Abstract
Prostate carcinoma (PC) is one of the most common cancers for males. However, the molecular mechanisms of PC progression are still to be uncovered. MicroRNA (miRNA) has been shown to be associated with the initiation and progression of prostate cancer. Among the identified tumor-promoting miRNAs, miR-96 has been well established to contribute to PC by reducing FOXO1 expression. This study is aimed to study if miR-96 can promote the progression of PC through other pathways. Our data reinforced the finding that the level of miR-96 was higher in PC samples and cell lines than in non-cancerous tissues and normal prostate epithelial cells. In addition, serum miR-96 abundance was also found to be elevated in PC patients. Decreasing miR-96 expression was able to suppress the proliferation, clonogenicity, and invasion of PC cells. Overexpressing miR-96 led to increased proliferation and colony formation of normal prostate epithelial cells. miR-96 level was found to be inversely associated with the abundance of metastasis suppressor protein 1 (MTSS1) messenger RNA (mRNA), which has been proved to be a tumor suppressor for PC. Predictive analysis indicated that there was a potential miRNA response elements (MREs) located within 3′UTR of MTSS1 mRNA. The changes in miR-96 expression can affect the levels of MTSS1 both at mRNA and protein levels. miR-96 also suppressed the activity of luciferase reporter under the regulation of 3′UTR of MTSS1. Further studies showed that MTSS1 restoration accounted for the effect of miR-96 reduction on PC cells. The overexpression of a recombinant MTSS1 resistant against miRNA regulation was also demonstrated to abolish the transforming effect of miR-96 on prostate epithelial cells. Taken together, we found that miR-96 has a higher abundance in serum samples of PC patients than healthy controls, implying that it may be used as a prognostic marker. MTSS1 is a new authentic target of miR-96 in PC. The above findings suggested that targeting miR-96 may be a promising strategy for PC treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate carcinoma (PC) is the most common malignancy of male urological systems and severely risks human health [1]. However, the molecular mechanisms underlying PC are still not completed explored yet.
As evolutionarily conserved non-coding RNA, microRNAs (miRNAs) can negatively regulate the expression of specific gene. The mechanisms involve the degradation of their messenger RNA (mRNA) or the inhibition of mRNA translation based on the binding between miRNAs and miRNA response elements (MREs) located with their 3′UTR [2]. miRNA has been well documented to participate in the development and homeostasis of mammals [3]. Recently, the implication of miRNA in the initiation, progression, metastasis, and recurrence of cancers has also been verified by several studies [4]. For instance, miR-96 is a well-recognized oncogenic miRNA in a variety of cancers [5, 6]. For prostate cancers, overexpression of miR-96 was shown to promote the survival, proliferation, and clonogenicity of PC cells [7–9]. Further studies revealed that FOXO1 is the downstream target that mediates the effect of miR-96 on PC [7–9].
However, it is still unknown if tumor suppressors other than FOXO1 are also involved with the action of miR-96 on PC cells. Thus, this study is aimed to identify new downstream targets of miR-96 that can mediate its effect on PC, in order to facilitate the molecular mechanism of PC progression.
Materials and methods
Cell culture
PNT2 human normal prostate epithelium cell line was purchased from Sigma Aldrich. PC3, LNCaP, and HEK293 cells were purchased from American Type Culture Collection. All the cells were cultured with RPMI 1640 media supplemented with 10 % FBS, 4 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5 % CO2 and humidified atmosphere at 37 °C.
PC specimen
Malignant PC and their corresponding noncancerous tissues were studied in this study. These samples were obtained from the PC patients with their written informed consent following the procedures approved by Ethical Review Board in China-Japan Union Hospital, Jilin University, Changchun (Changchun, China). These patients were subjected to surgery at Department of Urology, China-Japan Union Hospital, Jilin University, Changchun (Changchun, China).
Serum collection
We collected serum samples following the procedures previously described [10]. Briefly, blood were collected from vena and transferred into serum collection tubes. The samples were centrifuged at 3000g for 10 min at 4 °C, after 1 h coagulation at room temperature. Then, the serum samples were transferred into new tubes and stored at −80 °C for further analysis.
qPCR assay
The procedures for qPCR assay in this study were described previously [11]. Briefly, total RNA molecules were isolated from PC and non-cancerous tissues, cell lines, and serum samples with Trizol solution (Sigma Aldrich) according to manufacturer’s instructions. Reverse transcription reaction was done with All-in-One™ First-Strand cDNA Synthesis Kit (AORT-0020, GeneCopoeia) according to the manufacturer’s protocols. Quantitative PCR (qPCR) assay was performed with All-in-One™ miRNA qRT-PCR Detection Kit (AOMD-Q020, GeneCopoeia) with CFX96™ Real-Time PCR Detection System supplied with analytical software (Bio-Rad). U6 was selected as endogenous reference. The primers and probes for miR-96 and U6 were obtained from GeneCopoeia.
To determine the mRNA levels of MTSS1, total RNA was transcribed into complementary DNAs (cDNAs) with Rever Tra Ace qPCR RT Kit (Toyobo, Japan) according to the manufacturer’s instructions. cDNA was amplified by PCR, followed by agarose gel electrophoresis. The involved primers are as follows: MTSS1 forward: 5′- AGCAGCCTGAACAGTGTCAA-3′; MTSS1 reverse: 5′-GGGATGGTGACTTGGACTGG-3′; GAPDH forward: 5′-TCAGTGGTGGACCTGACCTG-3′; GAPDH reverse: 5′-TGCTGTAGCCAAATTCG TTG-3′.
miR-96 inhibitor and mimics
Cells were transduced with mirVana TM miRNA inhibitors (30 nM) for miR-96 or control molecules (30 nM) (Invitrogen) with LipofectamineTM 2000, 48 h prior to subsequent experiments. Thirty nanometers mirVana TM miRNA mimics for miR-96 or control molecules (30 nM) (Invitrogen) was transfected into HEK-293T cells together with different luciferase vectors using LipofectamineTM 2000.
Cell proliferation assay
5 × 103 PC3 and LNCaP cells were planted into each well of 96-well plates. Six hours later, cells were subjected to the indicated treatments. At the indicated time points (24, 48, 72, and 96 h), cells were treated with 10 μL MTT (5 mg/mL) for 4 h. MTT was then discarded and 150 μL DMSO was added. The absorbance was measured on a model 550 microplate reader at 570 nm with a reference wavelength of 655 nm. Viability (%) = absorbance value of treated cells/absorbance value of control cells.
The estimation of cell invasion
Transwell assays were used to study the effect of change in the expression level of miR-96 on prostate cancer cells. PC3 and LNCaP cells have different metastatic potentials. The metastatic potential of PC3 cells is high while it is low for the LNCaP. So we chose PC3 cells for cell invasion assay. PC3 cells were re-suspended in 200 mL serum-free media, followed by being plated into the upper chamber of 24-well hanging cell culture insert (Millipore, Billercia, MA, USA) pre-coated with Matrigel (BD Biosciences, Sparks, MD, USA). Nine hundred milliliters DMEM media containing 20 % FBS was added to lower chamber of each well. Forty-eight hours later, cells were fixed by 4 % paraformaldehyde and then dyed with crystal violet. The number of cells that passed through the membranes was counted under a microscope.
Contact-independent colony formation assay
Colony formation assay was used to determine the clonogenicity of cells following the protocols described previously [12]. Briefly, single cells were plated into low-attachment 96-well plates (Costar) at the concentration of 10 cells/well. After the indicated treatments, cells were cultured in stem cell medium. New media were replaced every 3 days. One percent Methyl cellulose (Sigma Aldrich) was used to prevent cell aggregation, ensuring the spheres were derived from a single cell. The experiment was incubated for 14 days, and then, the number of colonies which contained spheres with a diameter higher than 75 μm was counted. The experiments were performed for three times.
Immunoblot assay
Proteins were extracted from cells using M-PER® Mammalian Protein Extraction Reagent (Thermo Scientific), separated using polyacrylamide gel electrophoresis, and transferred onto 0.45-μm nitrocellulose membranes. The membrane was blocked with 5 % fat-free milk and then incubated with primary antibodies. Overnight, PBS with 0.5 % Tween-20 was used to wash the membrane, followed by incubation with appropriate secondary antibodies. The involved primary antibodies were all purchased from Cell Signaling Technology (Danvers, MA).
The identification of miR-96 target
The potential miR-96 targets were predicted using the algorithms TargetScan (http://www.targetscan.org/). MTSS1 was found to be on the list of miR-96 targets. To confirm if MTSS1 is an authentic miR-96 target, a luciferase reporter vector under the regulation of a 223 bp MTSS1 3′UTR fragment containing potential miR-96 binding site (GTGCCAA), which was constructed based on pMIR-REPORT vector (Applied Biosystems), was transfected into HEK-293 cells, as well as a luciferase vector containing the same MTSS1 3′UTR fragment with mutant miR-96 binding site (GTGCGTA) was generated and used as control. After miR-96 mimics was transfected into HEK-293T cells with LipofectamineTM 2000 (Invitrogen), detection of luciferase activity was performed with Dual-Luciferase® Reporter Assay kit (Promega, WI) following manufacturer’ instructions.
Exogenous MTSS1 overexpression
We employed a vector expressing MTSS1 to elevate its abundance in cells. pcDNA3.1-MTSS1, an eukaryotic expression vector for MTSS1, and control vector pcDNA3.1-GFP were kindly provided by Dr. Zhiwei Wang (Harvard Medical School, MA, USA). One nanogram pcDNA3.1-MTSS1 or pcDNA3.1-GFP was transfected into PNT2 cells with LipofectamineTM 2000 (Invitrogen). Forty-eight hours later, the cells were subjected to the subsequent experiments.
Statistical analysis
Two-tailed student’s test was used for statistical analysis in most parts of our study. The association between miR-96 level and MTSS1 mRNA abundance was estimated by Pearson analysis. The test for categorical variables was made by chi-square test. Asterisk means significant (P < 0.05) and double asterisk means very significant (P < 0.01).
Results
miR-96 level was increased in PC compared with non-cancerous tissue
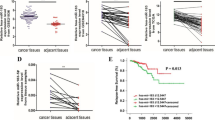
First of all, we collected a panel of PC patients (n = 45) and used qPCR assays to compare the expression level of miR-96 between their PC specimen and the matched adjacent non-cancerous tissues. The results showed that the abundance of miR-96 was significantly elevated in PC samples, compared with their matched adjacent non-cancerous tissues (P < 0.01, Fig. 1a). Similarly, higher expression of miR-96 was also detected in PC cell lines PC3 and LNCaP, compared with normal prostate epithelial cell lines PNT2 (P < 0.01, Fig. 1b).
miR-96 was overexpressed in PC tissues and associated with PC prognosis. a miR-96 levels in PC samples and adjacent noncancerous tissues (n = 45) were evaluated via qPCR. The lines indicated the average values of these samples. **P < 0.01 compared with adjacent noncancerous tissues. A adjacent noncancerous tissues, PC prostate cancer samples. b miR-96 levels in two PC cell lines, PC3, and LNCaP, as well as normal prostate epithelial cell, PNT2, were determined via qPCR. The bars represented means ± SD of three independent experiments. **P < 0.01 compared with normal prostate epithelial cell. c The abundance of miR-96 in blood samples of PC patients were also detected by qPCR assays. The bars represented means ± SD of three independent experiments. **P < 0.01 compared with control. d The correlation of miR-96 levels between PC tissue and serum samples were determined by Pearson analysis
miR-96 levels were significantly higher in the blood samples of PC patients
Subsequently, we collected serum samples from PC patients (n = 45) and evaluated the expression level of miR-96. Similar to its expression profile in PC tissues, miR-96 was also overexpressed in the blood samples from the selected PC patients. The findings make it possible that miR-96 may be used as biomarker for PC diagnosis (P < 0.01, Fig. 1c). We further compare the expression level of miR-96 between PC specimen and blood samples. Intriguingly, there is a positive correlation of miR-96 between PC specimen and blood samples (P < 0.01, Fig. 1d).
The suppression of miR-96 expression impaired the malignant phenotypes of PC cells
Next, we aimed to study if the change in the levels of miR-96 was able to affect the proliferation, invasion, and clonogenicity of PC cells. We employed synthetic inhibitors to suppress the expression of endogenous miR-96 in PC3 and LNCaP cells (P < 0.01, Fig. 2a). The proliferation of PC3 and LNCaP cells were both suppressed by the treatment of miR-96 inhibitors (P < 0.01, Fig. 2b). Colony formation assays also revealed that miR-96 downregulation partially abrogated the clonogenicity of prostate cancer cells (P < 0.01, Fig. 2c). Transwell assays revealed that miR-96 suppression also weakened the invasiveness of prostate carcinoma cells (P < 0.05, Fig. 2d).
miR-96 promoted the proliferation of PC cells. a PC3 and LNCaP cells were transfected with miR-96 inhibitor or control molecules. Forty-eight hours later, qPCR analysis was used to detect the expression level of miR-96. The bars showed means ± SD of three independent experiments. **P < 0.01 compared with control. b After miR-96 inhibitor was added, the proliferation rates of PC3 and LNCaP cells was detected by MTT assays, at the indicated time points. The dots indicated means ± SD of three independent experiments. **P < 0.01 compared with control. c The colonies of PC3 and LNCaP cells after the 14-day treatment of miR-96 inhibitors or controls was counted and shown as means ± SD of three independent experiments. **P < 0.01 compared with control. d The number of invasive PC3 and LNCaP cells were detected by transwell assays after transfected with miR-96 inhibitor or control molecules. The bars indicated means ± SD of three independent experiments. *P < 0.05 compared with control. The representative pictures were also shown (200-folds)
The above data showed that miR-96 played an important role in the maintenance of malignant phenotypes of prostate cancer cells.
miR-96 overexpression promoted the proliferation and colony formation of normal prostate cells
To further confirm the role of miR-96, PNT2 human normal prostate epithelium cell line was transfected with miR-96 mimics. qPCR assays demonstrated that miR-96 levels were elevated in PNT2 cells transfected with the mimics (P < 0.01, Fig. 3a). The proliferation rate of PNT2 cells was found to be increased when miR-96 was overexpressed (P < 0.01, Fig. 3b). And colony formation ability of PNT2 cells was also greatly enhanced by the treatment of miR-96 mimics (P < 0.01, Fig. 3c).
miR-96 contributed to the transformation of normal prostate cells. a Normal prostate cells, PNT2, were transfected with miR-96 mimics or controls. Forty-eight hours later, qPCR analysis was used to detect the expression level of miR-96. The bars showed means ± SD of three independent experiments. **P < 0.01 compared with control. b Under the same treatments, the proliferation rates of PNT2 cells were detected by MTT assays, at the indicated time points. The dots indicated means ± SD of three independent experiments. **P < 0.01 compared with control. c The colonies formed after the 14-day treatment of miR-96 mimics or controls was counted and shown as means ± SD of three independent experiments. **P < 0.01 compared with control
The expression levels of miR-96 is inversely associated with MTSS1 in prostate cancers
The expression of MTSS1, a putative tumor suppressor, was detected in prostate carcinoma specimen and cell lines. The results revealed that MTSS1 was underexpressed in prostate cancer samples, compared with noncancerous tissues (P < 0.01, Fig. 4a). Similarly, the abundance of MTSS mRNA was also found to be lower in prostate cancer cell lines than normal prostate epithelial cells (P < 0.01, Fig. 4b). Intriguingly, there is an inverse association between MTSS1 and miR-96 levels in the above samples (P < 0.01, Fig. 4c). Thus, it is of interest to further investigate the possible relationship between miR-96 and MTSS1.
The levels of MTSS1 is inversely correlated with miR-96. a MTSS1 mRNA levels in PC samples and adjacent noncancerous tissues (n = 45) were evaluated via qPCR. The lines indicated the average values of these samples. **P < 0.01 compared with adjacent noncancerous tissues. A adjacent noncancerous tissues, PC prostate cancer samples. b MTSS1 mRNA levels in two PC cell lines, PC3 and LNCaP, as well as normal prostate epithelial cell, PNT2, were determined via qPCR. The bars represented means ± SD of three independent experiments. **P < 0.01 compared with normal prostate epithelial cells. c The correlation between MTSS1 and miR-96 levels were determined by Pearson analysis
miR-96 suppressed the expression of tumor suppressor MTSS1 in prostate cancer cells
To verify the factors mediating the effect of miR-96 on prostate cancer, we aimed to identify the target of miR-96 with the help of online database. The prediction revealed that MTSS1 mRNA contained a potential miR-96 seed sequence within its 3′UTR (Fig. 5a). To determine if miR-96 had inhibitory effect on MTSS1 expression, we changed the expression level of miR-96 in prostate cancer and normal cell lines. The immunoblot assays revealed that the alteration in MTSS1 expression was inversely associated with miR-96 abundance (Fig. 5b, c). To further confirm this predicted miRNA response element (MRE), we generated a luciferase expression plasmid containing wide type or mutant MTSS1 3′UTR (report-MTSS1-wt and report-MTSS1-mut, respectively). Luciferase assay indicated that report-MTSS1-wt-expressed luciferase was suppressed by miR-96 overexpression, while report-MTSS1-mut was not affected under the same conditions (P < 0.01, Fig. 5d).
MTSS1 is an authentic target of miR-96 in prostate cancer cells. a MTSS1 3′UTR harbored miR-96 MRE highlighted in a white box. The numbers indicated the starting and ending points of this MRE. b PC3 and LNCaP cells were transfected with miR-96 inhibitor or control molecules. Forty-eight hours later, immunoblot assays were used to detect the expression level of MTSS1. c PNT2 and HEK-293T cells were transfected with miR-96 mimics or control molecules. Forty-eight hours later, immunoblot assays were used to detect the expression level of MTSS1. d PNT2 cells were transfected with luciferase reporter vectors containing wide type or mutant MTSS1 3′UTR (report-MTSS1-wt and report-MTSS1-mut, respectively). Forty-eight hours later, the expression levels of luciferase were determined. The bars showed means ± SD of three independent experiments. **P < 0.01 compared with control
MTSS1 restoration is required for the effect of miR-96 reduction on PC cells
It is of interest if MTSS1 overexpression is responsible for the effect of miR-96 suppression on PC cells. In order to address this issue, a MTSS1 small interfering RNA (siRNA) was employed to suppress the expression of MTSS1 induced by miR-96 reduction (Fig. 6a). MTT and colony formation assays showed that MTSS1 siRNA treatment was able to abrogate the effect of miR-96 suppression on the proliferation of PC cells (P < 0.01, Fig. 6b), as well as its ability of colony formation (P < 0.01, Fig. 6c). Transwell assay revealed that the invasiveness of PC3 cells was partially restored by MTSS1 siRNA treatment, after miR-96 expression was suppressed by inhibitors (P < 0.01, Fig. 6d).
The effect of miR-96 on PC cells is dependent on MTSS1. a PC3 cells were transfected with miR-96 inhibitor or control molecules (-) together with MTSS1 siRNA or control (-). Forty-eight hours later, immunoblot assays were used to detect the expression level of MTSS1. b Under the above treatments, the proliferation rates of PC3 cells were detected by MTT assays, at the indicated time points. The dots indicated means ± SD of three independent experiments. **P < 0.01 compared with control. c The colonies of PC3 cells after the 14-day treatment of miR-96 inhibitor or control molecules (-) together with MTSS1 siRNA or control (-) were counted and shown as means ± SD of three independent experiments. **P < 0.01 compared with control. d The number of invasive PC3 cells was detected by transwell assays under the same treatment. The bars indicated means ± SD of three independent experiments. **P < 0.01 compared with control. The representative pictures were also shown (200-folds)
miR-96 suppression of MTSS1 mediated its transforming activity on normal prostate cells
To determine if MTSS1 suppression is required for the promoting activity of miR-96 on normal prostate cells, we used a MTSS1-expressing plasmid to restore MTSS1 expression in prostate cells treated with miR-96 mimics (Fig. 7a). MTT assay showed that MTSS1 restoration was able to abolish the effect of miR-96 overexpression on the proliferation of PNT2 cells (P < 0.01, Fig. 7b). Similarly, the effect of miR-96 on the clonogenicity of normal prostate epithelial cells was also abrogated by MTSS1 overexpression (P < 0.01, Fig. 7c).
MTSS1 suppression mediated the transforming effect of miR-96 on normal prostate cells. a PNT2 cells were treated with miR-96 mimics alone or together with a MTSS1-expressing vector. Forty-eight hours later, MTSS1 expression was detected by immunoblot assays. An EGFP-expressing plasmid was used as control. b Under the same treatments, the proliferation rates of PNT2 cells were detected by MTT assays, at the indicated time points. The dots indicated means ± SD of three independent experiments. **P < 0.01 compared with control. c The colonies formed after the 14-day treatment of miR-96 mimics or controls was counted and shown as means ± SD of three independent experiments. **P < 0.01 compared with control
Correlation of miR-96 and MTSS1 expressions with clinical characteristics of prostate cancer patients
To explore the relationship between the expressions of miR-96, MTSS1, and prognosis of PC patients, we further analyzed the clinical characteristics of PC patients by using SPSS software. As shown in Table 1, the high expression of miR-96 in tissue of PC patients is closely correlated with operative high stage (P = 0.034), high PSA level (P = 0.02), and high Gleason score (P = 0.022). Also as shown in Table 2, the low expression of MTSS1 in tissue of prostate cancer patients is closely correlated with operative high stage (P = 0.027), high PSA level (P = 0.017), and high Gleason score (P = 0.013). No relationship was found between the levels of miR-96 and MTSS1 in terms of age (P > 0.05).
Discussion
Accumulated evidence indicated that miR-96 is associated with various types of cancers, such as hepatocellular carcinoma [13], breast cancer [14], non-small cell lung cancer [15], and prostate carcinoma [16]. The overexpression of miR-96 was found to promote the survival, proliferation, and clonogenicity of prostate carcinoma cells [7–9]. FOXO1 is verified as a mediator that links miR-96 with prostate cancer [7–9]. However, it is still unknown if there are new pathways or proteins other than FOXOs associated with the effect of miR-96 on the biology of prostate cancer. In this study, we provided evidence that MTSS1, a putative tumor suppressor, is also required for the oncogenic role of miR-96 in prostate cancers.
As initially identified as a metastasis suppressor [17], MTSS1 has been well documented to suppress almost all the aspects of cancers, such as proliferation [18], an epithelial-mesenchymal transition (EMT) [19]. The related molecular pathways include epithelial growth factor (EGF) [20], and Rho-family GTPases (Rac) [21]. Similar to other cancer types, MTSS1 is also shown to be relevant to the progression of prognosis of prostate cancer [22]. The reduction in the expression level of MTSS1 contributes to the growth, development, and metastasis of PC [23, 24]. Our data further reinforced the notion that MTSS1 is a tumor suppressor for prostate carcinoma.
The regulation of MTSS1 is also of interests for the study on prostate cancer biology. Zhong et al. reported that β-TRCP, an E3 ligase closely associated with cancer, reduced the expression level of MTSS1 in a ubiquitination-dependent fashion [25]. Also, miRNA was also found to participate in the regulation of MTSS abundance in prostate cancer cells. miR-182-5p overexpression in PC tissues can suppress the expression of MTSS1 by targeting the MREs within the 3′UTR of its mRNA [26]. However, it is still of interest if more mechanisms are involved with MTSS1 deregulation in prostate cancer. Our study revealed that MTSS downregulation may result from the increased expression of miR-96 in prostate cancer cells.
Furthermore, we found that the serum level of miR-96 is associated with the survival of PC patients. This finding makes it possible that miR-96 abundance in blood from PC patients can be used to predict the prognosis of these individuals. In fact, some other miRNAs have been applied for the prediction of patient survival. For instances, miR-187 and miR-182 levels in blood of PC patients can be used for the determination of the predicted patients’ survivals [27].
Collectively, miR-96 was shown to be associated with the prognosis of PC patients and promoted the growth of prostate carcinoma by targeting MTSS1. This finding implied that miR-96 is an effective therapeutic target for PC treatment. And more anti-tumor strategies are needed for therapeutic purposes by targeting this molecular pathway.
References
Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36.
Meister G. miRNAs get an early start on translational silencing. Cell. 2007;131:25–8.
Jung HJ, Suh Y. MicroRNA in aging: from discovery to biology. Curr Genomics. 2012;13:548–57.
Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7.
Bandres E, Cubedo E, Agirre X, Malumbres R, Zarate R, Ramirez N, et al. Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5:29.
Yan Z, Wang J, Wang C, Jiao Y, Qi W, Che S. miR-96/HBP1/Wnt/beta-catenin regulatory circuitry promotes glioma growth. FEBS Lett. 2014;588(17):3038–46.
Yu JJ, Wu YX, Zhao FJ, Xia SJ. miR-96 promotes cell proliferation and clonogenicity by down-regulating of FOXO1 in prostate cancer cells. Med Oncol. 2014;31:910.
Fendler A, Jung M, Stephan C, Erbersdobler A, Jung K, Yousef GM. The antiapoptotic function of miR-96 in prostate cancer by inhibition of FOXO1. PLoS One. 2013;8:e80807.
Haflidadottir BS, Larne O, Martin M, Persson M, Edsjo A, Bjartell A, et al. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS One. 2013;8:e72400.
Li Z, Ma YY, Wang J, Zeng XF, Li R, Kang W, et al. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Onco Targets Ther. 2015;9:139–48.
Ma L, Liu J, Shen J, Liu L, Wu J, Li W, et al. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol Ther. 2010;9:554–61.
Wang B, Liu J, Ma LN, Xiao HL, Wang YZ, Li Y, et al. Chimeric 5/35 adenovirus-mediated Dickkopf-1 overexpression suppressed tumorigenicity of CD44(+) gastric cancer cells via attenuating Wnt signaling. J Gastroenterol. 2013;48:798–808.
Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, et al. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–63.
Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–16.
Guo H, Li Q, Li W, Zheng T, Zhao S, Liu Z. miR-96 downregulates RECK to promote growth and motility of non-small cell lung cancer cells. Mol Cell Biochem. 2014;390(1–2):155–60.
Schaefer A, Jung M, Mollenkopf HJ, Wagner I, Stephan C, Jentzmik F, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126:1166–76.
Lee YG, Macoska JA, Korenchuk S, Pienta KJ. MIM, a potential metastasis suppressor gene in bladder cancer. Neoplasia. 2002;4:291–4.
Du P, Ye L, Ruge F, Yang Y, Jiang WG. Metastasis suppressor-1, MTSS1, acts as a putative tumour suppressor in human bladder cancer. Anticancer Res. 2011;31:3205–12.
Dawson JC, Bruche S, Spence HJ, Braga VM, Machesky LM. Mtss1 promotes cell-cell junction assembly and stability through the small GTPase Rac1. PLoS One. 2012;7:e31141.
Dawson JC, Timpson P, Kalna G, Machesky LM. Mtss1 regulates epidermal growth factor signaling in head and neck squamous carcinoma cells. Oncogene. 2012;31:1781–93.
Bompard G, Sharp SJ, Freiss G, Machesky LM. Involvement of Rac in actin cytoskeleton rearrangements induced by MIM-B. J Cell Sci. 2005;118:5393–403.
Hofer MD, Kuefer R, Varambally S, Li H, Ma J, Shapiro GI, et al. The role of metastasis-associated protein 1 in prostate cancer progression. Cancer Res. 2004;64:825–9.
Loberg RD, Neeley CK, Adam-Day LL, Fridman Y, St John LN, Nixdorf S, et al. Differential expression analysis of MIM (MTSS1) splice variants and a functional role of MIM in prostate cancer cell biology. Int J Oncol. 2005;26:1699–705.
Mustafa N, Martin TA, Jiang WG. Metastasis tumour suppressor-1 and the aggressiveness of prostate cancer cells. Exp Ther Med. 2011;2:157–62.
Zhong J, Shaik S, Wan L, Tron AE, Wang Z, Sun L, et al. SCF beta-TRCP targets MTSS1 for ubiquitination-mediated destruction to regulate cancer cell proliferation and migration. Oncotarget. 2013;4:2339–53.
Hirata H, Ueno K, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, et al. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS One. 2013;8:e55502.
Casanova-Salas I, Rubio-Briones J, Calatrava A, Mancarella C, Masia E, Casanova J, et al. Identification of miR-187 and miR-182 as biomarkers of early diagnosis and prognosis in patients with prostate cancer treated with radical prostatectomy. J Urol. 2014;192(1):252–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, L., Zhong, J., Guo, B. et al. miR-96 promotes the growth of prostate carcinoma cells by suppressing MTSS1. Tumor Biol. 37, 12023–12032 (2016). https://doi.org/10.1007/s13277-016-5058-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5058-2