Abstract
Dysregulation of Hippo-Yes-associate protein (YAP) signaling has important roles in the tumorigenesis of hepatocellular carcinoma (HCC). Our previous studies have shown that Cip1 interacting zinc finger protein 1 (CIZ1) activated YAP signaling in the HCC cells and promoted the growth and migration of cancer cells. However, the mechanisms for the activation of YAP signaling by CIZ1 are unknown. In this study, it was found that CIZ1 interacted with the transcriptional factor YAP in HCC cells. The nuclear matrix anchor domain of CIZ1 is responsible for its interaction with YAP. Moreover, CIZ1 enhanced the interaction between YAP and TEAD. Knocking down the expression of CIZ1 impaired the transcriptional activity as well as the biological functions of YAP. Taken together, our study demonstrated that CIZ1 is a positive regulator of YAP signaling, and CIZ1 might be a therapeutic target for HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hippo-Yes-associate protein (YAP) signaling is a conserved signaling cascade which controls the organ development through regulating of cell proliferation and apoptosis [1, 2]. Aberrant activation of Hippo-YAP signaling leads to the accumulation of YAP in the cytoplasm. Then YAP enters into the nucleus and formed a complex with TEAD to regulate the expression of various downstream genes, such as cyclin E and connective tissue growth factor (CTGF) [3]. In addition, YAP/TEAD complex interacted with multiple transcriptional regulators, and the cross-talk between the YAP/TEAD complex and these transcriptional regulators determines the ultimate output [4]. Investigating the regulation of Hippo-YAP signaling would benefit the clinical therapy for Hippo-YAP-associated diseases.
Upregulation of YAP/TEAD signaling has been found in various cancer types, including hepatocellular carcinoma (HCC) [5–8]. Numerous studies have demonstrated the important functions of YAP in the tumorigenesis of HCC [8, 9]. Knocking down the expression of YAP inhibited the proliferation of MHCC97H cells [10]. Moreover, Perra et al. have shown that YAP activation was an early event in the liver cancer development [11]. In addition, the protein level of YAP is an independent prognostic marker in hepatocellular carcinoma [12, 13]. All of these reports emphasized the pivotal roles of YAP in the tumorigenesis of HCC.
Recently, studies have identified multiple regulators for Hippo-YAP signaling. MicroRNAs, such as miR-375, miR-506, and miR-9-3p, have been reported to downregulate YAP signaling in HCC by targeting YAP or TAZ [14–16]. Also, cross-talking between YAP signaling and PIK3CA, MEK, and AXL kinase has been observed in HCC [17–19]. Identifying the novel regulators of YAP/TEAD signaling has been a hot area.
Cip1 interacting zinc finger protein 1 (CIZ1), a binding protein of p21Cip1/Waf1, is a regulator for the initiation of DNA replication and replication fork organization [20]. Dysregulation of CIZ1 was reported in breast cancer [21], medulloblastoma [22], Ewing sarcoma, and lung cancer [21]. Our previous study has shown that CIZ1 was upregulated in HCC and promoted the growth and migration of the cancer cells by activating YAP signaling. However, the detailed molecular mechanism remains unknown. Here, we examined the interaction between CIZ1 and YAP and elucidated the roles for CIZ1 in the regulation of YAP signaling.
Materials and methods
Cell culture
Two hepatocellular carcinoma cell lines (7404 and Hep3B) were purchased from American Type Culture Collection (ATCC). 7404 and Hep3B cells were cultured in DMEM (Invitrogen) supplemented with 10 % fetal bovine serum (FBS; PAA Laboratories, Pasching, Austria). 7404 and Hep3B cells were cultured in a humidified atmosphere containing 5 % CO2 at 37 °C.
Plasmid construction and transfection
CIZ1 complementary DNA (cDNA) was inserted into the expression vector pcDNA 3.1-myc. Human YAP cDNA was inserted into the expression vector pCMV-tag2B, resulting in the Flag-tagged YAP (Flag-YAP). For construction of the glutathione-S-transferase (GST)-YAP expression plasmid, YAP cDNA was inserted into the expression vector pGEX-4T-1.
Western blotting
Total cell lysate was separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The blotted membranes were blocked with Tris-buffered saline containing 0.05 % Tween 20 (TBST) and 5 % fat-free dry milk for 1 h at room temperature and incubated overnight with primary antibodies in TBST with 1 % bovine serum albumin. After washing with TBST, the membranes were further incubated for 1 h at room temperature with corresponding horseradish peroxidase-conjugated secondary antibody in appropriate dilution. The immunoreactive protein bands were visualized by ECL kit (Pierce). Antibodies to CIZ1 and YAP were purchased from Cell Signaling Technology. Antibodies to GAPDH, myc tag, and Flag tag were purchased from Santa Cruz Biotechnology.
Knocking down the expression of PRMT1 in hepatocellular carcinoma cells
RNAi lenti-virus particles (si con and si CIZ1) were purchased from GeneChem (China). Cells were incubated with the lenti-virus particles for 24 h and then selected with the medium containing puromycine.
Crystal violet assay
Equal number of control cells and experimental cells were seeded in 12-well plates and cultured in a medium supplemented with 10 % FBS at a density of 1000 cells/well. Medium was changed every other day. After 10 days of culture under the standard condition, the medium was removed and the cells were stained with 0.5 % crystal violet solution in 20 % methanol. After staining for 10 min, the fixed cells were washed with phosphate-buffered saline (PBS) and photographed.
Boyden chamber assay
Cells (2 × 105) suspended in 0.05 ml of a medium containing 1 % FBS were placed in the upper chamber, and the lower chamber was loaded with 0.152 ml of a medium containing 10 % FBS. Six hours later, cells which migrated to the lower surface of filters were detected with traditional H&E staining. The experiments were repeated thrice.
Immunoprecipitation assay
Cells were washed with ice-cold PBS and lysed in Tris-buffered saline (pH 7.4), containing 50 mM Tris, 150 mM NaCl, 1 % NP-40, 1 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 2.5 mg/ml aprotinin and leupeptin, 1 mM beta-glycerophosphate and 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), and 10 mM iodoacetate. Lysates were incubated on ice for 15 min before cellular debris and nuclei were removed by centrifugation at 10,000g for 20 min. Cell lysates were incubated with the corresponding primary antibodies overnight at 4 °C. Protein A-Sepharose (Amersham Biosciences, Piscataway, NJ, USA) beads in a 50:50 mixture in 50 mM Tris buffer, pH 7.0, were added and further incubated for another 4 h at 4 °C. The immunoprecipitates were washed four times in Tris-buffered saline and boiled for 5 min in 40 μl Laemmli buffer containing 0.02 % blue bromophenol and 2 % b-mercaptoethanol.
Luciferase assay
Cells were plated at a subconfluent density and co-transfected with 0.05 μg of the reporter plasmid, 0.5 μg of expression vectors, and 0.05 μg of Renilla luciferase pRL-TK as an internal control for transfection efficiency. Twenty-four hours later, cell lysates were prepared and the reporter activity was measured using the dual-luciferase reporter assay system (Promega). Transfections were performed in triplicate and repeated three times to ensure reproducibility.
GST pull-down assay
YAP1 was expressed as glutathione-S-transferase (GST) fusion proteins. pGEX-YAP1 plasmid was transfected into Escherichia coli. GST-YAP1 expression was induced by 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h at 37 °C. E. coli were harvested and resuspended in a solution containing 20 mM HEPES (pH 7.5), 120 mM NaCl, 10 % glycerol, 2 mM EDTA, and 10 μg/ml of leupeptin, aprotinin, and pepstatin A. Cells were lysed by sonication and centrifuged at 14,000 rpm at 4 °C for 15 min. The supernatants were incubated with glutathione-Sepharose beads for 1 h at 4 °C, and beads coupled with GST-YAP1 were washed six times with lysis buffer containing 0.5 % NP-40. Bound GST-YAP1 was eluted by boiling in SDS sample buffer, and the amount was determined by SDS-PAGE gel and Coomassie blue staining.
7404 cells were lysed and the cell lysates were incubated with 10 μg GST-YAP protein or GST protein overnight. Then, Sepharose 4B was added to the cell lysates and incubated for another 4 h. After centrifugation, the beads were collected and washed with the lysis buffer three times. Beads were boiled for 5 min in 40 μl Laemmli buffer containing 0.02 % blue bromophenol and 2 % b-mercaptoethanol. The proteins pulled down were examined by western blot.
Results
CIZ1 interacted with YAP in HCC cells
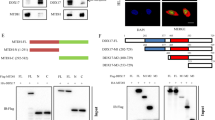
Our previous study has shown that CIZ1 promoted the growth as well as migration of hepatocellular carcinoma cells, and activating the YAP/TEAD signaling. However, the underlying molecular mechanism has been unknown. To explore the detailed molecular mechanism, we first investigated the interaction between CIZ1 and YAP. In the GST pull-down assay, the purified GST-YAP protein showed the binding activity with endogenous CIZ1 in 7404 cells (Fig. 1a). Next, the ectopic expression of the Flag-tagged YAP (Flag-YAP) and myc-tagged CIZ1 (myc-CIZ1) in 7404 cells was performed in 7404 cells. The following immunoprecipitation assay demonstrated the interaction between the exogenously expressed YAP and CIZ1 (Fig. 1b). Moreover, the interaction between the endogenously expressed CIZ1 and YAP was detected in the physiological condition (Fig. 1c). Also, co-localization of YAP and CIZ1 was observed in HCC tissues (Fig. 1d). These results demonstrated that YAP interacted with CIZ1 in the HCC cells.
CIZ1 formed a complex with YAP1. a CIZ1 interacted with GST-YAP1 fusion protein in the GST pull-down assay. GST-YAP fusion protein was purified and incubated with the 7404 cell lysate. The binding protein of GST-YAP was examined using anti-CIZ1 antibody. b The myc-CIZ1 and Flag-YAP1 plasmids were co-transfected into 7404 cells. Myc-CIZ1 was immunoprecipitated with anti-myc antibody. The immunoprecipitated protein was examined using anti-Flag antibody. c The endogenously expressed CIZ1 interacted with YAP1 in 7404 and Hep3B cells. d The co-localization of CIZ1 and YAP was found in HCC tissues. Green, CIZ1; red, YAP; blue, nucleus
The nuclear matrix anchor domain of CIZ1 is responsible for its interaction with YAP
Next, we mapped the domains of CIZ1 which mediated its interaction with YAP. Various CIZ1 expression vectors along with YAP expression vector were transfected into 7404 cells, and the interaction between YAP and CIZ1 different domains was examined using immunoprecipitation (Fig. 2a). It was found that YAP interacted with full-length CIZ1 and nuclear matrix anchor domain of CIZ1 but not the DNA replication domain of CIZ1 (Fig. 2b), suggesting the nuclear matrix anchor domain of CIZ1 mediated its interaction with YAP. We next examined whether CIZ1 modulated the interaction between YAP and TEAD. Overexpression of CIZ1 in 7404 cells promoted the interaction between YAP and TEAD, indicating that CIZ1 regulated the interaction between YAP and TEAD (Fig. 2c).
CIZ1 enhanced the interaction between YAP1 and TEAD. a Schematic graph of myc-CIZ1 protein. FL full length. b Nuclear matrix anchor domain of CIZ1 was responsible for the interaction with YAP. The indicated expression vectors were transfected into 7404 cells, and the myc-FL, myc-DNA replication domain, and myc-nuclear matrix anchor domain immunoprecipitated using anti-myc antibody. The interacted Flag-YAP was detected using anti-myc antibody. c CIZ1 promoted the interaction between YAP and TEAD in the immunoprecipitation assay
CIZ1 activated the transcriptional activity of YAP
The binding of YAP and CIZ1 prompted us to study whether CIZ1 regulated the transcriptional activity of YAP. As shown in Fig. 3a, downregulation of CIZ1 impaired the transcriptional activity of YAP in the luciferase assay in 7404 cells, while forced expression of CIZ1 enhanced the transcriptional activity of YAP in 7404 cells (Fig. 3a, b). Consistent with the observations from the luciferase assay, knocking down the expression of CIZ1 inhibited the expression of CTGF and Cyr61, two target genes downstream of YAP (Fig. 3c). To further demonstrate the roles of CIZ1 in regulating the expression of YAP target genes, we turned to the chromatin immunoprecipitation (ChIP) assay to examine the YAP/TEAD complex binding to the CTGF promoter. The results revealed that knocking down the expression of CIZ1 decreased binding of YAP to the CTGF promoter region (Fig. 3d). Taken together, these results suggested that knocking down of CIZ1 inhibited the transcriptional activity of YAP.
Knocking down the expression of CIZ1 impaired the transcriptional activity of YAP1. a Knocking down the expression of CIZ1 in 7404 cells impaired the transcriptional activity of YAP1 in the luciferase assay, while overexpression of CIZ1 enhanced the activity of YAP1 in 7404 cells. **P < 0.01. b Knocking down the expression of CIZ1 in 7404 cells impaired the expression of target genes downstream of YAP1. The indicated plasmids were transfected into 7404 cells. Total RNA was isolated and the mRNA levels of CTGF and cyr61 were examined. **P < 0.01. c Knocking down the expression of CIZ1 impaired the binding of YAP1 to the promoter region of CTGF
CIZ1 was necessary for the growth and migration of HCC cells induced by YAP
The roles of YAP in the growth and migration of HCC cells were reported in several studies. We next examined whether CIZ1 affected the functions of YAP in the HCC cells. Overexpression of YAP promoted the growth of 7404 and Hep3B cells in the crystal violet assay, while knocking down the expression of CIZ1 abolished the promoting effects of YAP on the growth of HCC cells (Fig. 4a, b). Consistent with these observations, downregulation of CIZ1 abolished the promoting effects of YAP on the migration of HCC cells in the Boyden chamber assay (Fig. 4c, d). Collectively, these results indicated that CIZ1 was necessary for the biology functions of YAP in HCC cells.
CIZ1 was necessary for the biological functions of YAP. a Knocking down the expression of CIZ1 abolished the growth advantage of 7404 HCC cell line and Hep3B cells overexpressing YAP. b Quantification of the results in (a). **P < 0.01. c Knocking down the expression of CIZ1 abolished the promoting effects of YAP1 on the migration of 7404 HCC cell line and Hep3B cells. d Quantification of the results in (c). **P < 0.01
Discussion
This study along with our previous study has identified CIZ1 as a cancer-promoting gene in HCC via positively regulating YAP/TEAD transcriptional complex activity. Through examining clinical HCC samples and paired adjacent noncancerous tissues, we found that CIZ1 was significantly increased in HCC. Taking advantage of HCC cancer cell culture system, we clearly showed that CIZ1 exhibited strong oncogenic roles in the growth and migration of HCC cells. Moreover, our data showed that CIZ1 acted as a novel positive regulator of YAP/TEAD transcriptional complex through enhancing the interaction between YAP and TEAD. Thus, our studies have provided a novel mechanism in HCC carcinogenesis through activating YAP/TEAD transcriptional activity by CIZ1.
Initially, the functions of CIZ1 were characterized in DNA replication [20]. Although dysregulation of CIZ1 has been reported in several types of cancer [23], the potential contribution of CIZ1 to the carcinogenesis of HCC and the detailed mechanism remains to be elucidated. Our study here provided strong evidence that CIZ1 was upregulated in HCC specimens and played an oncogenic role in HCC. Coincidently, a recent study has convincingly shown that CIZ1 promotes the growth and migration of gallbladder cancer cells [21]. Taken together, these findings suggested that CIZ1 might act as a common tumor-promoting regulator in a variety of human cancers including gallbladder cancer and liver cancer.
CIZ1 has been reported to activate beta-catenin/T cell factor (TCF) signaling in gallbladder cancer [21]. Taken our studies into consideration, CIZ1 might be a positive regulator for both beta-catenin/TCF signaling and TEAD/YAP signaling. Activation of beta-catenin/TCF signaling and TEAD/YAP signaling is very common in the initiation and progression of HCC [8, 24]. Inhibition of the functions of CIZ1 might be a novel strategy to target beta-catenin/TCF signaling and TEAD/YAP signaling simultaneously. Therefore, CIZ1 might be a therapeutic target for HCC.
References
Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–28.
Badouel C, McNeill H. SnapShot: the Hippo signaling pathway. Cell. 2011;145(3):484–484 e1.
Zhao B, Guan KL. Hippo pathway key to ploidy checkpoint. Cell. 2014;158(4):695–6.
Zhang W et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24(3):331–43.
Zhang K et al. YAP and TAZ take center stage in cancer. Biochemistry. 2015;54(43):6555–66.
Avril T, Chevet E. Proteostasis trumps YAP in colon cancer. Sci Signal. 2015;8(397):fs18.
Yang S et al. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget. 2015;6(34):36019–31.
Zhao B, Lei Q, Guan KL. Mst out and HCC in. Cancer Cell. 2009;16(5):363–4.
Jie L et al. The Hippo-yes association protein pathway in liver cancer. Gastroenterol Res Pract. 2013;2013:187070.
Wang C et al. Knockdown of yes-associated protein inhibits proliferation and downregulates large tumor suppressor 1 expression in MHCC97H human hepatocellular carcinoma cells. Mol Med Rep. 2015;11(6):4101–8.
Perra A et al. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61(5):1088–96.
Han SX et al. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res. 2014;2014:261365.
Li H et al. Yes-associated protein expression is a predictive marker for recurrence of hepatocellular carcinoma after liver transplantation. Dig Surg. 2014;31(6):468–78.
Liu AM, Poon RT, Luk JM. MicroRNA-375 targets Hippo-signaling effector YAP in liver cancer and inhibits tumor properties. Biochem Biophys Res Commun. 2010;394(3):623–7.
Wang Y et al. MiR-506 suppresses proliferation of hepatoma cells through targeting YAP mRNA 3'UTR. Acta Pharmacol Sin. 2014;35(9):1207–14.
Higashi T et al. miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in hepatocellular carcinoma cells. Br J Cancer. 2015;113(2):252–8.
Li X et al. Co-activation of PIK3CA and Yap promotes development of hepatocellular and cholangiocellular tumors in mouse and human liver. Oncotarget. 2015;6(12):10102–15.
Li L et al. MEK1 promotes YAP and their interaction is critical for tumorigenesis in liver cancer. FEBS Lett. 2013;587(24):3921–7.
Xu MZ et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30(10):1229–40.
Copeland NA et al. Cyclin-A-CDK2-mediated phosphorylation of CIZ1 blocks replisome formation and initiation of mammalian DNA replication. J Cell Sci. 2015;128(8):1518–27.
Zhang D et al. CIZ1 promoted the growth and migration of gallbladder cancer cells. Tumour Biol. 2015;36(4):2583–91.
Warder DE, Keherly MJ. Ciz1, Cip1 interacting zinc finger protein 1 binds the consensus DNA sequence ARYSR(0–2)YYAC. J Biomed Sci. 2003;10(4):406–17.
Higgins G et al. Variant Ciz1 is a circulating biomarker for early-stage lung cancer. Proc Natl Acad Sci U S A. 2012;109(45):E3128–35.
Takigawa Y, Brown AM. Wnt signaling in liver cancer. Curr Drug Targets. 2008;9(11):1013–24.
Acknowledgments
This work was supported by International Science and Technology Cooperation Program of the Ministry of Science and Technology (2011DFA32980), One Hundred Person Project of the Shanghai Health (XBR2013117), and the National Natural Science Foundation of China (NSFC81271694).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Lei, L., Wu, J., Gu, D. et al. CIZ1 interacts with YAP and activates its transcriptional activity in hepatocellular carcinoma cells. Tumor Biol. 37, 11073–11079 (2016). https://doi.org/10.1007/s13277-016-4866-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4866-8