Abstract
Cisplatin-based chemotherapy is commonly used for cervical cancer treatment. However, the development of chemoresistance is considered the main obstacle to the effectiveness of this therapeutic agent. MicroRNAs are illustrated to play a major role in the regulation of cancer cell chemosensitivity. Therefore, this study was aimed to investigate the potential therapeutic role of miRNA-143 in combination with cisplatin on cervical cancer cells. Then, CaSki cell line with low expression levels of miRNA-143 was selected for functional experiments. The cells were treated with miRNA-143 and cisplatin individually or in combination. The cell viability and apoptosis induction were evaluated by MTT, Annexin V-FITC/PI, and DAPI staining tests. Cell migration was further evaluated by wound healing assay. The effect of miRNA-143 and cisplatin combination on gene expression was quantified by real-time PCR. Furthermore, the combination therapy effect on cell cycle progression and autophagy induction was also evaluated by flow cytometry. Our results showed that miRNA-143 overexpression could increase cisplatin-induced apoptosis and increase the sensitivity of CaSki cells to low doses of this chemotherapeutic agent via modulating the expression of apoptosis-related genes including Bcl-2, Bax, and caspase-9. Besides, miRNA-143 and cisplatin were demonstrated to cooperatively increase the cell cycle arrest at the sub-G1 and G2-M phases, induce autophagy activation, and via downregulation of vimentin inhibit CaSki cell migration. Moreover, c-Myc as an important regulator of cell growth was downregulated in treatment groups compared to the control. In conclusion, regarding that miRNA-143 could sensitize cervical cancer cells to cisplatin, it may be considered a promising therapeutic strategy for the treatment of this malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer, as a gynecological malignancy, is one of the most fatal carcinomas and the fourth most common cancer with an estimated 570,000 new cases in 2018 among women, worldwide [1]. In countries with lower socioeconomic status, due to the unavailability of extensive screening, cervical cancer is the main leading cause of cancer-related death in women. To date, despite the advances in the early diagnosis of cervical cancer, including screening procedures based on the Papanicolaou test (Pap test) [2, 3] and improvement of therapeutic strategies, the prognosis of advanced-stage cervical cancer remains poor [4]. According to numerous findings, the major reasons leading to treatment failure and deaths related to cervical cancer are metastasis, invasion, and development of drug resistance, which are intimately related to tumor progression [5]. Therefore, identifying novel molecular mechanisms that are involved in metastasis and chemo-responsiveness of cervical cancer could be helpful for the development of new treatment strategies.
Cisplatin or CDDP as a major anticancer chemotherapy drug is extensively used in the treatment of solid malignancies containing cervical, ovarian, bladder, head and neck, colorectal, and lung cancers. Basically, in the platinum-based drugs, covalent binding with DNA purine bases through forming intrastrand and interstrand DNA adducts inhibits DNA replication and transcription which in turn causes cell cycle arrest and apoptosis [6]. Conversely, it should be considered that cisplatin chemotherapy has some adverse side effects, including hepatotoxicity, nephrotoxicity, and neurotoxicity. Additionally, intrinsic resistance to cisplatin develops in advanced stages of cervical cancer which is considered the main obstacle in the treatment of this malignancy with cisplatin [4]. However, recent evidence has confirmed that the combination with other molecular agents could enhance anticancer activity and reduce side effects of chemotherapeutic drugs including cisplatin [7,8,9,10].
Recent studies have increased attention to the function of microRNAs (miRNAs) in various aspects of carcinogenesis [11,12,13,14]. miRNAs, as the highly conserved small noncoding RNAs with ~21–25 nucleotides length post-transcriptionally, regulate gene expression mostly through binding to 3’UTR of target mRNAs [15,16,17,18], leading to repression of translation or their destruction [19,20,21]. Also, miRNAs have been demonstrated to play critical roles in the regulation of fundamental biological processes [22, 23], including cell apoptosis, proliferation, migration, invasion, and so forth [1, 16]. Dysregulation of miRNAs was revealed to be associated with the development of several human malignancies, including cervical cancer, particularly through regulating the chemosensitivity of tumor cells [1, 24]. Considering their targets and consequent effects, miRNAs may act as tumor suppressors or oncogenes, which indicates their considerable potential as therapeutic targets in human cancers. Restoring the expression of a downregulated tumor suppressor miRNA to reinstate its normal function is one of the miRNA-based therapeutic methods [25]. This strategy, known as miRNA replacement therapy, which was shown to be effective in reducing the side effects and improvement of chemotherapeutic efficiency [19], is suggested as a promising approach for the development of cancer therapeutic procedures [1, 24]. Compared to conventional methods, miRNA replacement therapy possesses great advantages, including high efficiency, specificity, and cost-effectivity [26].
Accumulating evidence has demonstrated that miRNA-143, as a promising tumor suppressor, is involved in the development of various malignancies, including cervical cancer [16, 27,28,29]. miRNA-143 is one of the best-characterized short regulatory noncoding RNAs [30] located on human chromosome 5 [31], which is downregulated in a wide range of human cancers, including colorectal, prostate, ovarian, and cervical cancers [27, 32]. In particular, the downregulation of miRNA-143 was shown to be correlated with tumor size and lymph node metastasis of cervical cancer [33]. Furthermore, it has been established that miRNA-143 is downregulated in cervical cancer cells, including HeLa and SiHa cell lines, and its overexpression could suppress cervical cancer progression and inhibit cell migration and invasion [16]. Besides, miRNA-143 was shown to participate in chemosensitivity of multiple malignancies, including colorectal [34], gastric [35], and bladder [36] cancers. However, the potential roles of miRNA-143 in the chemosensitivity of cervical cancer cells have not yet been investigated.
Therefore, in this study, we evaluated the effects of miRNA-143 combined with cisplatin on cervical cancer cells. The obtained results showed that exogenous overexpression of miRNA-143 could increase the chemosensitivity of cervical cancer cells and enhanced cisplatin-induced apoptosis by modulating the expression of Bax, Bcl-2, and caspase-9 as apoptosis-related genes. Furthermore, miRNA-143 and cisplatin cooperatively induced cell cycle arrest, reduced c-Myc expression, and inhibited cell migration via downregulation of vimentin, indicating that miRNA-143 and cisplatin combination could be regarded as a novel promising therapeutic strategy for cervical cancer treatment.
Materials and Methods
Cell Culture
HeLa and CaSki human cervical cancer cell lines were purchased from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran) and Iranian Biological Resource Center (IBRC) and cultivated in RPMI medium (Gibco, USA) supplement with FBS (10%, Gibco), antibiotics containing streptomycin (100 μg/mL), and penicillin (100 IU/mL). Cultivation was performed in an incubator providing 5% CO2 and humidity. The detachment of cells was done using 0.25% Trypsin-EDTA (Gibco) which was then deactivated by the complete medium containing 10% FBS as the trypsin inhibitor. The cells were subcultured, as they reached 70% confluence. The cell lines were initially passaged three times to reach sharp cellular morphology and consistent cell performance (log phase), and then they were subjected to experiments.
miRNA Transfection
The CaSki cell line, because of the lower expression level of miRNA-143, was selected for consequent experiments. miRNA-143 mimics (GenePharma Co, Shanghai) were transfected into cervical cells (1 × 106 cells/500 μl electroporation buffer in a 0.2-cm cuvette) in different amounts of 10, 20, and 40 pmol using Gene Pulser electroporation system (Bio-Rad), according to the protocols supplied (TC = 12.5 ms and Volts = 160 v), and afterward a total of 2 × 105 of the transfected cells were seeded into six-well plates. After 48 hours of cultivation, miRNA-143 cytotoxic effect was evaluated using MTT assay as explained in the following sections to verify the optimum concentration of miRNA-143 for the following experiments. The cells transfected with miR-control were considered negative control. Furthermore, as explained above, the cells were also transfected with the optimum dose of miRNA-143 and FITC-conjugated control miRNA (GenePharma Co, Shanghai) and then subjected to qRT-PCR and MACSQuant Analyzer 10 flow cytometry (Miltenyi Biotec, Germany) assays, respectively, to evaluate miRNA-143 expression levels and transfection efficiency.
Extraction of RNA and qRT-PCR
Total RNA was extracted by GeneAll Trizol RNA extraction kit (Korea) according to supplied procedures. Once extracted, RNA purity and concentration were measured by absorbance at 260 nm and 280 nm wavelengths using the NanoDrop spectrophotometer (Thermo Fisher Scientific Life Sciences, USA). Also, total RNA was electrophoresed on 1% agarose gel to evaluate its integrity. To quantify miRNA-143 expression, 1 μg of extracted RNA was utilized for complementary DNA (cDNA) synthesis by the Universal cDNA Synthesis miRCURY LNATM kit. Moreover, to estimate the expression levels of target genes, cDNA synthesis was performed using RT Master Mix (Takara PrimeScript). Then expression levels of Bcl-2, Bax, caspase-9, vimentin, c-Myc, and miRNA-143 were determined using a BioFACT™ 2X Real-Time PCR Master Mix (Korea) in the StepOnePlus Real-Time PCR System (Applied Biosystems, USA). GAPDH and U6 were used as the internal controls to normalize the expression of target genes and miRNA-143, respectively [37, 38]. In Table 1, the sequences of the oligonucleotides are shown.
MTT Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was employed to determine the cell viability and the half-maximal inhibitory concentration (IC50) of cisplatin. Briefly, 1.2 × 104 of CaSki cells were cultured in 96-well plates and treated with different doses of cisplatin ranging from 0.01 to 10 μg/mL. After 24 hours of incubation, the cells were incubated with 2 mg/ml MTT solution (50 μl) for 4 hours. To solubilize MTT formazan crystals, the medium was aspirated and dimethyl sulfoxide (100 μL) was added. Then, after a half-hour incubation, using a microplate reader (Tecan, Switzerland), the absorbance of each well was measured at 570-nm wavelength. To evaluate whether miRNA-143 sensitizes CaSki cells to cisplatin treatment, MTT assay was also used. Then, the cells were first transfected with miRNA-143 and cultivated for 24 hours. Then, they were treated with various doses of cisplatin (0.01 to 10 μg/mL) and, after a further 24 hours of cultivation, were subjected to MTT assay. All experiments were carried out in triplicates. The obtained IC50 in the combination group (0.21 μg/ml) was considered the cisplatin treatment concentration for subsequent assessments. Cell viability was calculated by the following formula:
Wound Healing (Scratch) Assay
To investigate the inhibition of cell migration through miRNA-143 and cisplatin combination, wound healing (scratch) assay was performed. CaSki cells were transfected with miRNA-143 mimics and 2 × 105 of transfected cells were seeded into each well of 24-well plates and incubated. After 24 hours, the cells were treated with cisplatin. Subsequently, cellular monolayers were scratched with a sterile yellow pipette tip from the center of cultured cells. Using an inverted microscope (Optika, XDS-3, Italy), the migrated cells to the wound area were followed at 0, 12, 24, and 48 hours after scratching.
Apoptosis Assay
For the evaluation of the apoptosis induction, CaSki cells were transfected with miRNA-143 mimics and seeded into 6-well plates at a density of 2 × 105 cells per well. After 24 hours of incubation, the cells were treated with cisplatin. Negative controls were cells without treatment and transfection. After detachment with trypsin/EDTA, the cells were harvested and the rate of apoptosis was evaluated using Annexin V/PI staining kit (Exbio, Czech) according to supplied protocols.
Also, to further explore apoptosis induction in treatment groups, based on nuclear fragmentation and chromatin condensation, DAPI staining was employed. In brief, following seeding into 96-well plates and treatments, CaSki cells were fixed with 4% paraformaldehyde at 37 °C for 1 hour and washed with PBS buffer several times. Subsequently, the cells were permeablized with 0.01% Triton X-100 for 10 minutes and then washed again with PBS. The cells were stained with DAPI solution (Sigma-Aldrich, MO) in the dark for 10 minutes, and ultimately cell morphology was visualized using Cytation 5 (DAPI channel, BioTek).
Cell Cycle Assay
miRNA-143 and cisplatin combination effect on cell cycle progression was also followed by flow cytometry. Given that, CaSki cells were transfected with miRNA-143 mimics and cultured in 6-well plates (at a density of 2.5 × 105 cells/well) for 24 hours and then treated with cisplatin. After a further 24 hours of cultivation, the cells were harvested and fixed with 70% ethanol at 4 °C overnight. Then, the cells were suspended with 500 μl of cold PBS containing 10 μg/ml propidium iodide (Sigma-Aldrich, Germany) and 1 mg/ml RNase A and incubated for 30 minutes in a dark place at room temperature. Finally, cell cycle status was evaluated by flow cytometry and analyzed with FlowJo software (version 7.6, TreeStar Inc., USA).
Autophagy
To investigate the autophagy activation in treatment groups, MDC (monodansylcadaverine) staining was performed. MDC staining is a quick and convenient method to evaluate autophagy in vitro. MDC because of the dansyl residue conjugated to cadaverine is considered an autofluorescent compound emitting blue fluorescence that accumulates in acidic autophagic vacuoles. Accumulation of MDC in autophagic vacuoles is the consequence of ion trapping mechanism and specific interactions with membrane lipids which are rich in autophagic vacuoles [39, 40]. Then, CaSki cells were transfected with miRNA-143 and seeded into 6-well plates (2 × 105 cells/well) and, after 24 hours, treated with cisplatin and incubated. After 24 hours of incubation, the cells were washed with PBS and then stained with the MDC (50 μM). After 10 minutes of incubation, the cells were washed with PBS again, detached by trypsin, and harvested. Consequently, the samples were instantly analyzed by flow cytometry.
Statistical Analysis
All statistical analyses were performed by GraphPad Prism version 6.0 (San Diego, CA). Flow cytometry data analysis was performed using FlowJo software. All values were presented as means ± standard deviation. To determine the statistical significance of intergroup differences, the T-test and one-way ANOVA test were used. p value < 0.05 was considered statistically significant.
Results
miRNA-143 Expression in Cervical Cell Lines
Initially, the expression levels of miRNA-143 were investigated in two cervical cell lines, including HeLa and CaSki cells. QRT-PCR results (Fig. 1) revealed that the expression level of miRNA-143 in CaSki cells was significantly (p < 0.05) lower than that of HeLa cell line, suggesting that the malignant features of CaSki cells may be more influenced by miRNA-143 expression. So, to better follow miRNA-143 restoration effects, CaSki cell line was chosen for the next experiments.
miRNA-143 Was Transfected Efficiently into CaSki Cells
After the transfection of CaSki cells with selected doses of miRNA-143, we evaluated the optimum amount of miRNA for further transfections using MTT assay. Consequently, in comparison with the control, the considerably higher cytotoxic effect of miRNA-143 on CaSki cells was observed at 20 pmol (p < 0.0001) compared to 10 pmol (nonsignificant) and 40 pmol (p < 0.01). Then, 20 pmol of miRNA-143 was selected as its effective amount for further experiments. There was no significant difference between the viability of control cells and cells transfected with miR-control (Fig. 2a).
miRNA-143 transfection into CaSki cell line. a Evaluation of the optimum dose of miRNA-143 by MTT assay. The dose of 20 pmol compared to 10 and 40 pmol had a higher cytotoxic effect on CaSki cells, so selected for further transfections; *p < 0.05 and ***p < 0.001. b The efficiency of miRNA-143 transfection was evaluated by flow cytometry. The transfection ratio was estimated at 99.9%. c miRNA-143 expression levels after transfection were assessed using qRT-PCR, which indicated its significant upregulation compared to control; ****p < 0.0001. Data are presented as the mean ± SD of triplicated experiments
Moreover, we used flow cytometry and qRT-PCR to evaluate the transfection efficiency and expression levels of miRNA-143. By using flow cytometry (Fig. 2b), the transfection rate of FITC-conjugated control miRNAs into CaSki cells was estimated at 99.9%. Also, as shown in Fig. 2c, qRT-PCR confirmed flow cytometry results and showed increased expression levels of miRNA-143 in CaSki cells after transfection (p < 0.0001). Hereinafter, the optimal amount of miRNA-143 for transfection in all subsequent experiments was determined to be 20 pmol.
miRNA-143 Sensitized CaSki Cells to Cisplatin
To evaluate cell viability and cisplatin inhibitory concentrations, MTT assay was performed. CaSki cells were treated with a range of 0.01 to 10 μg/mL of cisplatin, which led to a constant decrease in the CaSki cell viability. 5.02 μg/mL concentration, which diminished cell viability to 50% compared to the control group, was considered as IC50 of cisplatin. To investigate whether miRNA-143 transfection in combination with cisplatin, affects the viability of CaSki cells, MTT assay was also employed. As shown in Fig. 3, our results indicated that restoration of miRNA-143 could considerably (p<0.05) decrease IC50 of cisplatin from 5.02 μg/mL in the separately treated cells to 0.21 μg/mL in the combination group.
miRNA-143 and Cisplatin Combination Effect on Cell Apoptosis
To determine if miRNA-143 and cisplatin combination affects apoptosis induction, we used V-FITC/PI assay and DAPI staining. The obtained results established that miRNA-143 transfection and cisplatin treatment were separately able to significantly (p < 0.0001) induce apoptosis in CaSki cells. However, as depicted in Fig. 4, combination therapy increased cell apoptosis rate more effectively (p < 0.0001) compared to miRNA-143 and cisplatin treatment alone.
The effects of miRNA-143 and cisplatin combination on CaSki cell apoptosis. a The apoptosis induction in different treatment groups was evaluated via V-FITC/PI assay. b The ratio of apoptotic cells was calculated. The results showed that miRNA-143 could increase cisplatin-induced apoptosis. The results were signified as the mean ± SD (triplicated); ****p < 0.0001. c Chromatin fragmentation was followed by DAPI staining, which confirmed the apoptosis results achieved by flow cytometry
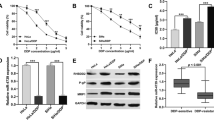
Furthermore, to investigate underlying mechanisms, we used real-time PCR and examined the expression levels of apoptosis modulators. Our results (Fig. 5) showed that Bax and caspase-9 as pro-apoptotic genes were upregulated in miRNA-143-transfected (p < 0.001 and p < 0.0001, respectively) and cisplatin-treated cells (p < 0.0001 and p < 0.0001, respectively) in comparison with the untreated-untransfected group. The upregulation of these genes in the combination group was significantly (p < 0.0001) higher than that of separately treated cells. Also, the Bcl-2 expression levels as a pro-survival gene were downregulated in treatment groups compared to the control (p < 0.0001). However, the lowest levels of Bcl-2 were observed through combination therapy compared to miRNA-143 transfection (p < 0.001) or cisplatin treatment (p < 0.0001) alone.
Combination of miRNA-143 and Cisplatin Inhibited CaSki Cell Migration
Wound healing assay was used to investigate miRNA-143 and cisplatin combination effect on cell migration. According to Fig. 6a–b, the obtained results demonstrated a significant reduction in the migration of miR-143-transfected cells compared to control cells. Also, cisplatin treatment was separately able to decrease CaSki cell migration rate. Results also indicated that 48 hours after wound creation, combination therapy shows a lower rate of migration compared to CaSki cells separately transfected with miRNA-143 (p < 0.01) or treated with cisplatin (p < 0.0001).
The combined effect of miRNA-143 and cisplatin on the inhibition of CaSki cell migration. a To investigate cell migration in treatment groups, a scratch assay was performed. b A graph presenting the number of cells migrated to wound area in treatment groups. c Analysis of vimentin gene expression levels via qRT-PCR. Each reaction was done in triplicate and the data were shown as the mean ± SD; **p < 0.01 and ****p < 0.0001
Moreover, to verify wound healing assay results, we assessed the vimentin expression levels as a metastasis-related gene and the direct target of miRNA-143. According to Fig. 6c, the expression levels of vimentin was downregulated in cells individually treated with miRNA-143 (p < 0.0001) or cisplatin (p < 0.0001) compared to the untreated group. Besides, the combination therapy could decrease vimentin expression more effectively than separate treatment with miRNA-143 (p < 0.01) and cisplatin (p < 0.0001).
Combination of miRNA-143 Regulates CaSki Cell Cycle Progression
Flow cytometry analysis was also employed to investigate the effects of miRNA-143 and cisplatin combination on cell cycle progression. Our results established that, compared to untreated-untransfected cells as negative controls, miRNA-143 upregulation arrested CaSki cells at the sub-G1 and G2-M phases. miRNA-143 increased the sub-G1 phase arrested cell percentage from 1.81 to 5.50% (p < 0.05) and G2-M phase cell population from 15.4 to 20.2% (p < 0.05). Also, treatment with cisplatin led to an increase in the population of sub-G1-phase cells by 1.81 to 9.78% (p < 0.05) and G2-M phase cells by 15.4 to 19.6% (p < 0.05) compared to controls. However, miRNA-143 in combination with cisplatin increased the percentage of sub-G1 and G2-M phase arrested cells from 1.81 to 18.3% (p < 0.05) and 15.4 to 27.1% (p < 0.05), respectively (Fig. 7a and b).
a The combined effect of miRNA-143 and cisplatin on the cell cycle was investigated by flow cytometry. The results showed that miRNA-143 and cisplatin cooperatively arrested the cell cycle at the sub-G1 and G2-M phases through treatments. b A graph representing cell cycle status in treatment groups. c c-Myc gene expression levels were evaluated using qRT-PCR in treatment groups. The data were signified as the mean ± SD (n = 3); *p < 0.05, ***p < 0.001, and ****p < 0.0001
To further investigate how miRNA-143 and cisplatin combination affects CaSki cell growth and proliferation, c-Myc expression levels, as the direct target of miRNA-143 and an important transcription factor regulating cell growth and proliferation, were evaluated. As demonstrated in Fig. 7c, miRNA-143 and cisplatin cooperatively (p < 0.0001) downregulated the expression of c-Myc in treatment groups compared to the controls. The expression levels of c-Myc were significantly lower in the combination group compared to cells separately transfected with miRNA-143 (p < 0.05) or treated with cisplatin (p < 0.001).
miRNA-143 and Cisplatin Combination Effect on Autophagy
We also used flow cytometry to detect the cells labeled with MDC and subsequently investigate the autophagy induction. As shown in Fig. 8a and b, the autophagy positive cell percentage (MDC POS) in miRNA-143-transfected and cisplatin-treated groups was significantly (p < 0.05 and p < 0.0001, respectively) increased to 3.52% and 9.19% compared to control (1.60%). Furthermore, combination therapy enhanced (p < 0.0001) the percentage of MDC POS cells to 15.1% in comparison with control. Thus, these results illustrated that miRNA-143 in combination with cisplatin could increase autophagy induction in CaSki cells compared to individually treated cells and control.
The combined effect of miRNA-143 and cisplatin on autophagy induction in CaSki cells. a Percentage of MDC positive cells was represented by flow cytometry analysis, showing that autophagy induction was increased through miRNA-143 and cisplatin combination compared to control and separate treatments. b Graph of autophagy induction rates in treatment groups. The data were presented as the mean ± SD of triplicated experiments; *p < 0.05, ****p < 0.0001
Discussion
One of the major concerns related to cancer is finding an effective treatment method. Subsequently, several types of treatment strategies have been suggested for cancer therapy, including chemotherapy as a common therapeutic strategy. Cisplatin, a platinum-based drug, is a major clinical chemotherapeutic agent used for cervical cancer. Despite tremendous efforts in cisplatin-based treatment, drug resistance is a major obstacle related to this therapeutic strategy [41]. Therefore, one of the critical points in the improvement of cervical cancer treatment is finding the molecular pathways or effectors that regulate the chemosensitivity of tumor cells [38]. Recent studies have explained the imperative functions of miRNAs in the regulation of genes involved in multiple biological processes, including drug resistance and responsiveness. miRNAs based on their expression patterns and target mRNAs are divided into two groups: the downregulated miRNAs in tumor cells, known as tumor suppressor miRNAs, and upregulated miRNAs, identified as oncomiRs [42]. It is broadly reported that the expression of miRNAs is dysregulated through malignancies, which is considered one of the main reasons for tumorigenesis, indicating the great potential of miRNAs as novel diagnostic, prognostic, and therapeutic targets. As previously reported, microRNA-143 as an anti-metastatic tumor suppressor miRNA is downregulated in cervical cancer and plays a remarkable role in the chemoresistance of multiple malignancies. Its downregulation was correlated with HPV16 infection, tumor size, and lymph node metastasis in cervical cancer patients [33]. Restoring miRNA-143 expression was also shown to promote apoptosis and inhibit tumor progression in cervical cancer cells via directly targeting Bcl-2 [27]. Furthermore, overexpression of miRNA-143 in gastric cancer cells was reported to inhibit cell proliferation and sensitize these cells to cisplatin-induced apoptosis [43]. Also, miRNA-143 was illustrated to be downregulated in the human bladder cancer tissues and cells, and its overexpression inhibited cell proliferation and enhanced the chemosensitivity of bladder cancer cells to gemcitabine [36], indicating that miRNA-143 plays an important role in drug responsiveness of malignant cells. However, the miRNA-143 effect on cervical cancer cell sensitivity to chemotherapy has not yet been investigated. Given that, in this study, the effect of miRNA-143 transfection in combination with cisplatin was examined.
According to MTT assay, our results indicated that miRNA-143 could increase the chemosensitivity of CaSki cells to cisplatin treatment. In other words, miRNA-143 and cisplatin combination decreased CaSki cell viability and proliferation more than cisplatin treatment alone. Consistently, miRNA-143 was previously reported to play a significant role in the regulation of cell survival and proliferation. It was established that miRNA-143 could inhibit cell proliferation and reduce cell survival rates in breast [44] and lung cancers [45] by targeting ERBB3 and ATG2B, respectively. Besides, miR-143 overexpression could downregulate HIF-1α expression levels and reduce the proliferation of HeLa cervical cancer cells [46]. More importantly, miRNA-143 was illustrated to diminish CRC cell survival and proliferation and sensitized these cells to 5-FU treatment as well [47]. Further analysis demonstrated that miRNA-143 could enhance cisplatin-induced apoptosis and by which increase the cisplatin cytotoxic effect on cervical cancer cells. To illustrate the underlying mechanism, we also evaluated the expression levels of genes involved in the apoptosis pathways. Bcl-2 gene family, including Bcl-2 as an anti-apoptotic regulator protein, and Bax as a pro-apoptotic regulator, and also caspase-9 as the initiator caspase of the intrinsic apoptosis pathway can modulate cell death through various mechanisms [48]. Our results showed that the overexpression of miRNA-143 separately or in combination with cisplatin could downregulate the expression of Bcl-2, as its previously validated direct target, and increase Bax and caspase-9 expression levels. Consistently, previous studies have illustrated that caspase-9 overexpression could lead to its activation and apoptosis induction in HeLa cells [49]. Zhang and colleagues also previously identified Bcl-2 as the direct target of miRNA-143 and demonstrated that restoration of this miRNA decreased cell viability, promoted cell apoptosis, and suppressed tumorigenesis in osteosarcoma via targeting Bcl-2 [50]. Moreover, the expression levels of Bcl-2 were shown to be negatively correlated with miRNA-143 levels in HeLa cells, revealing a molecular linkage between miRNA-143 and Bcl-2, as one of the mechanisms by which the downregulation of miRNA-143 may be involved in the pathogenesis of cervical cancer [32]. As mentioned, in agreement with our results, miRNA-143 via targeting Bcl-2 was also demonstrated to increase the sensitivity of gastric cancer and bladder cancer cells to cisplatin and gemcitabine, respectively [36, 43]. Shen et al. suggested that miRNA-143 possesses a great therapeutic potential in leukemia and could induce apoptosis and inhibit cell proliferation through the regulation of Bcl-2 and caspase-9 [51]. Furthermore, Zhang et al.’s studies on human epithelial cancers confirmed that miRNA-143 could induce apoptosis and inhibit tumor cell growth through the upregulation of Bax protein levels [52]. Therefore, it could be concluded that miRNA-143 could induce apoptosis and increase the chemosensitivity of cervical cancer cells via modulating major regulators of apoptosis pathways.
Furthermore, the results of scratch assay confirmed the remarkable reduction in the migration ability of CaSki cells through the combination of miRNA-143 and cisplatin compared to separate treatments. These results confirmed the connection between miRNA-143 expression level and the anti-migratory effect of cisplatin on cervical cancer cells. Consequently, we measured the expression levels of vimentin as a metastasis promoter in treatment groups to additionally investigate the mechanism by which miRNA-143 may function as an anti-metastasis agent in cervical cancer. Our data showed that vimentin was downregulated through the treatment of cells with miRNA-143 and cisplatin, separately or in combination. Vimentin as a member of cytoskeletal proteins and the mesenchymal intermediate filament is involved in cellular pathways regulating chemoresistance and metastasis. Consequently, it was shown that the expression of vimentin in chemoresistant pancreatic cancer cell lines was remarkably higher than that of drug-sensitive cell lines, suggesting its potential role in cancer cell drug resistance [53]. Its overexpression was also shown to be involved in the epithelial-mesenchymal transition (EMT) process which leads to tumorigenic events, including cell migration and invasion [54]. Furthermore, it was demonstrated that vimentin is overexpressed in cervical cancer tissues and its upregulation was correlated with lymph node metastasis and lymphatic invasion [55]. In agreement with this study, miRNA-143 was also demonstrated to inhibit cell migration by reduction of vimentin expression in breast adenocarcinoma cells as well [26].
Recent studies have demonstrated that miRNAs, including miR-143, play an imperative role in the regulation of cell cycle progression in cancer cells [56, 57]. It was shown that miRNA-143 could directly target CDK6 and regulate cell cycle progression through modulating the expression of cyclin D1, p-Rb, p21Cip1, and p27Kip1 in nasopharyngeal carcinoma cells [58]. Given that, to determine the antigrowth function of miRNA-143 and cisplatin combination in cervical cancer, we also investigated cell cycle status in treatment groups. According to achieved results, we illustrated that miRNA-143 alone or in combination with cisplatin led to cell cycle arrest at the sub-G1 and G2-M phases, suggesting that miRNA-143 could inhibit CaSki cell proliferation through induction of cell cycle arrest at these phases. Consistent with our results, Liu et al. found that miRNA-143 mimics could also decrease cell proliferation and induce the G0/G1 cell cycle arrest in HepG2 cells via targeting TLR2 [59]. Also, Peng Zhou et al. demonstrated an inhibitory effect of miRNA-143 on cell proliferation by cell cycle arrest at the G1/S transition in PC-3 human prostate cancer cells [60]. It was also established that miRNA-143 upregulation significantly decreased the proliferation rate of human gastric epithelium cells; indeed, miRNA-143 was illustrated to modulate cisplatin resistance of gastric cancer cells via targeting Bcl-2 and IGF1R signaling pathway as the well-characterized pathway in cell proliferation [43]. Guoping et al. also confirmed that exogenous overexpression of miRNA-143 in gastric cancer cells could inhibit cell proliferation and induce cell cycle arrest at the G0/G1 phase [61].
Moreover, the antigrowth function of miRNA-143 was investigated through the evaluation of c-Myc expression levels in treatment groups. c-Myc as a promoter of cell proliferation and inhibitor of cell differentiation is one of the commonly activated oncogenes involved in 20% of all human cancers [62]. QRT-PCR results showed the significant downregulation of c-Myc expression in cells treated with miRNA-143 and cisplatin alone; however, in combination therapy, the lowest expression of c-Myc was observed. In confirmation of our results, Zhu et al. performed a study about the contribution of epidermal growth factor receptors (EGFR) to tumorigenesis of colon cancer in vivo and in vitro. Their results illustrated that the transfection of miRNA-143 inhibited HCT116 cell growth via the downregulation of Myc as its direct target [63]. Furthermore, forced expression of miR-143 was reported to suppress c-Myc expression and inhibit in vivo development of small intestine tumors in ApcMin/+ mice [64].
Autophagy as an evolutionarily conserved catabolic process occurs in different cancers depends on tumor type. However, its function in cancer cells is not well determined and its relationship with miRNAs and anticancer therapy resistance is moderately complicated [65]. Autophagy as a caspase-independent cell death mechanism is suggested one of the important regulators involved in sensitizing cells to chemotherapy agents in certain cancers. Therefore, autophagy-dependent cell death could potentially enhance the efficiency of chemotherapy treatment in cancer cells [66]. So, we investigated the function of miRNA-143 in combination with cisplatin, in the induction of autophagy in CaSki cells. Our results also showed that miRNA-143 in combination with cisplatin could increase autophagy induction compared to the control, indicating that miRNA-143 functions in the regulation of autophagy in cervical cancer cells.
Conclusions
In summary, as depicted in Fig. 9, our results showed that restoration of miRNA-143 expression could induce apoptosis and increase the sensitivity of CaSki cells to cisplatin treatment via modulating the expression of apoptosis-related genes, including caspase-9, Bax, and Bcl-2. Besides, miRNA-143 and cisplatin were demonstrated to cooperatively increase the cell cycle arrest at the sub-G1 and G2-M phases. Furthermore, miRNA-143 combined with cisplatin inhibited the migration of CaSki cells via the downregulation of vimentin. Moreover, the expression levels c-Myc, as an important regulator of cell cycle and growth, was also reduced in treatment groups compared to the control, indicating the potential antiproliferative role of miRNA-143 in cervical cancer. Therefore, our findings suggested that miRNA-143 and cisplatin combination as a promising therapeutic strategy for the effective treatment of cervical cancer, demanding additional studies, including in vivo studies and clinical trials to further clarify underlying mechanisms and verify the value of this combination therapy as a potential therapeutic strategy.
References
Nahand JS, Taghizadeh-boroujeni S, Karimzadeh M, Borran S, Pourhanifeh MH, Moghoofei M, et al. microRNAs: new prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. J Cell Physiol. 2019;234(10):17064–99.
Rossetti D, Vitale SG, Tropea A, Biondi A, Lagana AS. New procedures for the identification of sentinel lymph node: shaping the horizon of future management in early stage uterine cervical cancer. Updat Surg. 2017;69(3):383–8.
Valenti G, Vitale SG, Tropea A. Tumor markers of uterine cervical cancer: a new scenario to guide surgical practice? Updat Surg. 2017;69:441–9.
Leekha A, Gurjar BS, Tyagi A, Rizvi MA, Verma AK. Vitamin C in synergism with cisplatin induces cell death in cervical cancer cells through altered redox cycling and p53 upregulation. J Cancer Res Clin Oncol. 2016;142(12):2503–14.
Wang L, Dai G, Yang J, Wu W, Zhang W. Cervical Cancer Cell Growth, Drug Resistance, and Epithelial-Mesenchymal Transition Are Suppressed by γ-Secretase Inhibitor RO4929097. Med Sci Monit. 2018;24:4046.
Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–84.
Asadzadeh Z, Mansoori B, Mohammadi A, Aghajani M, Haji-Asgarzadeh K, Safarzadeh E, et al. microRNAs in cancer stem cells: Biology, pathways, and therapeutic opportunities. J Cell Physiol. 2019;234(7):10002–17.
Jahanafrooz Z, Motamed N, Rinner B, Mokhtarzadeh A, Baradaran B. Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci. 2018;213:236–47.
Magee P, Shi L, Garofalo M. Role of microRNAs in chemoresistance. Ann Transl Med. 2015;3(21):332.
Yu M, Xu B, Yang H, Xue S, Zhang R, Zhang H, et al. MicroRNA-218 regulates the chemo-sensitivity of cervical cancer cells through targeting survivin. Cancer Manag Res. 2019;11:6511–9.
Babaei K, Shams S, Keymoradzadeh A, Vahidi S, Hamami P, Khaksar R, et al. An insight of microRNAs performance in carcinogenesis and tumorigenesis; an overview of cancer therapy. Life Sci. 2020;240:117077.
Osada H, Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis. 2007;28(1):2–12.
Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33(6):1126–33.
Rezaei T, Hejazi M, Mansoori B, Mohammadi A, Amini M, Mosafer J, et al. microRNA-181a mediates the chemo-sensitivity of glioblastoma to carmustine and regulates cell proliferation, migration, and apoptosis. Eur J Pharmacol. 2020;888:173483.
Rezaei T, Amini M, Hashemi ZS, et al. microRNA-181 serves as a dual-role regulator in the development of human cancers. Free Radic Biol Med. 2020;152:432–454.
Zhou M, Chen X, Wu J, He X, Ren R. MicroRNA-143 regulates cell migration and invasion by targeting GOLM1 in cervical cancer. Oncol Lett. 2018;16(5):6393–400.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24.
Kian R, Moradi S, Ghorbian S. Role of components of microRNA machinery in carcinogenesis. Exp Oncol. 2018;40(1):2–9.
Lee J-W, Kim B-G, Bae D-S. MicroRNAs in cervical carcinoma. In: MicroRNAs in Cancer Translational Research: Springer; 2011. pp. 189–99.
Viegas SC, Arraiano CM. Regulating the regulators: how ribonucleases dictate the rules in the control of small non-coding RNAs. RNA Biol. 2008;5(4):230–43.
Prabhakar B, Zhong X, Rasmussen TP. Focus: drug development: exploiting long noncoding RNAs as pharmacological targets to modulate epigenetic diseases. Yale J Biol Med. 2017;90(1):73–86.
Ghasabi M, Mansoori B, Mohammadi A, Duijf PHG, Shomali N, Shirafkan N, et al. MicroRNAs in cancer drug resistance: Basic evidence and clinical applications. J Cell Physiol. 2019;234(3):2152–68.
Wang J, Tong KS, Wong LL, Liew O-W, Raghuram D, Richards AM, et al. MicroRNA-143 modulates the expression of Natriuretic Peptide Receptor 3 in cardiac cells. Sci Rep. 2018;8(1):1–11.
Gambari R, Brognara E, Spandidos DA, Fabbri E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Νew trends in the development of miRNA therapeutic strategies in oncology. Int J Oncol. 2016;49(1):5–32.
Hosseinahli N, Aghapour M, Duijf PHG, Baradaran B. Treating cancer with microRNA replacement therapy: A literature review. J Cell Physiol. 2018;233(8):5574–88.
Tavanafar F, Safaralizadeh R, Hosseinpour-Feizi MA, Mansoori B, Shanehbandi D, Mohammadi A, et al. Restoration of miR-143 expression could inhibit migration and growth of MDA-MB-468 cells through down-regulating the expression of invasion-related factors. Biomed Pharmacother. 2017;91:920–4.
Zhang L, Zhang X, Zhang X, Lu Y, Li L, Cui S. MiRNA-143 mediates the proliferative signaling pathway of FSH and regulates estradiol production. J Endocrinol. 2017;234(1):1–14.
Li J, Liu Q, Clark LH, Qiu H, Bae-Jump VL, Zhou C. Deregulated miRNAs in human cervical cancer: functional importance and potential clinical use. Future Oncol. 2017;13(8):743–53.
Hatziapostolou M, Polytarchou C, Iliopoulos D. miRNAs link metabolic reprogramming to oncogenesis. Trends Endocrinol Metab. 2013;24(7):361–73.
Vacante F, Denby L, Sluimer JC, Baker AH. The function of miR-143, miR-145 and the MiR-143 host gene in cardiovascular development and disease. Vasc Pharmacol. 2019;112:24–30.
Wei Y-S, Xiang Y, Liao P-H, Wang J-L, Peng Y-F. An rs4705342 T> C polymorphism in the promoter of miR-143/145 is associated with a decreased risk of ischemic stroke. Sci Rep. 2016;6(1):1–6.
Liu L, Yu X, Guo X, Tian Z, Su M, Long Y, et al. miR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol Med Rep. 2012;5(3):753–60.
Chen Y, Ma C, Zhang W, Chen Z, Ma L. Down regulation of miR-143 is related with tumor size, lymph node metastasis and HPV16 infection in cervical squamous cancer. Diagn Pathol. 2014;9(1):88.
Qian X, Yu J, Yin Y, He J, Wang L, Li Q, et al. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12(9):1385–94.
Du F, Feng Y, Fang J, Yang M. MicroRNA-143 enhances chemosensitivity of Quercetin through autophagy inhibition via target GABARAPL1 in gastric cancer cells. Biomed Pharmacother. 2015;74:169–77.
Wang H, Li Q, Niu X, Wang G, Zheng S, Fu G, et al. miR-143 inhibits bladder cancer cell proliferation and enhances their sensitivity to gemcitabine by repressing IGF-1R signaling. Oncol Lett. 2017;13(1):435–40.
Jana SK, Banerjee P, Mukherjee R, Chakravarty B, Chaudhury K. HOXA-11 mediated dysregulation of matrix remodeling during implantation window in women with endometriosis. J Assist Reprod Genet. 2013;30(11):1505–12.
Hejazi M, Baghbani E, Amini M, Rezaei T, Aghanejad A, Mosafer J, et al. MicroRNA-193a and taxol combination: A new strategy for treatment of colorectal cancer. J Cell Biochem. 2020;121(2):1388–99.
Murugan S, Amaravadi RK. Methods for Studying Autophagy Within the Tumor Microenvironment. Adv Exp Med Biol. 2016;899:145–166.
Pattingre S, Petiot A, Codogno P. Analyses of Galpha-interacting protein and activator of G-protein-signaling-3 functions in macroautophagy. Methods Enzymol. 2004;390:17–31.
Ghasabi M, Majidi J, Mansoori B, Mohammadi A, Shomali N, Shirafkan N, et al. The effect of combined miR-200c replacement and cisplatin on apoptosis induction and inhibition of gastric cancer cell line migration. J Cell Physiol. 2019;234(12):22581–92.
Ghazanchaei A, Mansoori B, Mohammadi A, Biglari A, Baradaran B. Restoration of miR-152 expression suppresses cell proliferation, survival, and migration through inhibition of AKT–ERK pathway in colorectal cancer. J Cell Physiol. 2019;234(1):769–76.
Zhuang M, Shi Q, Zhang X, Ding Y, Shan L, Shan X, et al. Involvement of miR-143 in cisplatin resistance of gastric cancer cells via targeting IGF1R and BCL2. Tumor Biol. 2015;36(4):2737–45.
Yan X, Chen X, Liang H, Deng T, Chen W, Zhang S, et al. miR-143 and miR-145 synergistically regulate ERBB3 to suppress cell proliferation and invasion in breast cancer. Mol Cancer. 2014;13(1):1–14.
Wei J, Ma Z, Li Y, Zhao B, Wang D, Jin Y, et al. miR-143 inhibits cell proliferation by targeting autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol Med Rep. 2015;11(1):571–6.
Zhao Y, Liu X, Lu YX. MicroRNA-143 regulates the proliferation and apoptosis of cervical cancer cells by targeting HIF-1alpha. Eur Rev Med Pharmacol Sci. 2017;21(24):5580–6.
BorralhoPM K, Castro RE, da Silva IBM, Steer CJ, Rodrigues CMP. MicroRNA-143 reduces viability and increases sensitivity to 5-fluorouracil in HCT116 human colorectal cancer cells. FEBS J. 2009;276(22):6689–700.
Reed JC. Bcl-2 family proteins. Oncogene. 1998;17(25):3225–36.
Druškovič M, Šuput D, Milisav I. Overexpression of caspase-9 triggers its activation and apoptosis in vitro. Croat Med J. 2006;47(6):830–2.
Zhang H, Cai X, Wang Y, Tang H, Tong D, Ji F. microRNA-143, down-regulated in osteosarcoma, promotes apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol Rep. 2010;24(5):1363–9.
Shen J-Z, Zhang Y-Y, Fu H-Y, Wu D-S, Zhou H-R. Overexpression of microRNA-143 inhibits growth and induces apoptosis in human leukemia cells. Oncol Rep. 2014;31(5):2035–42.
Zhang J, Sun Q, Zhang Z, Ge S, Han ZG, Chen WT. Loss of microRNA-143/145 disturbs cellular growth and apoptosis of human epithelial cancers by impairing the MDM2-p53 feedback loop. Oncogene. 2013;32(1):61–9.
Lee J-G, McKinney KQ, Hwang S-I. Proteomic Differences and Linkages between Chemoresistance and Metastasis of Pancreatic Cancer Using Knowledge-Based Pathway Analysis. In: Molecular Diagnostics and Treatment of Pancreatic Cancer. Elsevier; 2014. pp. 221–44.
Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033–46.
Lin J, Lu J, Wang C, Xue X. The prognostic values of the expression of Vimentin, TP53, and Podoplanin in patients with cervical cancer. Cancer Cell Int. 2017;17(1):80.
Hydbring P, Wang Y, Fassl A, Li X, Matia V, Otto T, et al. Cell-cycle-targeting MicroRNAs as therapeutic tools against refractory cancers. Cancer Cell. 2017;31(4):576–90.
Hirakawa T, Nasu K, Abe W, Aoyagi Y, Okamoto M, Kai K, et al. miR-503, a microRNA epigenetically repressed in endometriosis, induces apoptosis and cell-cycle arrest and inhibits cell proliferation, angiogenesis, and contractility of human ovarian endometriotic stromal cells. Hum Reprod. 2016;31(11):2587–97.
He B, Xu Z, Chen J, Zheng D, Li A, Zhang L. Upregulated microRNA-143 inhibits cell proliferation in human nasopharyngeal carcinoma. Oncol Lett. 2016;12(6):5023–8.
Liu X, Gong J, Xu B. miR-143 down-regulates TLR2 expression in hepatoma cells and inhibits hepatoma cell proliferation and invasion. Int J Clin Exp Pathol. 2015;8(10):12738.
Zhou P, Chen W-G, Li X-W. MicroRNA-143 acts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am J Cancer Res. 2015;5(6):2056–63.
Guoping M, Ran L, Yanru Q. miR-143 inhibits cell proliferation of gastric cancer cells through targeting GATA6. Oncol Res. 2018;26(7):1023–9.
Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16(4):253–264.
Zhu H, Dougherty U, Robinson V, Mustafi R, Pekow J, Kupfer S, et al. EGFR signals downregulate tumor suppressors miR-143 and miR-145 in western diet–promoted murine colon cancer: role of G1 regulators. Mol Cancer Res. 2011;9(7):960–75.
Takaoka Y, Shimizu Y, Hasegawa H, et al. Forced expression of miR-143 represses ERK5/c-Myc and p68/p72 signaling in concert with miR-145 in gut tumors of Apc(Min) mice. PLoS One. 2012;7(8):e42137.
Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4(10):e838.
Bialik S, Dasari SK, Kimchi A. Autophagy-dependent cell death–where, how and why a cell eats itself to death. J Cell Sci. 2018;131(18):jcs215152.
Acknowledgment
The authors would like to thank Immunology Research Center, Tabriz University of Medical Sciences, and Urmia University for supporting the work.
Funding
We acknowledge the supports from the Immunology Research Center, Tabriz University of Medical Science (Grant no. 65542).
Author information
Authors and Affiliations
Contributions
Y.B.E. carried out the majority of experiments and data analysis. MA.D. assisted in the experiments and interpreted the results; Y.B.E. wrote the manuscript. M.A. revised the manuscript and contributed to carry out the molecular assays; B.B. revised the work critically for important intellectual content; SH.J. and N.M. contributed to cellular assay and statistical analysis. M. M. and A.M. designed and conducted the project.
Corresponding authors
Ethics declarations
Ethical consents
It’s not applicable for this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Esfandyari, Y.B., Doustvandi, M.A., Amini, M. et al. MicroRNA-143 Sensitizes Cervical Cancer Cells to Cisplatin: a Promising Anticancer Combination Therapy. Reprod. Sci. 28, 2036–2049 (2021). https://doi.org/10.1007/s43032-021-00479-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00479-5