Abstract
Minimally invasive surgery has gained worldwide acceptance in the treatment of colonic cancer in the last decades, thanks to its well-known advantages in short-term outcomes. Nevertheless, the penetrance of minimally invasive colorectal surgery still remains low. Few studies and metanalysis, to date, have analyzed the results of robotic versus laparoscopic colorectal surgery, often with conflicting conclusions. The robotic platform, thanks to its technological features, may potentially overcome the limitation of standard laparoscopy, especially when performing a complete mesocolic excision resection and an intracorporeal anastomosis. Robotic surgery could also shorten the learning curve of young novice surgeons, provided that strict protocols of structured training are applied. This paper is an update on the current available outcomes of robotic vs laparoscopic surgery in right colectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimally invasive surgery has gained worldwide acceptance in the treatment of colonic cancer in the last decades, thanks to its well-known benefits in terms of 30-day postoperative outcomes and equivalent long-term oncological results as shown by multicenter randomized controlled trials [1–3].
Nevertheless, the penetrance of laparoscopic colectomy still remains low [4–7] and the role of laparoscopic right colectomy is still controversial. This is probably due to the good outcomes obtained by standard open surgery, such as short operative time, a favourable length of stay and a short learning curve. Moreover, a main controversial and debated issue still remains about whether the anastomosis should be performed intra- or extracorporeally. To date, the majority of the published series dealing with minimally invasive right colectomy have reported an extracorporeal fashioning of the anastomosis (EA) [8].
Furthermore, the introduction of the concept of complete mesocolic excision (CME) by Hoenberger et al. [9], together with its potential long-term oncological advantages, has led to another matter of debate when dealing with laparoscopic right colectomy. The principle of CME with central vascular ligation with complete exposure and lymphadenectomy along the superior mesenteric axis may potentially increase the technical difficulties of minimally invasive surgery, especially when dealing with right colon cancer.
Robotic surgery may potentially overcome the limitations of straight laparoscopic instruments thanks to its technical features, with the aim of increasing the diffusion of minimally invasive right colectomy. To date, however, few comparative studies exist between robotic and laparoscopic right colectomy, and only in one report robotic and laparoscopic approach with intracorporeal anastomosis (IA) are analyzed [10].
This paper reports the current available data on laparoscopic versus robotic approach in right colectomy, focusing on some technical aspects as CME, IA and learning of the technique.
Robotic right colectomy: technical aspects
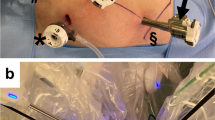
The patient is placed on the operating table in a supine position, with arms along the body and legs closed. Pneumoperitoneum is established via a Veress needle at the Palmer’s point and trocar are placed as shown in Fig. 1. A laparoscopic exploration of the peritoneal cavity, together with liver intraoperative ultrasound examination, is then carried out in order to rule out peritoneal seeding or liver metastasis. In order to achieve exposure of the right and transverse mesocolon, a slight Trendelenburg (5°–10°) and left tilt (5°–10°) are applied and the system is docked. Operative room setup is shown in Fig. 2. The three working arms carry a monopolar cautery hook/scissors in the left upper quadrant port (R1), bipolar forceps in the suprapubic port (R3) and Cadiere’s fenestrated forceps in the right lower quadrant port (R2). R2 is used to keep the superior mesenteric axis in traction, while R1 and R3 are used for dissection. After gentle cephalad traction on the transverse mesocolon with the grasp in R2, the ileocolic vessels are identified and lifted up with R3: the peritoneum is then opened just below their prominence and along the left side of the anterior aspect of the superior mesenteric vein. Ileocolic vessels, right colic vessels (if present), middle colic veins and the right branch of the middle colic artery can be easily and safely ligated at their roots along the right border of the superior mesenteric axis and at the Henle sinus. A CME is performed by sharp dissection of the posterior visceral fascial layer from the parietal one along Gerota’s and Fredet’s fascias, thus exposing the duodenum and the pancreatic head and providing a specimen with intact visceral fascial layers on both sides. When the hepatic flexure or the proximal transverse colon are involved (T3-N+ tumors), en-bloc resection of the right gastroepiploic lymphovascular chain is also performed close to the greater curvature of the stomach. After bowel transection and complete colonic detachment, a side-to-side isoperistaltic mechanical anastomosis (with a double-layered continuous suture closing the enterotomies carried out for the introduction of the 60 mm linear stapler) is performed. All the surgical steps are executed intracorporeally and the specimen, previously inserted into a plastic bag, is extracted via a mini-Pfannenstiel incision at the site of the suprapubic trocar. No abdominal drain is routinely left in place. Laparoscopic right colectomy is carried out following the same surgical principles, including intracorporeal anastomosis.
Intracorporeal (IA) vs extracorporeal (EA) anastomosis
Although minimally invasive colonic surgery has demonstrated better short-term outcomes and equivalent oncological results when compared to the open approach, LRC remains a procedure performed by a limited number of surgeons and mainly with EA [11], probably because of the good outcomes of the conventional open approach. It is technically considered more challenging than laparoscopic left-sided resections and the difficulty significantly increases when anastomosis is performed intracorporeally [12]. A systematic review comparing laparoscopic and open right colectomy found that on 17 comparative studies, 14 reported LRC with EA, while the remaining three did not report this information [8].
Data in the available literature are still conflicting: although two meta-analysis and one retrospective study have failed to clarify the debate between intra- and extra-corporeal anastomosis [13–15], other studies [16–18], on large cohorts of patients, have demonstrated several advantages in favor of intracorporeal anastomosis. Data are summarized in Table 1.
Feroci et al. [16] analyzed the results of five non-randomized studies comparing 202 patients who underwent to IA and 223 to EA and concluded that IA has a faster return to bowel function, earlier oral intake, a decreased use of analgesic as well as a shorter hospital stay. No differences were observed in terms of operative room time, incision length, number of harvested nodes and intra- and post-operative complications.
In the multicentric case–control Italian study [17], comparing 286 patients submitted to LRC with IA and 226 to LRC with EA, Milone et al., showed that the majority of complications and the severity of them (according to Clavien–Dindo classification), as well as the incidence of wound infections, were higher in EA group. In the IA’s group bowel function recovery was faster than EA’s group, while similar data concerning anastomotic leak, anastomotic bleeding and operative time were found in the two groups.
The faster recovery of bowel function associated with LRC with IA technique, theoretically, should be due to the reduction of visceral trauma, caused by tissue-stretching, particularly at the level of the mesenteric pedicle.
Another potential advantages of IA also include the possibility of performing a smaller abdominal incision for specimen extraction and in more convenient sites (usually Pfannenstiel incision), with better cosmetic results, decreased incisional hernia rates (ranging from 0 to 2 %) and less postoperative pain [19].
Moreover, although apparently operative time should be longer for LRC-IA than LRC-EA because it requires laparoscopic suturing skills, several studies have failed to show this finding [16]. In any case, the encountered difficulties to perform LRC-IA could be overcome by the use of the robotic system.
To date, few papers have compared robotic right colectomy with IA with both LRC with IA and EA.
Park et al., comparing robotic with laparoscopic right colectomy in a randomized clinical trial, found no advantages in the robotic group, but the study was jeopardized because laparoscopic and robotic cases were performed with a mixed anastomotic technique, with the majority of intraorporeal anastomosis in the robotic arm [20].
Morpurgo et al., comparing RRC-IA with LRC-EA in a case control study, found in the RRC-IA’s group a shorter time to first flatus, shorter length of hospital stay and lower anastomotic complication rates. On the other hand, Rawlings found no differences between the RRC-IA and LRC-EA in all of the examined outcomes [21].
In a recent retrospective multicentric study by Trastulli et al. [10], RRC-IA (102 pt) was compared with both LRC-IA (40 pt) and LRC-EA (94 pt). The authors reported for RRC-IA a significantly shorter time to first flatus and length of hospital stay compared to LRC-EA. Compared to LRC-IA, although robotic group has a shorter time to first flatus, no statistically difference was found in term of hospital stay between these two groups. Moreover the overall 30-day complication rate, anastomotic leak rate, wound infections and postoperative ileus did not significantly differ between the three groups.
In our experience, when comparing laparoscopic right colectomy with IA vs robotic right colectomy with IA (69 laparoscopic, 53 robotic), we found no anastomotic leak in the robotic group (0 vs 7.2 %, unpublished data). Noticeably, a consistent proportion of right colectomy with IA were performed by a novice colorectal surgeon only in the robotic arm (10 out of 53, 18 %).
Learning curve: overview of the literature and personal remarks
The learning curve in minimally invasive colorectal surgery is often ill-defined and the quantification to evaluate surgeons’ proficiency is variable. A single parameter (i.e. operative time) is often used to establish surgical competence, but this unfortunately offers only a subjective, incomplete and simplistic view on the real level of proficiency achieved.
According to the recent review by Barrie et al. [22], currently three types of studies exist in literature defining the learning curve in MI colonic resection: studies that use a single parameter, studies using multiple parameters and finally studies performing a cumulative sum (CUSUM) analysis.
If all this types of studies are matched together, the number of operations necessary to complete the learning curve ranged from 5 to 310 cases for laparoscopic and from 15 to 30 cases for robotic surgery. The wide range reported clearly shows that a multidimensional analysis with the CUSUM method should be performed, thus assessing at the same time trends in multiple and significant surgical outcomes (OR time, conversion rates, post-operative complications, oncological outcomes, case complexity, surgeon experience and level of teaching/mentoring provided). Although operation time and conversion rate have historically been the most widely used criteria for learning curve assessment, Chen et al. [23] demonstrated that, especially for left side resection, a shorter operative time is related to a much higher morbidity rate.
On the other hand, when taking into account only studies in which the CUSUM method was used, the variability clearly decreases and a shorter learning curve for robotic colorectal surgery, if compared to its laparoscopic counterpart, is confirmed (robotic group: 15–30 cases vs laparoscopic group 60–80 cases) [22].
Unfortunately, given the relatively recent introduction of robotic surgery, it’s important to underline that the evidence on robotic colorectal learning curve is still limited. Furthermore, most studies focused the attention on robotic rectal surgery [22, 24–31] and no paper is available, to date and to the best of our knowledge, on right hemicolectomy.
In any case, structured educational programs are mandatory to certify robotic surgical proficiency and the steps of our own training schedule for young and novice surgeons are summarized in Table 2.
Obviously, the first steps of the learning curve are focused on the correct use of the console, in order to achieve complete and safe control of master controllers and pedals (simulators, dry lab). Then, the porcine model is very useful to understand the spatial relationship of the robotic instruments with the patient’s body and to face the adaptation to the loss of tactile and tensile feedback through animal tissue handling.
Then, procedure-specific case observation in specialized centers is fundamental to reach proficiency in trocar placement, cart docking and arm positioning. When starting with the first case, that should be carefully selected (low BMI, no prior abdominal surgery, no bulky masses), a proctor should be available on site. The list of procedures to be performed, according to surgical complexity, is summarized in Table 3.
In our experience, a young novice surgeon, who completed is residency in a specialized center and performed more than 150 colorectal operations as table assistant (but without having performed open or laparoscopic colorectal surgery as leading surgeon), was able to perform 10 robotic right colectomy (intracorporeal anastomosis) with acceptable operative times, no conversions and no intra- or post-operative complications. Obviously, a continuous quality assessment is mandatory.
Discussion
Data on potential advantages of robotic vs laparoscopic right colectomy are, to date, still lacking. Nevertheless, there is potential room for investigation and improvement when dealing with CME, intracorporeal anastomosis and the learning curve of young novice surgeons.
If compared to left-sided resections, dissection of the vascular pedicle and intracorporeal anastomosis are considered the most complex steps of laparoscopic right colectomy [12]. The relatively recent introduction of the CME concept [9] has further highlighted this issue, since some single-center series [9, 32] and population-based studies [33] have shown that this approach could potentially improve long-term oncological outcomes. Following the CME concept, the level of vessel ligation and central lymphadenectomy with complete exposure of SMV and pancreatic head can be difficult steps during minimally invasive right colectomy [34].
There have been a number of studies looking at the feasibility of performing a CME laparoscopically [35–40]: though feasible and safe, the evidence for oncological adequacy in hepatic flexure and transverse colon cancers still remains lacking [34, 41].
To date, however, no study has directly focused on robotic CME for right-sided colonic malignancies. Noticeably, in our experience of 122 minimally invasive right colectomy with IA (69 laparoscopic, 53 robotic), a reduction in conversion rates (1.8 vs 8.7 %) was observed in favour of the robotic group (unpublished data). The lower conversion rate, as a technical outcome, might suggest that minimally invasive right colectomy with CME could be more easily carried out under robotic assistance. Further high-quality studies are necessary to confirm these preliminary findings. Moreover, due to potential technical advantages in central vascular dissection (i.e., middle colic pedicle), future studies should also focus on its role in the treatment of hepatic flexure/proximal transverse colon cancers.
Intracorporeal vs extracorporeal anastomosis in right colectomy remains another debated issue. Recent studies have highlighted some benefits of intracorporeal anastomosis, if compared to the extracorporeal technique, in terms of faster recovery of bowel function, shorter time to oral intake, shorter length of stay and lower complication rates [10, 16, 17]. In our experience, we had no anastomotic leakage in the robotic group (0 vs 7.2 %), even if these preliminary findings need to be confirmed by larger studies. Surgeons that routinely perform an extracorporeal anastomosis after a LRC and are not familiar with laparoscopic intracorporeal suturing could maximize the advantages of using the robotic system thanks to the wristed instruments and their suturing capabilities.
Robotic surgery could also potentially reduce the learning curve process in minimally invasive colorectal surgery [22], even if, to date, no study has selectively addressed the issue of right colectomy.
The robotic platform with the dual console offers an invaluable tool for fast, safe and optimal training. It could potentially shorten the learning curve in colorectal surgery and facilitate the widespread diffusion of the minimally invasive approach even among young and novice surgeons. This could be an added value especially when considering that the penetrance on minimally invasive colorectal surgery is still quite low (about 30–40 %) in most countries [4–7].
To conclude, the robotic approach, if compared to its laparoscopic counterpart, could potentially improve short-term clinical outcomes by reducing anastomotic leak and conversion rate and shorten the learning process of young novice surgeons through structured teaching programs. The added value of CME on oncological outcomes should be further evaluated before recommending its adoption in routine practice.
References
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82. doi:10.1002/bjs.8945
Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 10(1):44–52. doi:10.1016/s1470-2045(08)70310-3
Clinical Outcomes of Surgical therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350(20):2050–2059. doi:10.1056/NEJMoa032651
Kwon S, Billingham R, Farrokhi E, Florence M, Herzig D, Horvath K, Rogers T, Steele S, Symons R, Thirlby R, Whiteford M, Flum DR (2012) Adoption of laparoscopy for elective colorectal resection: a report from the Surgical Care and Outcomes Assessment Program. J Am Coll Surg 214(6):909–918. doi:10.1016/j.jamcollsurg.2012.03.010
Delaney CP, Chang E, Senagore AJ, Broder M (2008) Clinical outcomes and resource utilization associated with laparoscopic and open colectomy using a large national database. Ann Surg 247(5):819–824. doi:10.1097/SLA.0b013e31816d950e
Simorov A, Shaligram A, Shostrom V, Boilesen E, Thompson J, Oleynikov D (2012) Laparoscopic colon resection trends in utilization and rate of conversion to open procedure: a national database review of academic medical centers. Ann Surg 256(3):462–468. doi:10.1097/SLA.0b013e3182657ec5
Klugsberger B, Haas D, Oppelt P, Neuner L, Shamiyeh A (2015) Current state of laparoscopic colonic surgery in Austria: a national survey. J Laparoendosc Adv Surg Tech Part A 25(12):976–981. doi:10.1089/lap.2015.0373
Rondelli F, Trastulli S, Avenia N, Schillaci G, Cirocchi R, Gulla N, Mariani E, Bistoni G, Noya G (2012) Is laparoscopic right colectomy more effective than open resection? A meta-analysis of randomized and nonrandomized studies. Colorectal Dis 14(8):e447–e469. doi:10.1111/j.1463-1318.2012.03054.x
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis 11(4):354–364. doi:10.1111/j.1463-1318.2008.01735.x (discussion 364–355)
Trastulli S, Coratti A, Guarino S, Piagnerelli R, Annecchiarico M, Coratti F, Di Marino M, Ricci F, Desiderio J, Cirocchi R, Parisi A (2015) Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: a retrospective multicentre study. Surg Endosc 29(6):1512–1521. doi:10.1007/s00464-014-3835-9
Carnuccio P, Jimeno J, Pares D (2014) Laparoscopic right colectomy: a systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech Coloproctol 18(1):5–12. doi:10.1007/s10151-013-1029-4
Jamali FR, Soweid AM, Dimassi H, Bailey C, Leroy J, Marescaux J (2008) Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg 143(8):762–767. doi:10.1001/archsurg.143.8.762 (discussion 768)
Stein SA, Bergamaschi R (2013) Extracorporeal versus intracorporeal ileocolic anastomosis. Tech Coloproctol 17(Suppl 1):S35–S39. doi:10.1007/s10151-012-0937-z
Cirocchi R, Trastulli S, Farinella E, Guarino S, Desiderio J, Boselli C, Parisi A, Noya G, Slim K (2013) Intracorporeal versus extracorporeal anastomosis during laparoscopic right hemicolectomy––systematic review and meta-analysis. Surg Oncol 22(1):1–13. doi:10.1016/j.suronc.2012.09.002
Lee KH, Ho J, Akmal Y, Nelson R, Pigazzi A (2013) Short- and long-term outcomes of intracorporeal versus extracorporeal ileocolic anastomosis in laparoscopic right hemicolectomy for colon cancer. Surg Endosc 27(6):1986–1990. doi:10.1007/s00464-012-2698-1
Feroci F, Lenzi E, Garzi A, Vannucchi A, Cantafio S, Scatizzi M (2013) Intracorporeal versus extracorporeal anastomosis after laparoscopic right hemicolectomy for cancer: a systematic review and meta-analysis. Int J Colorectal Dis 28(9):1177–1186. doi:10.1007/s00384-013-1651-7
Milone M, Elmore U, Di Salvo E, Delrio P, Bucci L, Ferulano GP, Napolitano C, Angiolini MR, Bracale U, Clemente M, D’Ambra M, Luglio G, Musella M, Pace U, Rosati R, Milone F (2015) Intracorporeal versus extracorporeal anastomosis. Results from a multicentre comparative study on 512 right-sided colorectal cancers. Surg Endosc 29(8):2314–2320. doi:10.1007/s00464-014-3950-7
Grams J, Tong W, Greenstein AJ, Salky B (2010) Comparison of intracorporeal versus extracorporeal anastomosis in laparoscopic-assisted hemicolectomy. Surg Endosc 24(8):1886–1891. doi:10.1007/s00464-009-0865-9
DeSouza A, Domajnko B, Park J, Marecik S, Prasad L, Abcarian H (2011) Incisional hernia, midline versus low transverse incision: what is the ideal incision for specimen extraction and hand-assisted laparoscopy? Surg Endosc 25(4):1031–1036. doi:10.1007/s00464-010-1309-2
Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP (2012) Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 99(9):1219–1226. doi:10.1002/bjs.8841
Morpurgo E, Contardo T, Molaro R, Zerbinati A, Orsini C, D’Annibale A (2013) Robotic-assisted intracorporeal anastomosis versus extracorporeal anastomosis in laparoscopic right hemicolectomy for cancer: a case control study. J Laparoendosc Adv Surg Tech Part A 23(5):414–417. doi:10.1089/lap.2012.0404
Barrie J, Jayne DG, Wright J, Murray CJ, Collinson FJ, Pavitt SH (2014) Attaining surgical competency and its implications in surgical clinical trial design: a systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann Surg Oncol 21(3):829–840. doi:10.1245/s10434-013-3348-0
Chen W, Sailhamer E, Berger DL, Rattner DW (2007) Operative time is a poor surrogate for the learning curve in laparoscopic colorectal surgery. Surg Endosc 21(2):238–243. doi:10.1007/s00464-006-0120-6
Kim HJ, Choi GS, Park JS, Park SY (2014) Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon’s experience. Dis Colon Rectum 57(9):1066–1074. doi:10.1097/dcr.0000000000000174
Melich G, Hong YK, Kim J, Hur H, Baik SH, Kim NK, Sender Liberman A, Min BS (2015) Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc 29(3):558–568. doi:10.1007/s00464-014-3698-0
Park EJ, Kim CW, Cho MS, Kim DW, Min BS, Baik SH, Lee KY, Kim NK (2014) Is the learning curve of robotic low anterior resection shorter than laparoscopic low anterior resection for rectal cancer?: a comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Medicine 93(25):e109. doi:10.1097/md.0000000000000109
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29(7):1679–1685. doi:10.1007/s00464-014-3855-5
Buchs NC (2015) Robotic technology: optimizing the outcomes in rectal cancer? World J Clin Oncol 6(3):22–24. doi:10.5306/wjco.v6.i3.22
Byrn JC, Hrabe JE, Charlton ME (2014) An initial experience with 85 consecutive robotic-assisted rectal dissections: improved operating times and lower costs with experience. Surg Endosc 28(11):3101–3107. doi:10.1007/s00464-014-3591-x
Foo CC, Law WL (2015) The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg. doi:10.1007/s00268-015-3251-x
Formisano G, Marano A, Bianchi PP, Spinoglio G (2015) Challenges with robotic low anterior resection. Miner Chir 70:341–354
Cho MS, Baek SJ, Hur H, Soh Min B, Baik SH, Kyu Kim N (2015) Modified complete mesocolic excision with central vascular ligation for the treatment of right-sided colon cancer: long-term outcomes and prognostic factors. Ann Surg 261(4):708–715. doi:10.1097/sla.0000000000000831
Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Iversen ER, Kristensen B, Gogenur I (2015) Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 16(2):161–168. doi:10.1016/s1470-2045(14)71168-4
Chow CF, Kim SH (2014) Laparoscopic complete mesocolic excision: West meets East. World J Gastroenterol 20(39):14301–14307. doi:10.3748/wjg.v20.i39.14301
Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2014) Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol 21(7):2288–2294. doi:10.1245/s10434-014-3614-9
Huang JL, Wei HB, Fang JF, Zheng ZH, Chen TF, Wei B, Huang Y, Liu JP (2015) Comparison of laparoscopic versus open complete mesocolic excision for right colon cancer. Int J Surg 23:12–17. doi:10.1016/j.ijsu.2015.08.037
Kang J, Kim IK, Kang SI, Sohn SK, Lee KY (2014) Laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 28(9):2747–2751. doi:10.1007/s00464-014-3521-y
Melich G, Jeong DH, Hur H, Baik SH, Faria J, Kim NK, Min BS (2014) Laparoscopic right hemicolectomy with complete mesocolic excision provides acceptable perioperative outcomes but is lengthy–analysis of learning curves for a novice minimally invasive surgeon. Can J Surg 57(5):331–336
Mori S, Baba K, Yanagi M, Kita Y, Yanagita S, Uchikado Y, Arigami T, Uenosono Y, Okumura H, Nakajo A, Maemuras K, Ishigami S, Natsugoe S (2015) Laparoscopic complete mesocolic excision with radical lymph node dissection along the surgical trunk for right colon cancer. Surg Endosc 29(1):34–40. doi:10.1007/s00464-014-3650-3
Shin JW, Amar AH, Kim SH, Kwak JM, Baek SJ, Cho JS, Kim J (2014) Complete mesocolic excision with D3 lymph node dissection in laparoscopic colectomy for stages II and III colon cancer: long-term oncologic outcomes in 168 patients. Tech Coloproctol 18(9):795–803. doi:10.1007/s10151-014-1134-z
Gouvas N, Pechlivanides G, Zervakis N, Kafousi M, Xynos E (2012) Complete mesocolic excision in colon cancer surgery: a comparison between open and laparoscopic approach. Colorectal Dis 14(11):1357–1364. doi:10.1111/j.1463-1318.2012.03019.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Giampaolo Formisano, Pasquale Misitano, Giuseppe Giuliani, Giulia Calamati, Lucia Salvischiani, Sofia Esposito and Paolo Pietro Bianchi have no conflict of interests or financial ties to disclose.
Ethical approval
Our study was conducted in accordance with the Italian government ethical guidelines for the treatment of humans and with Institutional Review Board approval.
Research involving human participants and/or animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Formisano, G., Misitano, P., Giuliani, G. et al. Laparoscopic versus robotic right colectomy: technique and outcomes. Updates Surg 68, 63–69 (2016). https://doi.org/10.1007/s13304-016-0353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-016-0353-4