni F (2011) A halotolerant, Thermotolerant, and facultative biosurfactant producer: identification and molecular characterization of a bacterium and evolution of emulsifier stability of a Lipopeptide biosurfactant. Biotechnol Bioproc E 16:72–80
Jagoueix S, Bove JM, Garnier M (1994) The phloem-limited bacterium of greening disease of citrus Is a member of the a subdivision of the proteobacteria. international journal of systematic bacteriology 44 (3):379–386
Jia ZC, Sun YM, Yuan L, Tian QY, Luo KM (2010) The chitinase gene (Bbchit1) from Beauveria bassiana enhances resistance to Cytospora chrysosperma in Populus tomentosa Carr. Biotechnol Lett 32:1325–1133
Jiang HX, Wang XH, Xiao CZ, Wang WY, Zhao X, Sui JK, Sa RB, Guo TL, Liu XL (2015) Antifungal activity of Brevibacillus laterosporus JX-5 and characterization of its antifungal components. World J Microbiol Biotechnol 31:1605–1618
Khyati V, Pathak, Hareshkumar K (2014) Application of extracellular lipopiptide biosurfactant produced by endophytic Bacillus subtilis K1, isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR). 3 Biotech 4:41–48
Lee SC, Kim SH, Park IH, Chung SY, Chandra MS, Choi YL (2010) Isolation, purification, and characterization of novel Fengycin S from Bacillus amyloliquefaciens LSC04 degrading-crude oil. Biotechnol Bioproc E 15:246–253
Ma Z, Zhu JL, Sun ZQ, Liang J, Zhang ZX, Zhang LM, Sun LJ, Li WJ (2015) The influences of biotic and abiotic factors on the occurrence and severity of poplar canker disease in Qingfeng County, China and the management implications. J For Res 26:1025–1034
Meng Y, Zhao W, You J, Gang HZ, Liu JF, Yang SZ, Ye RQ, Mu BZ (2016) Structural analysis of the lipopeptide produced by the Bacillus subtilis mutant R2-104 with mutagenesis. Appl Biochem Biotechnol 179:973–985
Ongena M, Jacques P, Toure Y, Destain J, Jabrane A, Thonart P (2005) Involvement of fengycin-type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis. Appl Microbiol Biotechnol 69:29–38
Pathak KV, Keharia H (2014) Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR). 3 Biotech 4:41–48
Pathak KV, Bose A, Keharia H (2014) Characterization of novel Lipopeptides produced by Bacillus tequilensis P15 using liquid chromatography coupled Electron spray ionization tandem mass spectrometry (LC–ESI–MS/MS). Int J Pept Res Ther 20:133–143
Rautela R, Singh AK, Shukla A, Cameotra SS (2014) Lipopeptides from Bacillus strain AR2 inhibits biofilm formation by Candida albicans. Antonie Van Leeuwenhoek 105:809–821
Vanittanakom N, Loeffler W, Koch U, Jung G (1986) Fengycin-a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot 39:888–901
Villegas-Escobar V, Ceballos I, Mira JJ, Argel LE, Orduz Peralta S, Romero-Tabarez M (2013) Fengycin C produced by Bacillus subtilis EA-CB0015. J Nat Prod 76:503–509
Wang J, Liu J, Wang X, Yao J, Yu Z (2004) Application of electrospray ionization mass spectrometry in rapid typing of fengycin homologues produced by Bacillus subtilis. Lett Appl Microbiol 39:98–102
Williams BH, Hathout Y, Fenselau C (2002) Structural characterization of lipopeptide biomarkers isolated from Bacillus globigii. J Mass Spectrom 37:259–264
Yang H, Li X, Li X, Yu HM, Shen ZY (2015) Identification of lipopeptide isoforms by MALDI-TOF-MS/MS based on the simultaneous purification of iturin, fengycin, and surfacetin by RP-HPLC. Anal Bioanal Chem 407:2529–2542
Sources of funding
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (No. 201503112), the Special Fund for Forest Scientific Research in the Public Welfare (No. 201304212), and Funds of Shandong “Double Tops” Program (No. 2017010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that no conflicts of interest (potential or otherwise, financial or non-financial) are associated with this study.
Rights and permissions
About this article
Cite this article
Sa, RB., An, X., Sui, JK. et al. Purification and structural characterization of fengycin homologues produced by Bacillus subtilis from poplar wood bark. Australasian Plant Pathol. 47, 259–268 (2018). https://doi.org/10.1007/s13313-018-0552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-018-0552-1