Abstract
MiR-21 is an important microRNA (miRNA) modulating radiosensitivity of cervical cancer cells. However, the underlying mechanism of miR-21 upregulation in radioresistant cervical cancer has not been fully understood. In addition, autophagy may either promote or alleviate radioresistance, depending on the types of cancer and tumor microenvironment. How autophagy affects radiosensitivity in cervical cancer and how miR-21 is involved in this process has not been reported. This study showed that miR-21 upregulation in radioresistant cervical cancer is related to HIF-1α overexpression. MiR-21 overexpression decreases PTEN, increases p-Akt, and subsequently increases HIF-1α expression, while miR-21 inhibition results in increased PTEN, decreased p-Akt, and then decreased HIF-1α. Therefore, we inferred that there is a HIF-1α-miR-21 positive feedback loop through the PTEN/Akt/HIF-1α pathway in cervical cancer cells. In addition, we also demonstrated that miR-21 confers decreased autophagy in cervical cancer cells after IR via the Akt-mTOR signaling pathway. Decreased autophagy is one of the potential mechanisms of increased radioresistance in cervical cancer cells. These findings expand our understanding of radioresistance development in cervical cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the one of the most common female malignancies [1]. Combined radiotherapy and chemotherapy (chemoradiotherapy) is recommended as either a primary or an adjuvant treatment for the patients with cervical cancer staging from 1B2 to stage 4A [2–4]. Although the administration of chemoradiotherapy can significantly improve patient survival, local recurrence is still common due to radioresistance [5]. However, the underlying mechanism of radioresistance is still not fully understood. Our previous study observed that miR-21 is an important microRNA (miRNA) modulating radioresistance development in HR-HPV positive cervical cancer cells through downregulating LATS1 [6].
Previous studies reported that viral oncoprotein E6 can facilitate the transcription of miR-21 [7]. The association between miR-21 overexpression and increased radioresistance was also observed in some types of cancer [8, 9]. Our previous study observed that miR-21 was further increased in radioresistant cervical cancer [6]. However, only limited studies reported the mechanisms underlying miR-21 upregulation and radioresistance. Hypoxia is a common feature of many solid tumors including cervical cancer. Some previous studies reported that hypoxia inducible factor 1α (HIF-1α), a transcription factor induced under hypoxia might protect cervical cancer cells from irradiation (IR) induced apoptosis via multiple mechanisms, such as increasing VEGF and decreasing P53 expression [10] and suppressing radiation-induced Bax expression [11]. In fact, HIF-1α is an important transcription factor that can facilitate transcription of many genes through binding to the hypoxia responsive elements (HREs) [12, 13]. In this study, we firstly explored the association between HIF-1α expression and miR-21 in cervical cancer cells.
Autophagy may also play a role in the development of radioresistance [14]. It may either promote or alleviate radioresistance, depending on the types of cancer and tumor microenvironment [14]. Due to the wide influence of miR-21 on cell cycle, DNA damage repair, apoptosis, autophagy, and hypoxia, it has been recognized as a novel promising target in cancer radiation therapy [9]. However, how autophagy affects radiosensitivity in cervical cancer and how miR-21 is involved in this process has not been reported. Therefore, this study further investigates the association among miR-21, autophagy, and radioresistance in cervical cancer.
Methods
Patients and tissue samplings
This study was approved by the ethics committee of Cangzhou City Central Hospital. All of the patients never received preoperative radiotherapy and/or chemotherapy before this study. Cervical tissue samples were obtained from 22 patients histologically diagnosed as infiltrating Ib-IIa and IIb-IIIa cervical cancer. The patients were administrated with radiotherapy by giving standard, pelvic radiation therapy and brachytherapy with a total point A dose 75–80 Gy according to 2014 NCCN Clinical Practice Guideline in Oncology for Cervical Cancer. In addition, the patients also received concurrent chemotherapy of weekly cisplatin 40 mg/m2 [15]. The radiosensitive and radioresistant cases were assessed by histological examination of residual tumor tissues by colposcopically directed biopsy 6 months after completion of radiotherapy. Histological assessment was performed by pathologist without authorship in this study. All of the samples contained at least 70 % tumor cells were used for further studies.
Cell culture
Human cervical cancer cell line HeLa and SiHa cells were grown in RPMI‑1640 medium (Gibco‑BRL, USA) supplemented with 10 % fetal bovine serum (HyClone, USA). All cells were cultured in a humidified atmosphere containing 5 % CO2 at 37 °C.
Cell transfection
Chemically synthesized miR-21 mimics, miR-21 inhibitors, and the corresponding scramble negative controls were purchased from Ribobio Life Science (Shanghai, China). To overexpress miR-21 or to knockdown of endogenous miR-21, siHa and Hela cells were transfected with 50 nM miR-21 mimics or 100 nM miR-21 inhibitors by using lipofectamine 2000 (Invitrogen, USA). To study the effect of autophagy on radiosensitivity, siHa and Hela cells with or without miR-21 overexpression were treated with 50 μM rapamycin (Rapa, Sigma-Aldrich, USA) or 5 mM 3-MA (Sigma-Aldrich, USA) 1 h before IR, for a duration of 24 h. Then, the cells were subjected to analysis of autophagy, apoptosis, and GFP accumulation. HIF-1α expression vector, pCMV-HIF-1α (RG202461) was purchased from OriGene. siHa and Hela cells were transfected with pCMV-HIF-1α or the empty negative control vector using Lipofectamine 2000 (Invitrogen).
QRT-PCR analysis of miR-21 expression
Total RNAs from tissue and cell samples were extracted using TRIzol reagent (Invitrogen). MiRNAs specific complementary DNA (cDNA) was synthesized using the stem-loop primers and the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, USA). MiR-21 expression was quantified by using the TaqMan MicroRNA Assays Kit (Life Technologies, USA), with RNU6B as a control gene in an ABI Prism 7500 (Applied Biosystems). The 2-ΔΔ Ct method was used to calculate relative miR-21 expression.
Total RNAs in the cell samples were extracted using the TRIzol reagent (Invitrogen) according to manufacturer’s instructions. Then the first strand cDNA was synthesized using the PrimeScript® RT reagent kit (TaKaRa, Japan). To quantify the expression of HIF1-α mRNA, qRT-PCR was performed using the following primers: F, 5′-CGTTCCTTCGATCAGTTGTC-3′; R, 5′-TCAGTGGTGGCAGTGGTAGT-3′ and SYBR® Premix DimerEraser kit (TaKaRa) in an ABI Prism 7500 (Applied Biosystems). The 2-ΔΔCt method was used to calculate relative HIF1-α mRNA expression.
Western blot analysis
Western blot analysis was performed as described in one of our previous study [6]. Briefly, the primary antibodies used included anti-HIF1-α (1:1000, ab16066, Abcam, USA), anti-Cleaved Caspase-3 (1:1000, #P42574, Cell Signaling, USA), anti-PTEN (1:1000, ab32199, Abcam), anti-phospho-AKT (1:1000, #9271, Cell Signaling), anti-AKT (1:1000, EPR16798, Abcam), anti-LC3B (1:3000, ab51520, Abcam), anti-p62/SQSTM1 (1:1000, #8025, Cell Signaling), anti-phospho-mTOR (1:2000, ab109268, Abcam), anti-phospho-p70S6 (1:1000, #6198, Cell Signaling), anti-p70S6 (1:1000, #9202, Cell Signaling) and anti-β-actin (1: 2000, ab8227, Abcam). The second HRP conjugated secondary antibodies were purchased from Abcam. The blot signals were visualized using the ECL Western blotting substrate (Promega, USA).
Colony formation and apoptosis analysis
1 × 105 SiHa or Hela cells were seeded in six-well plates and prepared for transfection the next day. 48 h after transfection, the plates were irradiated with 137Cs (Nordion, Ottawa, ON, Canada), with a dose rate of 1.25 Gy/min. The plates were treated with a dose of 0, 2, 4, 6, and 8 Gy in a single fraction. Then, the plates were further incubated in a cell incubator for 10 to 13 days and then the cells were fixed with 10 % paraformaldehyde and stained with 1 % crystal violet in 70 % ethanol. Colonies (with minimal 50 cells) were counted. Survival fraction was defined as the number of colonies/(cells inoculated × plating efficiency) and radiation survival curve was drew by using the multi-target single-hit model: SF = 1-(1-exp(-x/D0))^N.
Flow cytometry analysis of cell cycle and cell apoptosis
Cells were fixed in 70 % ethanol at −20 °C for 24 h and then washed using PBS. The fixed cells were firstly incubated with 100 μg/mL RNase A in PBS for 30 min at 37 °C and then 10 μg/mL propidium iodide (PI) (Sigma-Aldrich) was added to the cell suspension for another 30 min incubation in dark. DNA content was analyzed using a FACSCaliber (BD Biosciences, USA). Cell apoptosis was detected by using Annexin V-FITC Apoptosis Detection Kit (ab14085, Abcam) and the apoptosis rates were measured by using a FACSCalibur. Each test was performed with at least three repeats.
Preparation of Hela cells with stable GFP-LC3 expression
Briefly, Hela cells were transfected with pSELECT-GFP-LC3 (InvivoGen) were screened for stable clones using 250 μg/mL Zeocin (Sigma-Aldrich) up to 3 weeks. Then, the cells with stable GFP expression were transfected with 50 nM miR-21 mimics or 100 nM miR-21 inhibitors. 48 h after transfection, the cells were subjected to IR (6 Gy). The cells without transfection were treated with 5 mM 3-MA or 50 μM rapamycin 1 h before radiation, for a duration of 24 h. After that, the accumulation of GFP-LC3 puncta was captured using an inverted microscope (TS100F, Nikon, Japan).
Statistical analysis
Experimental data are presented as mean ± SD based at least three repeats. All statistical analyses were performed using SPSS 18.0 software (SPSS, USA). One-way ANOVA was performed to compare means of multiple group experiments. Comparison between groups was performed by using non-paired Student’s t test. P value of <0.05 was considered significant.
Results
MiR-21 is significantly upregulated in radioresistant cervical cancer in a HIF-1α-dependent manner
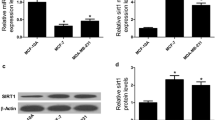
Our previous study observed that miR-21 is an oncogenic miRNA which confers radioresistance of HR-HPV positive cervical cancer cells through targeting LATS1 [6]. However, how miR-21 is dysregulated in radioresistant cervical cancer has not been reported yet. After 6 months of chemoradiotherapy, histological assessment showed that there were 13 radiosensitive and 9 radioresistant cases among the 22 cervical cancer patients. We firstly quantified miR-21 expression in both radiosensitive and radioresistant cancer tissues. The results showed that the radioresistant cases generally had significantly higher miR-21 expression than the radiosensitive counterparts (Fig. 1a). Cervical cancer is a type of solid tumor usually with increased expression of HIF-1α [11]. In addition, one previous study also reported that HIF-1α can bind to the hypoxia response element (HRE) in the promoter region of miR-21 promoter region and enhance its expression [16]. Therefore, we hypothesized that the elevated expression of miR-21 in the radioresistant cases might be associated with dysregulated HIF-1α. By performing Western blot analysis, we confirmed that the radioresistant tissues had significantly higher HIF-1α (Fig. 1b). To further explore the regulative role of HIF-1α in miR-21 expression, siHa and Hela cells were transfected with HIF-1α expression vector for overexpression (Fig. 1c). Then, we further detected the change of miR-21 after HIF-1α overexpression. QRT-PCR analysis showed that miR-21 increased substantially 48 h after HIF-1α transfection (Fig. 1d). These results suggest that MiR-21 is significantly upregulated in cervical cancer in a HIF-1α dependent manner.
MiR-21 is significantly upregulated in radioresistant cervical cancer in a HIF-1α-dependent manner. a. QRT-PCR analysis of miR-21 expression in radiosensitive (n = 13) and radioresistant (n = 9) cervical cancer tissues. b. Western blot analysis of HIF-1α expression in four randomly selected radiosensitive and four randomly selected radioresistant cervical cancer tissues. c. Western blot analysis of HIF-1α expression in siHa and Hela cells transfected with HIF-1α expression vectors 48 h after transfection. d. QRT-PCR analysis of miR-21 expression in siHa and Hela cells transfected with HIF-1α expression vectors 72 h after transfection. *p < 0.05, **p < 0.01
PTEN/Akt/HIF-1α feedback loop is involved in radioresistance associated miR-21 upregulation
Some previous studies reported that miR-21 might also indirectly affect HIF-1α expression [17, 18]. Therefore, we decided to further investigate whether there is a feedback regulation between miR-21 and HIF-1α. In both siHa and Hela cells, enforced miR-21 expression increased HIF-1α mRNA, while miR-21 inhibitor decreased HIF-1α mRNA level (Fig. 2a). Then, we detected the change of PTEN, p-Akt, and HIF-1α protein level after miR-21 overexpression. Western blot analysis showed that miR-21 overexpression decreased PTEN. In fact, PTEN is an inhibitor of the PI3K/Akt pathway by removing the 3′ phosphate of phosphatidylinositol 3,4,5-trisphosphate (PIP3) [19]. Our results confirmed that PTEN downregulation is associated with increased p-Akt and HIF-1α in both siHa and Hela cells (Fig. 2b). These results are consistent with previous findings which reported that HIF-1α is a downstream target of PTEN/PI3K/Akt signaling pathway involved in tumorigenesis as well as in response to hypoxia [20, 21]. Therefore, we infer that there is a HIF-1α-miR-21 positive feedback loop through the PTEN/Akt/HIF-1α pathway (Fig. 2c).
PTEN/Akt/HIF-1α feedback loop is involved in radioresistance associated miR-21 upregulation. a. QRT-PCR analysis of HIF-1α mRNA expression in siHa and Hela cells transfected with 50 nM miR-21 mimics or 100 nM miR-21 inhibitor (IH) 48 h after transfection. b. Western blot analysis of PTEN, p-Akt, Akt, and HIF-1α expression in siHa and Hela cells transfected with 50 nM miR-21 mimics or 100 nM miR-21 inhibitor (IH) 48 h after transfection. c. Schematic representation of the proposed model of PTEN/Akt/HIF-1α/miR-21 feedback loop in cervical cancer cells. *p < 0.05, **p < 0.01
MiR-21 reduces autophagy after IR in cervical cancer cells
One recent study suggests that the administration of rapamycin helps to reduce radioresistance of radioresistant Hela cells [22]. Considering the strong autophagy inducing effect of rapamycin, we decided to further study the association between autophagy and radioresistance. By detecting LC3B and p62 protein, we observed that the radioresistant tissues had significantly higher expression of LC3-II and reduced p62 level (Fig. 3a). We then studied whether miR-21 is involved in autophagy regulation in siHa and Hela cells after IR. Western blot analysis showed that IR significantly induced autophagy in both siHa and Hela cells (Fig. 3b, c). Knockdown of endogenous miR-21 elevated autophagy after IR. In contrast, miR-21 overexpression significantly reduced autophagy, the effect of which was similar to that of 3-MA (Fig. 3b, c). To further investigate the effect of miR-21 on autophagy, Hela cells with stable expression of LC3-GFP were induced. IR induced significant formation and accumulation of LC3-GFP puncta (Fig. 3d). In contrast, miR-21 overexpression significantly reduced the puncta formation and accumulation, while miR-21 knockdown substantially enhanced the formation and accumulation (Fig. 3d). 3-MA treatment reduced the lipidation of LC3, while rapamycin increased the lipidation (Fig. 3d). These results suggest that MiR-21 can reduce autophagy after IR.
MiR-21 reduces autophagy after IR in cervical cancer cells. a. Western blot analysis of LC3-I, LC3-II, and p62 expression in 4 randomly selected radiosensitive and 4 randomly selected radioresistant cervical cancer tissues. b and c. siHa (b) and Hela (c) cells with miR-21 overexpression or knockdown or treated with 5 mM 3-MA were exposed to 6 Gy IR. 24 h after IR, Western blot analysis was performed to detect LC3-I, LC3-II, and p62 expression. d. Hela cells (with stable GFP-LC3 expression) with indicated treatments were exposed to 6 Gy. The images of GFP-LC3 puncta accumulation were captured using a fluorescence microscope 24 h later
MiR-21 upregulation contributes to increased radioresistance partly through decreasing autophagy
Since we confirmed that miR-21 can modulate autophagy in both siHa and Hela cells after IR, we decided to further investigate the association among miR-21, autophagy, and radiosensitivity in the cervical cancer cells. By performing colony formation assay, we observed that miR-21 overexpression increase cell survival after IR (Fig. 4a, b), while miR-21 knockdown significantly reduced cell survival (Fig. 4c, d). Then, we performed flow cytometry analysis to measure the proportion of apoptotic cells after IR. MiR-21 overexpression significantly reduced the rate of apoptosis after IR (Fig. 4e, f). However, the protective effect of miR-21 was fully abrogated by pre-treatment of rapamycin (Fig. 4e, f). Following Western blot analysis also confirmed that siHa and Hela cells with miR-21 overexpression had reduced expression of cleaved caspase-3, while the cells with miR-21 knockdown had increased expression of cleaved caspase-3 (Fig. 4g). Since radiosensitivity is closely related to the distribution of cells in each cell phases, we then measured the change of cell cycle distribution of siHa and Hela cells after miR-21 overexpression. MiR-21 overexpression significantly reduced IR-induced cell cycle arrest at G2 phase before mitosis (Fig. 4h, i). However, Rapamycin treatment significantly enhanced G2/M phase arrest (Fig. 4h, i).
MiR-21 upregulation contributes to increased radioresistance partly through decreasing autophagy. a–d. The survival fraction of siHa (a and c) and Hela (b and d) cells with miR-21 overexpression (a and b) or miR-21 knockdown (c and d) or pretreated with rapamycin (c and d). e. Representative images of apoptosis in siHa and Hela cells with miR-21 overexpression alone or in combination with rapamycin treatment 24 h after 6 Gy IR. f. Quantification of the proportion of apoptotic cells in figure e. g. Western blot analysis of cleaved caspase-3 in siHa and Hela cells with miR-21 overexpression, miR-21 knockdown, or rapamycin treatment 24 h after 6 Gy IR. h. Representative images of cell cycle distribution of siHa and Hela cells with miR-21 overexpression alone or in combination with rapamycin treatment 24 h after 6Gy IR. i. Quantification of the G2/M arrest in figure panel h. *p < 0.05, **p < 0.01
The Akt-mTOR signaling pathway is significantly enhanced by miR-21
Since miR-21 exerts protective effects on IR induce cell death at least partly through suppressing autophagy, we further investigated whether the suppression occurred via mTOR inhibition. The Akt-mTOR pathway was evaluated in both siHa and Hela cells. mTOR acts as an important regulator in autophagy induction. In addition, the PI3K/Akt pathway can activate mTOR [23]. Therefore, we measured the expression of p-Akt, p-mTOR, and phospho-p70S6 (p-p70S6), all of which are mTOR substrates in both siHa and Hela cells after IR. The results showed that miR-21 overexpression significantly enhanced the expression of p-mTOR and p-p70S6, while miR-21 inhibitor remarkably reduced the expression of p-mTOR and p-p70S6 in both siHa and Hela cells (Fig. 5a, b). Based on these results, we inferred that the Akt-mTOR signaling pathway was significantly enhanced by miR-21 (Fig. 5c), which is an underlying mechanism of miR-21 mediated autophagy inhibition.
The Akt-mTOR signaling pathway is significantly enhanced by miR-21. a, b Western blot analysis of p-Akt, Akt, p-mTOR, mTOR, p-p70S6, and p70S6 expression in siHa cells 48 h after miR-21 mimics or miR-21 inhibitors (IH) transfection in siHa (a) and Hela (b) cells. c. Schematic representation of the proposed model of The Akt-mTOR signaling pathway miR-21 mediated autophagy inhibition in cervical cancer cells
Discussion
Although the oncogenic role of miR-21 in pathological development of cervical cancer [7, 24] and its role in radioresistance development [6, 9] has been reported in previous studies, how it is dysregulated in radioresistant cervical cancer is still not quite clear. One recent study reported that HIF-1α is significantly co-localized with miR-21 in glioma cells [25]. More importantly, HIF-1α can also bind to the promoter of miR-21 by recruiting transcriptional coactivator CBP/p300 and enhance its transcription in cardiomyocytes [16]. In this study, we investigated the association between miR-21 and HIF-1α in cervical cancer and observed that HIF-1α overexpression directly resulted in miR-21 upregulation.
Interestingly, miR-21 overexpression may induce HIF-1α overexpression through AKT and ERK activation, which contributes to enhanced angiogenesis and tumor angiogenesis [17]. MiR-21 may also induce epithelial-mesenchymal transition and forming of breast cancer stem cell-like cells via promoting HIF-1α expression [18]. Therefore, we further explored how miR-21 overexpression and knockdown affected HIF-1α expression in cervical cancer cells. The results confirmed that miR-21 overexpression decreased PTEN, increased p-Akt, and subsequently increased HIF-1α expression, while miR-21 inhibition resulted in increased PTEN, decreased p-Akt, and following decreased HIF-1α. In fact, PTEN is a well confirmed direct target of miR-21, which plays a critical inhibitive role in PI3K/Akt signaling pathway [26, 27]. Therefore, we inferred that there is a HIF-1α-miR-21 positive feedback loop through the PTEN/Akt/HIF-1α pathway in cervical cancer cells. These findings partly revealed how miR-21 is further upregulated in radioresistant cervical cancer.
MiR-21 is also a miRNA related to autophagy regulation. Silencing of miR-21 confers enhanced autophagy in malignant glioma cell lines [28]. It can inhibit autophagy via the PTEN/Akt pathway in hepatocellular carcinoma cells [29]. In fact, dysregulated autophagy is associated with altered radiosensitivity of some types of cancer [14]. For example, enhancing autophagy may enhance radiosensitivity of malignant glioma cell lines [28]. Autophagy inhibition in breast cancers and lung cancer cells may enhances radiosensitivity [30, 31]. However, whether miR-21 modulates autophagy in cervical cancer and how autophagy affects radiosensitivity of the cervical cancer cells is not clear. One recent study suggests that administration of rapamycin helps to reduce radioresistance of radioresistant Hela cells [22]. In fact, rapamycin has strong autophagy inducing effects. Therefore, we hypothesized that miR-21 might decrease autophagy and thus confer increased radioresistance. By overexpression or knockdown of miR-21 in siHa and Hela cells, we found that knockdown of endogenous miR-21 elevated autophagy after IR. In contrast, miR-21 overexpression has similar effect as 3-MA in inhibiting autophagy, while miR-21 knockdown presented similar effect in promoting autophagy as rapamycin. Following colony formation assay, flow cytometry and Western blot analysis confirmed that miR-21 induced lower autophagy and increased radioresistance in both siHa and Hela cells.
Since the use of rapamycin fully abrogated the autophagy inducing effects of miR-21 in siHa and Hela cells, we further investigated whether the suppressing effects occurred via mTOR inhibition. mTOR acts as an important regulator in autophagy induction. In addition, the PI3K/Akt pathway can activate mTOR [23]. By performing Western blot analysis, we further confirmed that the Akt-mTOR signaling pathway was significantly enhanced by miR-21, which is an underlying mechanism of miR-21 mediated autophagy inhibition. Similar mechanism was observed in hepatocellular carcinoma, in which miR-21 participates in the acquired resistance of sorafenib by suppressing autophagy through the Akt/PTEN pathway [29].
Conclusion
This study revealed that miR-21 upregulation in radioresistant cervical cancer is at least partly caused by HIF-1α overexpression and is further enhanced via the PTEN/Akt/HIF-1α feedback loop. In addition, miR-21 confers decreased autophagy in cervical cancer cells after IR via the Akt-mTOR signaling pathway. Decreased autophagy is one of the potential mechanisms of increased radioresistance in cervical cancer cells. These findings expand our understanding of radioresistance development in cervical cancer.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Ariga T, Toita T, Kasuya G, Nagai Y, Inamine M, Kudaka W, et al. External beam boost irradiation for clinically positive pelvic nodes in patients with uterine cervical cancer. J Radiat Res. 2013;54:690–6.
Rogers L, Siu SS, Luesley D, Bryant A, Dickinson HO. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev. 2012;5:CD007583.
Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–25.
Powell ME. Modern radiotherapy and cervical cancer. Int J Gynecol Cancer. 2010;20:S49–51.
Liu S, Song L, Zhang L, Zeng S, Gao F. Mir-21 modulates resistance of hr-hpv positive cervical cancer cells to radiation through targeting lats1. Biochem Biophys Res Commun. 2015;459:679–85.
Shishodia G, Verma G, Srivastava Y, Mehrotra R, Das BC, Bharti AC. Deregulation of microRNAs let-7a and mir-21 mediate aberrant stat3 signaling during human papillomavirus-induced cervical carcinogenesis: role of e6 oncoprotein. BMC Cancer. 2014;14:996.
Zhang J, Zhang C, Hu L, He Y, Shi Z, Tang S, et al. Abnormal expression of mir-21 and mir-95 in cancer stem-like cells is associated with radioresistance of lung cancer. Cancer Investig. 2015;33:165–71.
Liu J, Zhu H, Yang X, Ge Y, Zhang C, Qin Q, et al. MicroRNA-21 is a novel promising target in cancer radiation therapy. Tumour Biol. 2014;35:3975–9.
Fu Z, Chen D, Cheng H, Wang F. Hypoxia-inducible factor-1alpha protects cervical carcinoma cells from apoptosis induced by radiation via modulation of vascular endothelial growth factor and p53 under hypoxia. Med Sci Monit. 2015;21:318–25.
Liu J, Zhang J, Wang X, Li Y, Chen Y, Li K, et al. HIF-1 and NDRG2 contribute to hypoxia-induced radioresistance of cervical cancer Hela cells. Exp Cell Res. 2010;316:1985–93.
Song Z, Ren H, Gao S, Zhao X, Zhang H, Hao J. The clinical significance and regulation mechanism of hypoxia-inducible factor-1 and mir-191 expression in pancreatic cancer. Tumour Biol. 2014;35:11319–28.
Xue M, Li X, Li Z, Chen W. Urothelial carcinoma associated 1 is a hypoxia-inducible factor-1α-targeted long noncoding RNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion. Tumour Biol. 2014;35:6901–12.
Yang Y, Yang Y, Yang X, Zhu H, Guo Q, Chen X, et al. Autophagy and its function in radiosensitivity. Tumour Biol. 2015;36:4079–87.
Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, et al. Cervical cancer, version 2.2015. J Natl Compr Cancer Netw. 2015;13:395–404. quiz 404.
Liu Y, Nie H, Zhang K, Ma D, Yang G, Zheng Z, et al. A feedback regulatory loop between HIF-1α and mir-21 in response to hypoxia in cardiomyocytes. FEBS Lett. 2014;588:3137–46.
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, et al. MiR-21 induced angiogenesis through akt and erk activation and HIF-1α expression. PLoS One. 2011;6:e19139.
Han M, Wang Y, Liu M, Bi X, Bao J, Zeng N, et al. MiR-21 regulates epithelial-mesenchymal transition phenotype and hypoxia-inducible factor-1α expression in third-sphere forming breast cancer stem cell-like cells. Cancer Sci. 2012;103:1058–64.
Luo H, Yang Y, Duan J, Wu P, Jiang Q, Xu C. PTEN-regulated akt/foxo3a/bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis. 2013;4:e481.
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–5.
Miyazaki M, Miyazaki K, Chen S, Chandra V, Wagatsuma K, Agata Y, et al. The E-id protein axis modulates the activities of the PI3K-AKT-mTORC1-hif1a and c-myc/p19arf pathways to suppress innate variant TFH cell development, thymocyte expansion, and lymphomagenesis. Genes Dev. 2015;29:409–25.
Kuwahara Y, Mori M, Kitahara S, Fukumoto M, Ezaki T, Mori S, et al. Targeting of tumor endothelial cells combining 2 gy/day of x-ray with everolimus is the effective modality for overcoming clinically relevant radioresistant tumors. Cancer Med. 2014;3:310–21.
Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–14.
Yao T, Lin Z. Mir-21 is involved in cervical squamous cell tumorigenesis and regulates ccl20. Biochim Biophys Acta. 1822;2012:248–60.
Hermansen SK, Nielsen BS, Aaberg-Jessen C, Kristensen BW. Mir-21 is linked to glioma angiogenesis: a co-localization study. J Histochem Cytochem. 2016;64:138–48.
Richart A, Loyer X, Neri T, Howangyin K, Guerin CL, Ngkelo A, et al. Microrna-21 coordinates human multipotent cardiovascular progenitors therapeutic potential. Stem Cells. 2014;32:2908–22.
Yang X, Cheng Y, Li P, Tao J, Deng X, Zhang X, et al. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 2015;36:383–91.
Gwak HS, Kim TH, Jo GH, Kim YJ, Kwak HJ, Kim JH, et al. Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS One. 2012;7:e47449.
He C, Dong X, Zhai B, Jiang X, Dong D, Li B, et al. Mir-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/AKT pathway. Oncotarget. 2015;6:28867–81.
Sun Q, Liu T, Yuan Y, Guo Z, Xie G, Du S, et al. Mir-200c inhibits autophagy and enhances radiosensitivity in breast cancer cells by targeting ubqln1. Int J Cancer. 2015;136:1003–12.
Liu Z, Huang S. Inhibition of mir-191 contributes to radiation-resistance of two lung cancer cell lines by altering autophagy activity. Cancer Cell Int. 2015;15:16.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, L., Liu, S., Zhang, L. et al. MiR-21 modulates radiosensitivity of cervical cancer through inhibiting autophagy via the PTEN/Akt/HIF-1α feedback loop and the Akt-mTOR signaling pathway. Tumor Biol. 37, 12161–12168 (2016). https://doi.org/10.1007/s13277-016-5073-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5073-3