Abstract
Hypoxia promotes tumor invasion and metastasis via multiple mechanisms, including epithelial-mesenchymal transition (EMT). Twist, an EMT regulator, has been disclosed to associate with invasion and metastasis as well as poor prognosis of many malignancies. However, it remains undefined whether Twist is involved in invasion and metastasis of hypoxic non-small cell lung cancer (NSCLC). In this study, protein levels of Twist, hypoxia-inducible factor-1α (HIF-1α), and EMT markers (E-cadherin and vimentin) were examined by immunohistochemistry in 76 lung cancer tissues from NSCLC patients. Expression of Twist and its correlation with HIF-1α, E-cadherin, and vimentin were analyzed. Small interfering RNA (siRNA) against Twist was used to knockdown Twist expression in hypoxic NSCLC cells, A549 and NCI-H460. Cellular invasion and protein levels of Twist, E-cadherin, and vimentin were evaluated by matrigel invasion assay and Western blot, respectively. Our results showed that in clinical samples, there was a significant association between Twist expression and differentiation degree, lymph node metastasis, and TNM stage. Correlation analysis demonstrated that expression of Twist was negatively correlated with E-cadherin expression, but positively associated with HIF-1α and vimentin expression. In cultured NSCLC cells, Twist messenger RNA (mRNA) and protein levels were upregulated under hypoxia, while knockdown of Twist suppressed potentiated invasion and expression of mesenchymal marker vimentin induced by hypoxia. Protein level of increased epithelial marker E-cadherin was shown along with Twist downregulation. These findings suggest that Twist promoting hypoxic invasion and metastasis of NSCLC may be associated with altered expression of EMT markers. Inhibition of Twist may be of therapeutic significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis is one of the major biological characteristics of malignant tumors and contributes nearly 90 % of cancer-related deaths. It is a complex multi-step process and influenced by the interactions of cell-cell and cell-matrix. A large number of studies have shown that epithelial-mesenchymal transition (EMT) is closely correlated with tumor invasion and metastasis [1–3]. Through EMT, epithelial cells lose polarity and cell-cell junctions, undergo dramatic remodeling of the cytoskeleton, change morphology, and eventually acquire migratory capacity. During EMT process, epithelial markers such as E-cadherin and β-catenin are downregulated; mesenchymal markers such as vimentin and fibronectin expression are increased [4, 5].
Twist, a highly conservative basic helix-loop-helix (bHLH) transcription factor, is overexpressed in a variety of human tumors and associated with tumor invasion, metastasis, and poor prognosis [6–11]. Moreover, Twist also plays an important role in multiply processes, including angiogenesis, resistance to apoptosis, multidrug resistance, and EMT [10–12]. In breast cancer and gastric carcinoma, Twist was overexpressed and correlated with EMT [13, 14].
Hypoxia, a common phenomenon during the development of malignancy, correlates with EMT and tumor metastasis, but the detailed mechanism remains undefined. Non-small cell lung cancer (NSCLC) accounts for more than 80 % of lung cancer and is one of the leading causes of death worldwide. Hypoxia plays a crucial role during NSCLC EMT and metastasis [15, 16]. As yet, how hypoxia mediates EMT of NSCLC is ambiguous, whether the EMT regulator Twist is involved in invasion induced by hypoxia and relevant molecular mechanisms are largely unknown. For the first time, the present study disclosed the relationship between Twist, EMT, and hypoxic metastasis in NSCLC from both clinical tissues and in vitro cultured NSCLC cells. In NSCLC patients, the expression of Twist protein detected by immunohistochemistry was significantly associated with differentiation degree, lymph node metastasis, and TNM stage. Correlation analysis showed that Twist had positive and negative correlations with E-cadherin and vimentin expression, respectively. In vitro study showed that silencing of Twist in A549 and NCI-H460 cells suppressed hypoxic invasion, accompanied by increased E-cadherin expression and diminished vimentin expression. Our data provide a basis for Twist becoming a potential molecular target for treatment in hypoxic metastasis of lung cancer.
Materials and methods
Patients and specimens
A total of 76 primary NSCLC specimens were collected from patients with NSCLC undergoing surgery resection at the Shandong Cancer Hospital and Institute. All patients had not received radiotherapy or chemotherapy prior to the surgery. The study was approved by the institutional review board, and all patients enrolled in this study provided informed consent for the use of these tissues for clinical research. Among them, 55 were men and 21 were women, with the median age of 62 years old (range 38 to 76 years). The clinicopathological features of patients, including age, gender, differentiation degree, histology, TNM stage, and lymph node metastasis are shown in Table 1.
Immunohistochemistry analysis
Formalin-fixed, paraffin-embedded lung cancer tissues were cut at a thickness of 4 μm. The sections were treated with xylene and rehydrated, and then, endogenous peroxidase activity was eliminated with 3 % hydrogen peroxide. Antigen retrieval was performed in microwave oven via boiling assay, and 5 % bovine serum albumin was applied to block nonspecific binding site. Slides were incubated with primary antibody, including rabbit anti-Twist (1:100, Abcam), mouse anti-HIF-1α (1:50, Abcam), rabbit anti-E-cadherin (1:100, Santa Cruz Biotechnology), and rabbit anti-vimentin (1:500, Abcam) antibody at 4 °C overnight. The following procedures were performed according to the protocol of PV-9000 two-step staining kit (Zhongshan, Beijing, China). Diaminobenzidine (DAB) served as chromogen. Two independent-experienced pathologists assessed blindedly all the sections according to the following immunoreactive score (IRS): IRS = SI (staining intensity) × PP (percentage of positive cells). SI was determined as 0 negative, 1 weak, 2 moderate, and 3 strong. PP was defined as 0 0 %, 1 1–10 %, 2 11–50 %, 3 51–80 %, 4 81–100 %. IRS value ≥4 was considered as a positive staining result [17].
Cell lines and culture conditions
Human A549 and NCI-H460 lung cancer cells (American Type Culture Collection, ATCC, Manassas, VA, USA) were both maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) containing 10 % fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin in a 37 °C humidified incubator and an atmosphere of 5 % CO2 in air. Cells were cultured on sterilized culture dishes and passaged every 2–3 days to maintain exponential growth. Hypoxic treatment was performed under 0.5 % oxygen, 5 % CO2, and 94.5 % nitrogen (Galaxy R CO2 incubator, RS Biotech, UK) at 37 °C for 24 h. Meanwhile, normoxic cells were grown in a 19 % O2 and 5 % CO2 incubator (BBD6220, Heraeus, Germany) until harvest.
Twist small interfering RNA treatment
Knockdown of Twist expression was obtained using transfection of Twist small interfering RNA (siRNA). The sequences for the Twist siRNA were as follows: sense: 5′-GCUGAGCAAGAUUCAGACCTT-3′ and antisense: 5′-GGUCUGAAUCUUGCUCAGCTT-3′. For the control siRNA, the sequences were sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense: 5′-ACGUGACACGUUCGGAGAATT-3′. Above siRNA was synthesized by Genepharma (Shanghai, China). The cells were transfected for 48 h under normoxic conditions, using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocols, and then exposed to hypoxia (0.5 % O2) for 24 h prior to assays.
RNA isolation and real-time PCR
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen, San Diego, CA, USA) according to the manufacturer’s instructions. A total of 1 μg RNA was used for reverse transcription with Super-Script II reverse transcriptase (TaKaRa RT kit, Dalian, China). The PCR primers used in the study were Twist: 5′-AGTCCGCAGTCTTACGAGGAG-3′ (forward) and 5′-GACCTGGTAGAGGAAGTCGATG-3′(reverse) and β-actin: 5′-TTAGTTGCGTTACACCCTTTC-3′(forward) and 5′-GCTGTCACCTTCACCGTTC-3′ (reverse). Quantitative real-time PCR was set up in triplicate for each sample using TaKaRa SYBR® Premix Ex Taq™ quantitative PCR kit. β-actin was used as the reference gene. Ct values of the samples were calculated, and the relative level of Twist messenger RNA (mRNA) was expressed with 2−ΔΔCt value.
Western blot analysis
Total cellular protein was extracted with a lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM PMSF, 2 mM EDTA (pH 8.0), 0.5 % Triton X-100, 5 mM DTT, 0.2 mM PMSF, and 2 μg/ml aprotinin. Protein concentration was quantified using the Bradford method. And then, 50 μg of protein underwent SDS-PAGE and transferred to Polyvinylidene difluoride (PVDF) membrane using an electronic Bio-Rad transfer apparatus. The PVDF membranes were blocked for 2 h at room temperature with 5 % fat-free milk and then probed overnight at 4 °C with the primary antibodies, including mouse anti-human Twist and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA, dilution 1: 2000) antibodies, rabbit anti-human E-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA, USA, dilution 1:100) antibodies, and vimentin (Abcam, UK, dilution 1:100) antibodies. Then, the membrane was washed with TBST three times for a total of 30 min. The secondary antibody, horseradish peroxidase-labeled rabbit anti-mouse or goat anti-rabbit immunoglobulin G secondary antibody (1:2000, Santa Cruz Biotechnology), was added and incubated at room temperature for 90 min. The membrane was washed, reacted with an ECL plus reagent (Beyotime, China) for 5 min, and then exposed onto Kodak imaging film. Radiographs were scanned and analyzed by the use of Multi-image light cabinet and software (Alpha Innotech Corporation, San Leandro, CA, USA). Relative protein level was quantified using β-actin as a loading control.
Matrigel invasion assay
In vitro invasion ability of cells was measured at a 24-well transwell chamber with a pore size of 8 μm (Costar, NewYork, NY, USA). Briefly, NSCLC cells were transfected with Twist siRNA and control siRNA for 48 h under normoxic condition and then seeded into the upper matrigel chamber containing 100 μl serum-free medium DMEM. DMEM containing 10 % fetal calf serum was added into the lower chamber as the chemoattractant. Following 24 h incubation under hypoxic conditions, cells that failed to migrate through the membrane were removed with a cotton swab. The invaded cells were fixed and stained using 0.1 % crystal violet. The numbers of invaded cells were pictured and counted in five separate fields under microscope (×100).
Statistical analysis
Statistical analysis was performed with SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The relationship between Twist expression and clinicopathological variables was evaluated using chi-square test. The nonparametric Spearman rank correlation coefficient was applied to analyze the relationship between Twist and EMT markers and HIF-1α. All statistical tests were two-sided, and a p value smaller than 0.05 was defined as significant.
Results
Expression of Twist, HIF-1α, and EMT markers in NSCLC
We assessed the expressions of Twist, HIF-1α, E-cadherin, and vimentin in 76 lung cancer tissues via immunohistochemistry approach. Twist and HIF-1α protein were expressed mainly in the nucleus of tumor cells (Fig. 1a, b), while E-cadherin and vimentin were located in cell membrane and cytoplasm, respectively (Fig. 1c, d). According to the criteria established for our immunostaining, 52.6 % (40/76) and 55.3 % (42/76) of tumors were positive for Twist and HIF-1α staining, respectively. Positive E-cadherin expression was observed in 51.3 % (39/76) of the NSCLC tissue samples, and aberrant mesenchymal protein expression frequency was 39.5 % (30/76) for vimentin.
Association of twist with clinicopathological characteristics of NSCLC
As shown in Table 1, the expression of Twist protein was significantly correlated with histological differentiation, TNM stage, and lymph node metastasis of NSCLC patients (all p < 0.05). Nevertheless, there were no significant correlations between Twist expression and age, gender, and tumor pathology (all p > 0.05).
Twist correlated with the levels of EMT markers and HIF-1α in NSCLC tissues
Table 2 summarizes the relationships between Twist expression and the levels of EMT markers as well as HIF-1α in NSCLC patients. The expression of Twist was negatively correlated with E-cadherin (p < 0.001) and positively associated with both vimentin (p = 0.014) and HIF-1α (p = 0.001) expression, indicating that Twist was correlated with EMT and hypoxia.
Twist expression in NSCLC cells were induced by hypoxia
In order to evaluate the influence of hypoxia treatment on Twist expression in NSCLC cells, we exposed A549 and NCI-H460 cells to normoxia (19 % O2) or hypoxia (0.5 % O2, 5 % CO2, and 94.5 % N2) for 24 h and detected the expression at levels of Twist mRNA and protein, by real-time PCR and Western blot methods, respectively. Compared with normoxic conditions, hypoxia upregulated both Twist mRNA (Fig. 2a) and protein expression (Fig. 2b), indicating that hypoxia increases Twist expression on transcriptional and post-transcriptional levels.
Influence of hypoxia on Twist expression in non-small cell lung cancer (NSCLC) cells. Cells were exposed to normoxia (19 % O2) or hypoxia (0.5 % O2) for 24 h. Twist mRNA and protein expression were assessed by real-time PCR and Western blot, respectively. a Twist mRNA expression in A549 and NCI-H460 cells. Relative fold of Twist expression was determined by comparison to cells cultured under 19 % O2 and arbitrarily set at 1.0. b Protein expression of Twist was detected by Western blot
Twist downregulation suppressed invasion induced by hypoxia in NSCLC cells
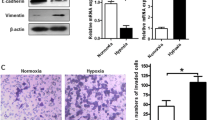
Twist siRNA was used to downregulate Twist expression. NSCLC cells transfected with Twist siRNA showed diminished Twist mRNA and protein expressions (Fig. 3a, b). Meanwhile, another Twist siRNA sequence also demonstrated similar RNAi effect (data not shown). The sequence for this Twist siRNA was as follows: sense: 5′-GAUGGCAAGCUGCAGCUAUTT-3′ and antisense: 5′-AUAGCUGCAGCUUGCCAUCTT-3′. In the aspect of cell invasion, hypoxia significantly promoted invasion potential. In comparison to control siRNA, Twist siRNA transfection suppressed cell invasion induced by hypoxia in A549 and NCI-H460 cells. The reduction rates were 32 % in A549 and 38 % in NCI-H460, respectively (Fig. 3c, d). The present findings indicated that Twist may be involved in hypoxic invasion of NSCLC cells.
Silencing of Twist diminished the invasion of NSCLC cells under hypoxic conditions. At 48 h after transfection, cells were exposed to hypoxia (0.5 % O2) for 24 h and collected for Twist mRNA and protein analysis and in vitro invasion potential. a Twist mRNA expression was analyzed by real-time PCR and normalized to β-actin expression. b Twist protein level was determined by Western blot. β-actin was used as loading control. Relative Twist protein expression was determined by comparison to control siRNA transfected cells and arbitrarily set at 1.0. c Cells in transwell chamber were incubated under normoxic or hypoxic conditions for 24 h following transfection of cells with control or Twist siRNA. The data are standardized against control in normoxia and presented as relative cell invasion. *p < 0.05
Twist downregulation reversed the expression of decreased E-cadherin and increased vimentin induced by hypoxia in NSCLC cells
EMT is associated with the local invasion and distant metastasis of tumor. The most important phenotype molecule change during EMT process is a reduced or lost expression of E-cadherin, an epithelial marker. In order to determine the relationship between Twist and EMT induced by hypoxia in NSCLC cells, we observed the effect of hypoxia and Twist intervention on protein expression of E-cadherin and vimentin, two important EMT phenotype molecules. The results showed that hypoxia induced decreased E-cadherin and increased vimentin expression in NSCLC cells. Compared with non-transfected hypoxic cells, cells transfected with control siRNA displayed similar protein expression of E-cadherin and vimentin, while knockdown of Twist expression reversed protein expression of decreased E-cadherin and elevated vimentin induced by hypoxia (Fig. 4). The present data demonstrated that EMT contributed to hypoxic invasion associated with Twist.
Twist downregulation canceled hypoxia-induced downregulation of E-cadherin and upregulation of vimentin in NSCLC cells. At 48 h after transfection under a normoxic state, NSCLC cells were exposed to hypoxia (0.5 % O2) for the following 24 h and collected for E-cadherin and vimentin protein analysis. β-actin was used as an internal control. *p < 0.05
Discussion
To the best of our knowledge, this study is the first report showing that Twist is involved in hypoxic invasion, metastasis of NSCLC, and correlates with EMT. In patients with NSCLC, we found that the expression of Twist protein was significantly correlated with poor tumor differentiation, TNM stage, and lymph node metastasis. Moreover, Twist expression was negatively correlated with E-cadherin and positively associated with vimentin and HIF-1α, indicating that Twist was related to hypoxia and EMT of NSCLC patients. Consistently, in vitro experiments demonstrated that NSCLC cells exposed to hypoxia showed increased Twist mRNA and protein expressions. Meanwhile, silencing of Twist suppressed the increased invasive potential and vimentin protein expression induced by hypoxia, along with upregulated E-cadherin protein expression. All these results suggest that Twist may be important for hypoxia mediated EMT and invasion.
Emerging evidence suggests that Twist functions as an oncoprotein, and its expression is positively correlated with metastasis and poor clinical prognosis in a wide range of human cancers [6–11]. Consistent with our study, Hui and coworker demonstrated that overexpression of Twist in NSCLC patients was correlated with TNM stage, differentiation, and nodal status, which implies that Twist plays an important role in NSCLC progression and serves as an independent biomarker for a poor clinical prognosis [18].
EMT was closely related to metastasis in multiple cancers, and hypoxia is an important factor to induce EMT. Lee and partners reported that Twist overexpression correlated with hepatocellular carcinoma metastasis through induction of EMT [19]. However, in NSCLC patients, whether Twist expression is related to hypoxia and EMT has not been fully evaluated. The present study showed that the positive expression of Twist was negatively correlated with E-cadherin and positively associated with HIF-1α and vimentin expression (all p < 0.05), indicating that Twist was correlated to hypoxia and EMT in NSCLC. A regulation mechanism may exist between Twist and EMT phenotype molecule, E-cadherin, and vimentin. Similar with our study, Nakashima reported that in cultured lung cancer cells, Twist was involved in phenotype alterations through EMT [20].
In order to further clarify the relationship among Twist and hypoxic metastasis and EMT in NSCLC, we performed intensive study in cultured cells. Due to the functional HRE located in the proximal promoter of the Twist gene, Twist has been shown to be a direct target of HIF-1 [21]. Similar with previous reports [21, 22], the present data demonstrated that Twist expression was upregulated by hypoxia. Moreover, silencing of Twist can effectively suppress the increased invasiveness induced by hypoxia. For the first time, this study established a link between Twist and hypoxic invasion in NSCLC. As yet, the mechanisms involved in Twist promoting tumor metastasis remains unclear. Some researchers have reported that Twist can identify E-box motif in target gene promoter, inhibit target genes transcription including E-cadherin and Akt2, and finally induce EMT [23, 24]. E-cadherin, a Ca2+-dependent cell adhesion molecule, located in adherent junctions of epithelia, plays a crucial role in the suppression of tumor invasion. Downregulation or loss of E-cadherin expression is an important event during EMT process in multiple cancers and coincides with increased tumor malignancy [25, 26]. Molecular mechanisms of Twist leading to downregulated expression of E-cadherin are not fully understood. Direct binding to E-box locus or inhibiting activity of some regulation factors by Twist may involve in this process. Mironchik and partners disclosed that Twist overexpression induces angiogenesis in vivo and correlates with chromosomal instability in breast cancer [27]. Sun et al. also disclosed that Twist plays an important role in vasculogenic mimicry of hepatocellular carcinoma [28]. Moreover, Twist is capable of regulating miRNAs expression profile. For example, Twist can induce miR-10b expression, which plays an important role in invasion and progression of breast cancer [29]. In prostate cancer cells PC-3 and metastatic intrahepatic cholangiocarcinoma tissues, Twist has been shown to have negative associations with miR-29b and miR-214, respectively [30, 31]. In addition to EMT pathway, whether Twist affects other signaling pathways and what roles these pathways played in NSCLC hypoxic metastasis needs to be further explored. Moreover, relevant in vivo assay is required to verify the relationship between Twist and hypoxic metastasis.
Taken together, in the current study, we disclosed that Twist is associated with hypoxic metastasis and EMT of NSCLC. Twist downregulation can attenuate the increased invasiveness and vimentin level as well as decreased E-cadherin expression induced by hypoxia. The present study provides evidence for Twist serving as a potential therapeutic target in hypoxic lung cancer.
References
Lili LN, Matyunina LV, Walker LD, Wells SL, Benigno BB, McDonald JF. Molecular profiling supports the role of epithelial-to-mesenchymal transition (EMT) in ovarian cancer metastasis. J Ovarian Res. 2013;6(1):49.
Hu M, Chen X, Zhang J, Wang D, Fang X, Wang X, et al. Over-expression of regulator of G protein signaling 5 promotes tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma cells. J Surg Oncol. 2013;108(3):192–6.
Zhang H, Liu J, Yue D, Gao L, Wang D, Zhang H, et al. Clinical significance of E-cadherin, beta-catenin, vimentin and S100A4 expression in completely resected squamous cell lung carcinoma. J Clin Pathol. 2013;66(11):937–45.
Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65(14):5991–5.
Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42.
Gong T, Xue Z, Tang S, Zheng X, Xu G, Gao L, et al. Nuclear expression of Twist promotes lymphatic metastasis in esophageal squamous cell carcinoma. Cancer Biol Ther. 2012;13(8):606–13.
Yu L, Lu S, Tian J, Ma J, Li J, Wang H, et al. TWIST expression in hypopharyngeal cancer and the mechanism of TWIST-induced promotion of metastasis. Oncol Rep. 2012;27(2):416–22.
Wushou A, Pan HY, Liu W, Tian Z, Wang LZ, Shali S, et al. Correlation of increased twist with lymph node metastasis in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2012;70(6):1473–9.
Ru GQ, Wang HJ, Xu WJ, Zhao ZS. Upregulation of Twist in gastric carcinoma associated with tumor invasion and poor prognosis. Pathol Oncol Res. 2011;17(2):341–7.
Feng Z, Gan H, Cai Z, Li N, Yang Z, Lu G, et al. Aberrant expression of hypoxia-inducible factor 1alpha, TWIST and E-cadherin is associated with aggressive tumor phenotypes in endometrioid endometrial carcinoma. Jpn J Clin Oncol. 2013;43(4):396–403.
Wang WS, Yu SL, Yang XS, Chang SD, Hou JQ. Expression and significance of twist and E-cadherin in ovarian cancer tissues. Asian Pac J Cancer Prev. 2013;14(2):669–72.
Motegi S, Yamada K, Ishikawa O. Twist1 in tumor cells and alpha-smooth muscle actin in stromal cells are possible biomarkers for metastatic giant basal cell carcinoma. J Dermatol. 2013;40(8):661–3.
D’Angelo RC, Liu XW, Najy AJ, Jung YS, Won J, Chai KX, et al. TIMP-1 via TWIST1 induces EMT phenotypes in human breast epithelial cells. Mol Cancer Res. 2014;12(9):1324–33.
Yang Z, Zhang X, Gang H, Li X, Li Z, Wang T, et al. Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochem Biophys Res Commun. 2007;358(3):925–30.
Nurwidya F, Takahashi F, Kobayashi I, Murakami A, Kato M, Minakata K, et al. Treatment with insulin-like growth factor 1 receptor inhibitor reverses hypoxia-induced epithelial-mesenchymal transition in non-small cell lung cancer. Biochem Biophys Res Commun. 2014;455(3-4):332–8.
Ruan J, Zhang L, Yan L, Liu Y, Yue Z, Chen L, et al. Inhibition of hypoxia-induced epithelial mesenchymal transition by luteolin in non-small cell lung cancer cells. Mol Med Rep. 2012;6(1):232–8.
Shi Y, Wu H, Zhang M, Ding L, Meng F, Fan X. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn Pathol. 2013;8:89.
Hui L, Zhang S, Dong X, Tian D, Cui Z, Qiu X. Prognostic significance of twist and N-cadherin expression in NSCLC. PLoS One. 2013;8(4), e62171.
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12(18):5369–76.
Nakashima H, Hashimoto N, Aoyama D, Kohnoh T, Sakamoto K, Kusunose M, et al. Involvement of the transcription factor twist in phenotype alteration through epithelial-mesenchymal transition in lung cancer cells. Mol Carcinog. 2012;51(5):400–10.
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295–305.
Sun S, Ning X, Zhang Y, Lu Y, Nie Y, Han S, et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009;75(12):1278–87.
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67(5):1979–87.
Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367(2):235–41.
Hong J, Zhou J, Fu J, He T, Qin J, Wang L, et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71(11):3980–90.
Zhong K, Chen W, Xiao N, Zhao J. The clinicopathological significance and potential drug target of E-cadherin in NSCLC. Tumour Biol. 2015;36(8):6139–48.
Mironchik Y, Winnard Jr PT, Vesuna F, Kato Y, Wildes F, Pathak AP, et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65(23):10801–9.
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, et al. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51(2):545–56.
Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–8.
Ru P, Steele R, Newhall P, Phillips NJ, Toth K, Ray RB. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther. 2012;11(5):1166–73.
Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist. FEBS J. 2012;279(13):2393–8.
Acknowledgements
This work was supported by grants from Shandong Province Science Foundation (ZR2010HM083, ZR2009CM141) and Shandong Health Department (2011HZ095, 2015WS0155).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Wei, L., Sun, JJ., Cui, YC. et al. Twist may be associated with invasion and metastasis of hypoxic NSCLC cells. Tumor Biol. 37, 9979–9987 (2016). https://doi.org/10.1007/s13277-016-4896-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4896-2