Abstract
RNA-binding protein Lin28 was originally found as a heterochronic gene which played a significant role in the development of Caenorhabditis elegans. The tumor suppressor let-7 is a downstream target of Lin28, which has a wide variety of target genes which are involved in many aspects of cellular activities. By inhibition of let-7 and directly binding the target RNAs, Lin28 plays an important role in different biological and pathological processes including differentiation, metabolism, proliferation, pluripotency, and tumorigenesis. Overexpression of Lin28 has been reported in several kinds of cancers and is correlated with poor outcomes. It has been shown that Lin28 could affect the progression of cancers in several ways, such as promoting proliferation, increasing glucose metabolism, and inducing epithelial-mesenchymal transition (EMT) and cancer stem cells. Decrease of Lin28 expression or reactivation of let-7 in cancer cells could induce a reverse effect, indicating their therapeutic values in developing novel strategies for cancer treatment. Here, we will overview the regulatory mechanisms and functions of Lin28 in cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Regulation mechanisms of Lin28
RNA-binding proteins (RBPs) interact with messenger RNAs (mRNAs) and non-coding RNAs to form dynamic complex structures called messenger ribonucleoprotein particles (mRNPs). The mRNPs regulate gene expression through modulating almost all processes of mRNAs, such as processing, exportation, localization, translation, and degradation [1, 2]. Dysregulation of RBPs’ expression is an important pathogenic mechanism of cancer [2]. In this review, we will focus on the role of Lin28 in cancer.
Lin28 was initially identified as a heterochronic gene which manipulated the developmental timing of Caenorhabditis elegans [3]. Mutations of Lin28 could induce a precocious development in C. elegans [4]. Lin28 has two homologs including Lin28a and Lin28b. Lin28a gene locates in human chromosome 1p36.11 and encodes a protein which has 209 amino acids, while Lin28b is sited in 6q21 and encodes a protein of 250 amino acids [5]. Both of them contain a unique cold-shock domain (CSD) and a zinc knuckle domain consisting of two CysCysHisCys (CCHC) type zinc fingers, which mediate the combination of Lin28 and its target RNAs. Lin28 is broadly expressed in the early development and stem cells. During the process of differentiation, the expression of Lin28 decreases, which is finally absent in most differentiated cells of the adult [6]. In pluripotent mammalian cells, it has also been found that Lin28 localizes to mRNP complexes, cytoplasmic processing bodies, and stress granules where mRNAs and microRNAs were regulated [7]. Furthermore, studies have revealed some differences between Lin28a and Lin28b [8–10]. For example, in mouse testis, the expression and distribution patterns of Lin28a and Lin28b vary disparately during postnatal development, which suggests the distinct functional roles of them [10]. Moreover, recent studies have shown that Lin28a and Lin28b regulate let-7 through different mechanisms [9]. Nevertheless, researchers have speculated that they commonly bind to the same target sites on RNAs and share similar functions. However, further investigations of the differences and relationships between Lin28a and Lin28b are still needed.
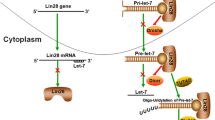
The mechanism of Lin28 function remains an attractive issue. Current studies mainly focus on two regulation patterns. One is the Lin28-let-7-dependent regulation axis, which is a significant regulation pattern of Lin28. Let-7 is also found as a heterochronic gene in C. elegans and plays an essential role in embryo development. Let-7 family contain 12 different members including let-7a-1, -2, -3; let-7b; let-7c; let-7d; let-7e; let-7f-1; let-7f-2; let-7g; let-7i; and miR-98 in human species. All of them can be suppressed by Lin28. And contrary to Lin28, let-7 family is barely expressed in the embryonic stages and is upregulated at the end of embryonic development [8]. As one of the most well-known microRNAs (miRNAs), let-7 is identified as a tumor suppressor. It has multiple target genes which are involved in many aspects of cellular activities [11]. Both let-7 and Lin28 are indispensible in the differentiation of stem cells [3]. Lin28 can bind to the G-rich element (GGAG, GAAG, or AGGG) located at the 3′ loop structure of the primary and precursor of let-7 transcripts. Then, Lin28 mediates the uridylation of the transcripts, which blocks the following DROSHA and DICER1 RNases III processing [8, 12–14]. The uridylation process is performed by polymerase TUTase4, which is recruited by Lin28, and then adds an oligouridine tail to the transcripts [13, 15]. Later, the exoribonuclease DIS3L2 specifically targets the uridylated tail and initiates the degradation of pre-let-7 from 3′ to 5′ terminal direction [16]. Unlike Lin28a, the function of Lin28b is mediated through sequestering primary let-7 transcripts from cleavage in the nucleus. This distinction might result from the different subcellular localization of Lin28a and Lin28b. Lin28a mainly distributes in cytoplasm. While due to the nuclear localization signals in Lin28b, it primarily locates in nucleoli [9]. Furthermore, researchers have found a double-negative feedback loop between Lin28 and let-7 that let-7 can also bind to the 3′ UTR of Lin28 and repress its expression [17, 18].
Directly binding to target RNAs is another crucial functional mechanism of Lin28, which is a let-7-independent pattern. More than 6000 genes have been found to possess Lin28-responsive elements (LREs) in human embryonic stem cells and somatic cells. Not only the 5′- and 3′-untranslated regions of mRNAs but also the open reading frames (ORFs) contain the LREs [19, 20]. Many mRNAs have been proven to bind Lin28 directly, such as cyclin A/B/D, cyclin-dependent kinase 2/4 (Cdk2/4), cell division cycle 2/20 (Cdc2 and Cdc20), and insulin-like growth factor-II (IGF2) [20]. Recently, the GGAGA(U) motif has been reported as a significant binding site of Lin28 and is enriched in interacted mRNAs’ loop structures [21]. However, the detailed mechanisms by which Lin28 stabilizes its target RNAs are still unclear. Furthermore, the auto-regulation of both let-7 and Lin28 increases the complexity of the regulatory network. Mature let-7 helps to the combination of Argonaute and primary let-7 transcript, which increases the mature let-7 level through promoting the processing events of the pri-let-7 [22]. And, Lin28 can also directly bind to its own mRNA and enhance the protein expression [21].
Aberrant expression of Lin28 correlates with tumorigenesis
Several experiments have shown that overexpression of Lin28 could result in Wilms tumor, neuroblastoma, hepatoblastoma, and hepatocellular cancer in mouse models [23–25]. Aberrant expression of Lin28 has also been found in many kinds of cancers, such as hepatocellular [26], lung [27], kidney [28], colon carcinoma, glioma [29], breast cancer [30, 31], gastric cancer [32], bladder cancer [33], oral squamous cell carcinoma [34], germ cell carcinoma [35], and ovarian carcinoma [36, 37]. Another study has discovered that Lin28 was reactivated in 7.1–17.1 % of epithelial tumors in a total of 369 different kinds of specimens. And, suppression of the endogenous Lin28 could inhibit cell growth [38]. These discoveries imply that Lin28 may function as an important oncogene, and dysregulation of Lin28 wildly exists in cancers.
The tumor-promoting effect of Lin28 is correlated with both the downstream targets and upstream signals. Oncogenes like Myc [39, 40], Ras, and embryonic gene high-mobility group A2 (HMGA2), which are involved in the regulation of different cancers, are classical downstream targets of Lin28/let-7 axis [41, 42]. Lin28 can derepress Myc expression via repressing let-7. And, Myc can interact with its partner protein Myc-associated protein X (MAX) and other co-factors to form heterodimers and thus activating transcription of target genes. Dysregulation of Myc promotes the development of cancer by promoting cell proliferation and angiogenesis, catalyzing DNA replication, and inhibiting differentiation [43]. Furthermore, Myc can increase Lin28 expression at transcriptional level, forming a positive feedback loop between Lin28 and Myc [24, 44, 45]. As to RAS, all the three members of RAS family, HRAS, KRAS, and NRAS, contain multiple let-7 complementary sites in their 3′-untranslated regions of mRNAs [46]. RAS genes also control several aspects of cellular behaviors through interacting with different signal pathways. Point mutation or defects in the signaling pathway of RAS protein contribute to the initiation of cancer by inducing proliferation, invasion, and angiogenesis [47]. HMGA2, another target of Lin28, has been found in a great number of cancers and is associated with cancer metastasis, chemoresistance, and poor survival [48]. On the other hand, Lin28 can mediate the upstream tumorigenic signals. For example, dysregulation of miR-125, miR-9, miR-30, tristetraprolin, let-7, c-Myc, nuclear factor kappa B (NF-κB), and the eukaryotic initiation factor (eIF5A) are important upstream signals of Lin28 which are closely related to tumorigenesis [20, 49, 50]. Take eIF5A for example; eIF5A is involved in the initiation and elongation process. It is elevated in many cancers and correlated with the clinical characteristics of cancer patients [51]. And, elF5A is a direct regulator of Lin28/let-7 axis. Treatment of cancer cells with difluoromethylornithine (DFMO), a drug which inhibited polyamine biosynthesis to modulate the transcriptional modification of eIF5A, can restore the balance of Lin28/let-7 axis and inhibit glycolytic metabolism. These findings suggest that Lin28 might be involved in the tumorigenic effect of eIF5A [52, 53]
Lin28 also plays an important role in bridging inflammation and cancer. Chronic inflammation and tumor-elicited inflammation are correlated with the initiation, progression, and metastatic of cancers. This effect is mediated by many related chemokines, cytokines, and transcription factors such as NF-κB and signal transducer and activator of transcription factor 3 (STAT3) [54]. Lliopoulos and his colleagues demonstrated a positive feedback loop between inflammation and cell transformation concerning Lin28 and let-7. After the transient activation of an inflammatory signal mediated by the oncoprotein Src, the NF-κB expression is increased and elicits a higher level of Lin28 and a decrease of let-7. The increase of Lin28 leads to a higher expression of IL-6. On the other hand, IL-6 can promote the activation of NF-κB, thereby forms a positive feedback loop among these factors. IL-6-mediated activation of STAT3 is an essential element for the transformation process [55]. In the serum of non-small cell lung cancer (NSCLC) patients, the level of proinflammatory cytokine IL-1β is dramatically elevated. IL-1β can repress miR-101 through cyclooxygenase 2 (COX2)-HIF pathway. And, miR-101 is a negative regulator of Lin28. Thus, IL-1β can upregulate Lin28b by repressing miR-101. COX2 inhibitors like aspirin or celecoxib could abrogate the effects on Lin28 and miR-101 mediated by IL-1 [27]. All the results above highlight the importance of Lin28/let-7 axis in the regulation of inflammation and cancers [27] (Fig. 1).
Elevated Lin28 expression promotes cancer cell proliferation in different ways
Lin28 contributes to enhanced glucose metabolism
Cancer cells exhibit a high rate of glucose uptake and glycolysis to adapt increased energy requirement, even though in a condition of sufficient oxygen, this phenomenon is called Warburg effect [56]. Increasing evidences have showed that Lin28 is associated with elevated glucose metastasis in cancers, thus promoting the cell proliferation [57]. Shyh-Chang et al. have found that transgenic mouse with constitutive low level of Lin28 expression shows resistance to obesity, enhancement in insulin sensibility, glucose metabolic, increased body size, and faster repair after tissue injuries [58, 59]. Meanwhile, let-7 is also closely related to glucose homeostasis. These information suggest that dysregulation of Lin28/let-7 axis might contribute to the enhanced metabolism of cancer cells [60]. Firstly, Lin28 derepresses the targets of let-7 such as insulin-like growth factor 1 receptor (IGF1R), insulin receptor (INSR), and insulin receptor substrate 2/4 (IRS2/4), which are components of insulin-PI3K-mTOR signaling pathway, thus increasing glucose metabolism and promoting cell growth [61–63]. Moreover, c-Myc also can increase the uptake of glucose and glycolysis, contributing to the Warburg effect [45, 64]. Further investigations have found that Lin28 promotes both oxidative phosphorylation and glycolysis. RNA immunoprecipitation assay has also shown that Lin28a directly binds to mRNAs and increases the translation of oxidative enzymes including phosphofructokinase (Pfkp), pyruvatedehydrogenase 1 (Pdha1), isocitrate dehydrogenase (Idh3b), succinate dehydrogenase (Sdha), and NADH dehydrogenase 3 and 8 (Ndufb3/8). Enhanced translations of these oxidative enzymes accelerate oxidative metabolism and reprogram cellular bioenergetics. In this process, downregulation of let-7 is a necessary but insufficient factor [59]. More recently, pyruvate dehydrogenase kinase 1 (PDK1) has been found as another important target of Lin28 in regulating aerobic glycolysis. In brief, Lin28 is tightly correlated with the glucose metabolism of cancer cells [65].
Lin28 promotes translation of ribosomal proteins, RNA metabolic enzymes, and other factors in concert with RNA helicase A
It has been found that elevated Lin28 protein level would increase the association of RNA helicase A (RHA) with polysomes. RHA belongs to DEAD-box RNA helicase family and exists ubiquitously in cells. RHA catalyzes RNA-RNA and RNA-protein rearrangements and thus facilitates mRNA translation, especially for mRNA with highly structured RNA elements [66]. Studies have found that point mutation of either CDS or CCHC domain can abrogate the regulation ability of Lin28 to target mRNAs, while it does not affect its interaction with RHA. Furthermore, both the amino and carboxyl terminus of RHA have Lin28 interacting domains which simultaneously interact with Lin28. RHA acts as an important co-factor of Lin28 to enhance the translation of the target mRNAs of Lin28 [19].
Another underlying mechanism of the tumor-promoting effect of Lin28 might be related to the downstream targets concerning regulation of RNA metabolic processes. Wilbert’s study found that increased Lin28 level would cause a widespread change in alternative splicing patterns. Besides, the gene ontology (GO) analysis of their work also identified 1386 target genes concerning the regulation of RNA metabolic processes, 234 genes relating to RNA splicing and 87 genes involved in RNA localization [21]. For instance, TDP-43, FUS/TLS, and TIA-1 were controlled by Lin28. The TDP-43 and FUS/TLS played important roles in the regulation of mRNA and miRNA. TIA-1 was a key factor in the formation of stress granules [21]. Another convinced evidence for this notion came from a genome-wide study. It demonstrated that Lin28 selectively bound to a relatively small subset of mRNAs concerning with cell growth and survival. They chose the top 268 genes which enriched at least 2.5-fold in Lin28 immunoprecipitation (IP) and carried out the GO analysis to classify them into different groups. Results showed that these mRNAs represented genes concerning encoding RNP protein, participating in biosynthesis, translation, cellular metabolism, and other crucial cellular progressions [19]. Overall, overexpression of Lin28 could induce high level of protein that were related to the survival and growth of cancer cells. These results suggest that with the help of RHA, enhanced translation of ribosomal proteins, metabolic enzymes, and other factors is another important mechanism by which Lin28 promotes cancer progression [7, 19, 21, 66, 67].
Lin28 regulates the cell cycle proteins
It has been found that Lin28 is closely connected with cell cycle regulators. In mouse embryonic stem cells, disturbed Lin28 expression mainly influenced the cell cycle in G2/M transition [68]. However, in cancer cells, researchers have found that Lin28a promotes proliferation of tumor cells through regulating the G0/G1 transition in cell cycle [38]. This can partly be explained by the fact that Lin28 promotes cell division through different cell cycle proteins in ES cells and cancer cells. Studies have demonstrated that Lin28 can increase the expression of cyclin D1 and 2 (CCND1/2), cell division cycle 25 homolog A (CDC25A), Cdc34, Cdk6, and other cell cycle-related factors by repressing let-7. Furthermore, Lin28 can also increase the translation of cyclin A/B/C, Cdc2/20, and Cdk 1/2/4 by directly binding to the mRNAs [20]. In addition to the direct interaction with cell cycle regulators, Lin28/let-7 axis can also affect other oncogenes and signal pathways like Myc, Ras, and PI3K/Akt signaling to increase cell proliferation [20, 69]. Thus, Lin28 promotes cell proliferation in multiple checkpoints of the cell cycle progression through different mechanisms [20, 38, 68].
Lin28 is involved with induction of EMT and cancer stem cells
The epithelial-mesenchymal transition (EMT) is the process of epithelial cells detaching from the epithelial sheet and obtaining a migratory mesenchymal phenotype. It is believed that the cancer cells which undergo an EMT and migrate to blood vessels are key factors of the initiation of cancer metastasis [31, 70–73]. In breast cancer, Lin28 has a high expression profile in mesenchymal-type cells compared with epithelial-type cells. Besides, overexpression of Lin28 increases vimentin, a mesenchymal marker, and decreases the epithelial marker E-cadherin via the suppression of let-7. And, the cell morphology also changes toward a mesenchymal phenotype [31]. Researchers have found that Lin28 participates in the EMT which are driven from oncostatin M (OSM), a member of the IL-6 family, in a STAT3-dependent way. STAT3 binds to the promoter of Lin28 and increases its expression, thus upregulates the HMGA2 level via suppression of let-7. Inhibition of HMGA2 would impede the OSM-induced EMT, suggesting that HMGA2 might act as a key performer for EMT [74].
Cancer stem cells can be identified as a minority of cancer cells which have the abilities of indefinitely self-renewal to perpetuate themselves and generate new tumors. The invasion, metastasis, relapse, and drug resistance of cancer are tightly related to the cancer stem cells. Lin28, together with Oct4, Sox2, and Nanog, can reprogram human somatic cells into pluripotent stem cells (iPSC) which shares several features similar to human embryonic stem cells [75]. Cancer stem cells are believed to arise through reprogramming mechanisms which are similar to the generation of iPSC [76]. Researches have demonstrated that acetaldehyde dehydrogenase 1 (ALDH1) could be identified as a biomarker of both normal and malignant mammary progenitor cells. High activity of ALDH1 always predicts poor outcomes in breast cancers [77]. And, Lin28/let-7 axis plays an important role in regulating ALDH1+ cancer stem cells [17, 18, 30]. Lin28 expression is positively correlated with the ALDH1+ cell percentage and is essential to maintain the ALDH1+ tumor cells. And, let-7 decreases the population of ALDH1+ tumor cell [18, 30, 76]. The positive correlation between Lin28 expression and percentage of ALDH1+ tumor cells has been found in clinical specimens and cell lines in vitro [17]. Moreover, Lin28 upregulates the expression of Oct4 and Sox2, which is mediated by the derepression of AT-rich interaction domain molecule 3B (ARID3B) and HMGA2, respectively [76]. Ectopic Lin28 can increase cancer stem-like properties and reprogramming efficiency in oral squamous carcinoma cells. These findings suggest that Lin28/let-7 axis is closely related to the generation of cancer stem cells.
Interestingly, after the induction of an EMT on the non-tumorigenic, immortalized human mammary epithelial cells (HMLEs), the mesenchymal-like cells obtain stem cell markers and increased mammosphere-forming ability. And, stem-like cells isolated from mammary carcinomas also contain markers that indicate the cells that undergone EMT progression [71]. Thus, EMT and cancer stem cells are interconnected. The links between EMT and cancer stem cells are involved in many molecular mechanisms [72]. For example, the double-negative feedback loop of miR-200 and the ZEB families (ZEB1 and ZEB2) is involved in the program of EMT and mesenchymal-epithelial transition (MET). Interestingly, miR-200 can inhibit Lin28 directly, and Lin28 activates miR-200 by increasing the expression of Oct4. Based on the interaction of Lin28/let-7 and miR-200/ZEB in regulating the stemness and EMT, researchers devised a theoretical framework model to elucidate the interplay of EMT and stemness [72, 78]. They demonstrated that the relative strength of the couplings (inhibition of Lin28 by miR-200 and inhibition of ZEB by let-7) determines which phenotypes can gain stemness [31, 78–81].
Lin28 contributes to the resistance of radiation treatment and chemotherapy
Radiotherapy and chemotherapy are two important treatments for cancer patients. Resistance to radiotherapy and chemotherapy has long been a great challenge for effective treatment. However, the precise mechanism which mediated the resistance is still unclear. Abundant evidences have shown that overexpression of Lin28 contributed to cancer cell radiotherapy and chemotherapy resistance. For instance, several researches have demonstrated that reactivation of Lin28 in cancer cells would decrease the radiotherapy and chemotherapy sensitivity [55, 82–86]. Firstly, as discussed above, Lin28 can promote the induction of the cancer stem cells, which is a crucial underlying mechanism of the therapy resistance. Secondly, researchers have also demonstrated that the let-7 family, as well as other relative factors such as RB, p21, Bcl-xL, caspase, and miR-107 can be regulated by Lin28 and participate in the mechanism of radio/chemoresistance [55, 82–86]. On the other hand, restored let-7 abundance or knockdown of Lin28 can reverse the resistance of cancer cells in vitro. Rebalance of Lin28/let-7 might be an effective method to overcome the radio/chemoresistance.
Lastly, rebalance of Lin28/let-7 axis also shows a promising prospect in cancer cells with positive expression of epidermal growth receptor 2 (HER2) or androgen receptor (AR). Lin28 can promote the growth, invasion, and soft agar colony forming of prostate cancer (PCa) cells. And in PCa, the expression of Lin28 is increased, while let-7 is suppressed. Further researches have also demonstrated that Lin28 could increase the expression of AR and activate the AR-dependent signaling [87–89]. AR is a steroid hormone receptor involved in both normal functions of target organs and pathological processes of PCa. The amplifications of AR gene and protein contribute to the survival of PCa cells. Besides, high abundance of AR is tightly connected with castration-resistant PCa. On the contrary to the role of Lin28, let-7 can inhibit PCa growth and suppress AR expression by targeting c-Myc [88]. Thus, rebalance of the Lin28/let-7 axis increases the efficacy of endocrine therapy which targets androgen synthesis and AR pathway in prostate cancer [90]. As to HER2 protein, which is overexpressed in several types of cancers, is a famous target of the cancer targeted therapy with trastuzumab (Herceptin). Lin28 has been identified to increase HER2 expression through binding to HER2 mRNA at post-transcriptional level [91, 92]. Both Lin28 and HER2 are correlated with tumor invasion and poor clinical prognosis in gastric cancer [91]. Similarly, silence of Lin28 combined with Herceptin may be a promising therapeutic tactics for cancer. Further studies of Lin28 are still needed to understand its diagnostic and prognostic values.
Prospective
As a RNA-binding protein, Lin28 was originally found to play a significant role in the development of C. elegans. Then, Lin28 is found to play an essential role in several biological and pathological activities including differentiation, metabolism, proliferation, and tumorigenesis in both let-7-dependent and let-7-independent manners. Elevated expression of Lin28 has been found in different kinds of cancers and is connected with different progressions of cancers. For example, Lin28 contacts with both upstream and downstream oncogenic targets and signals. And, Lin28 can increase glucose metabolism, promote translation of ribosomal protein, and regulate cell cycle regulators. Thus, Lin28 promotes the proliferation of cancer cells in different ways. Furthermore, the induction of EMT and cancer stem cells is closely related to Lin28. Lin28 can also induce the resistance to radiation treatment and chemotherapy (Fig. 2). Decreased expression of Lin28 or reactivation of let-7 in cancer cells could induce a reverse effect. Briefly, Lin28 acts as an important oncogene concerning different aspects of tumor progressions, and it can be used as an effective target for cancer treatment. However, further researches of its functions and regulation mechanisms in cancers are still in great need.
References
Mitchell SF, Parker R. Principles and properties of eukaryotic mRNPs. Mol Cell. 2014;54(4):547–58.
Wurth L, Gebauer F. RNA-binding proteins, multifaceted translational regulators in cancer. Biochim Biophys Acta. 2015;1849(7):881–6.
Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88(5):637–46.
Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226(4673):409–16.
Zhou J, Ng SB, Chng WJ. LIN28/LIN28B: an emerging oncogenic driver in cancer stem cells. Int J Biochem Cell Biol. 2013;45(5):973–8.
Yang DH, Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns. 2003;3(6):719–26.
Balzer E, Moss EG. Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 2007;4(1):16–25.
Hafner M, Max KE, Bandaru P, Morozov P, Gerstberger S, Brown M, et al. Identification of mRNAs bound and regulated by human LIN28 proteins and molecular requirements for RNA recognition. RNA. 2013;19(5):613–26.
Piskounova E, Polytarchou C, Thornton JE, LaPierre RJ, Pothoulakis C, Hagan JP, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147(5):1066–79.
Gaytan F, Sangiao-Alvarellos S, Manfredi-Lozano M, Garcia-Galiano D, Ruiz-Pino F, Romero-Ruiz A, et al. Distinct expression patterns predict differential roles of the miRNA-binding proteins, Lin28 and Lin28b, in the mouse testis: studies during postnatal development and in a model of hypogonadotropic hypogonadism. Endocrinology. 2013;154(3):1321–36.
Kolenda T, Przybyla W, Teresiak A, Mackiewicz A, Lamperska KM. The mystery of let-7d - a small RNA with great power. Contemp Oncol (Pozn). 2014;18(5):293–301.
Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14(8):1539–49.
Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138(4):696–708.
Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor microRNA. Mol Cell. 2008;32(2):276–84.
Lim J, Ha M, Chang H, Kwon SC, Simanshu DK, Patel DJ, et al. Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell. 2014;159(6):1365–76.
Ustianenko D, Hrossova D, Potesil D, Chalupnikova K, Hrazdilova K, Pachernik J, et al. Mammalian DIS3L2 exoribonuclease targets the uridylated precursors of let-7 miRNAs. RNA. 2013;19(12):1632–8.
Yang X, Lin X, Zhong X, Kaur S, Li N, Liang S, et al. Double-negative feedback loop between reprogramming factor LIN28 and microRNA let-7 regulates aldehyde dehydrogenase 1-positive cancer stem cells. Cancer Res. 2010;70(22):9463–72.
Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10(8):987–93.
Peng S, Chen LL, Lei XX, Yang L, Lin H, Carmichael GG, et al. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. 2011;29(3):496–504.
Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12(4):395–406.
Wilbert ML, Huelga SC, Kapeli K, Stark TJ, Liang TY, Chen SX, et al. LIN28 binds messenger RNAs at GGAGA motifs and regulates splicing factor abundance. Mol Cell. 2012;48(2):195–206.
Zisoulis DG, Kai ZS, Chang RK, Pasquinelli AE. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature. 2012;486(7404):541–4.
Urbach A, Yermalovich A, Zhang J, Spina CS, Zhu H, Perez-Atayde AR, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes Dev. 2014;28(9):971–82.
Molenaar JJ, Domingo-Fernandez R, Ebus ME, Lindner S, Koster J, Drabek K, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44(11):1199–206.
Nguyen LH, Robinton DA, Seligson MT, Wu L, Li L, Rakheja D, et al. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell. 2014;26(2):248–61.
You X, Liu F, Zhang T, Lv N, Liu Q, Shan C, et al. Hepatitis B virus X protein upregulates Lin28A/Lin28B through Sp-1/c-Myc to enhance the proliferation of hepatoma cells. Oncogene. 2014;33(4):449–60.
Wang L, Zhang LF, Wu J, Xu SJ, Xu YY, Li D, et al. IL-1beta-mediated repression of microRNA-101 is crucial for inflammation-promoted lung tumorigenesis. Cancer Res. 2014;74(17):4720–30.
Rakheja D, Chen KS, Liu Y, Shukla AA, Schmid V, Chang TC, et al. Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nat Commun. 2014;2:4802.
Qin R, Zhou J, Chen C, Xu T, Yan Y, Ma Y, et al. LIN28 is involved in glioma carcinogenesis and predicts outcomes of glioblastoma multiforme patients. PLoS One. 2014;9(1), e86446.
Xie R, Wang Y, Nie W, Huang W, Song W, Wang Z, et al. Lin28B expression correlates with aggressive clinicopathological characteristics in breast invasive ductal carcinoma. Cancer Biother Radiopharm. 2014;29(5):215–20.
Liu Y, Li H, Feng J, Cui X, Huang W, Li Y, et al. Lin28 induces epithelial-to-mesenchymal transition and stemness via downregulation of let-7a in breast cancer cells. PLoS One. 2013;8(12):e83083.
Hu Q, Peng J, Liu W, He X, Cui L, Chen X, et al. Lin28B is a novel prognostic marker in gastric adenocarcinoma. Int J Clin Exp Pathol. 2014;7(8):5083–92.
Li Y, Liu H, Lai C, Du X, Su Z, Gao S. The Lin28/let-7a/c-Myc pathway plays a role in non-muscle invasive bladder cancer. Cell Tissue Res. 2013;354(2):533–41.
Wu T, Jia J, Xiong X, He H, Bu L, Zhao Z, et al. Increased expression of Lin28B associates with poor prognosis in patients with oral squamous cell carcinoma. PLoS One. 2013;8(12):e83869.
Murray MJ, Saini HK, Siegler CA, Hanning JE, Barker EM, van Dongen S, et al. LIN28 Expression in malignant germ cell tumors downregulates let-7 and increases oncogene levels. Cancer Res. 2013;73(15):4872–84.
Peng S, Maihle NJ, Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene. 2010;29(14):2153–9.
Lu L, Katsaros D, Shaverdashvili K, Qian B, Wu Y, de la Longrais IA, et al. Pluripotent factor lin-28 and its homologue lin-28b in epithelial ovarian cancer and their associations with disease outcomes and expression of let-7a and IGF-II. Eur J Cancer. 2009;45(12):2212–8.
Li N, Zhong X, Lin X, Guo J, Zou L, Tanyi JL, et al. Lin-28 homologue A (LIN28A) promotes cell cycle progression via regulation of cyclin-dependent kinase 2 (CDK2), cyclin D1 (CCND1), and cell division cycle 25 homolog A (CDC25A) expression in cancer. J Biol Chem. 2012;287(21):17386–97.
Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67(20):9762–70.
Dang CV. Therapeutic targeting of Myc-reprogrammed cancer cell metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:369–74.
Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21(9):1025–30.
Boyerinas B, Park SM, Shomron N, Hedegaard MM, Vinther J, Andersen JS, et al. Identification of let-7-regulated oncofetal genes. Cancer Res. 2008;68(8):2587–91.
Deng K, Guo X, Wang H, Xia J. The lncRNA-MYC regulatory network in cancer. Tumour Biol. 2014;35(10):9497–503.
Wang Z, Lin S, Li JJ, Xu Z, Yao H, Zhu X, et al. MYC protein inhibits transcription of the microRNA cluster MC-let-7a-1∼let-7d via noncanonical E-box. J Biol Chem. 2011;286(46):39703–14.
Buechner J, Tomte E, Haug BH, Henriksen JR, Lokke C, Flaegstad T, et al. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105(2):296–303.
Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–47.
Downward J. Targeting RAS, signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22.
Pallante P, Sepe R, Puca F, Fusco A. High mobility group a proteins as tumor markers. Front Med (Lausanne). 2015;2:15.
Kim CW, Vo MT, Kim HK, Lee HH, Yoon NA, Lee BJ, et al. Ectopic over-expression of tristetraprolin in human cancer cells promotes biogenesis of let-7 by down-regulation of Lin28. Nucleic Acids Res. 2012;40(9):3856–69.
Zhong X, Li N, Liang S, Huang Q, Coukos G, Zhang L. Identification of microRNAs regulating reprogramming factor LIN28 in embryonic stem cells and cancer cells. J Biol Chem. 2010;285(53):41961–71.
Mathews MB, Hershey JW. The translation factor eIF5A and human cancer. Biochim Biophys Acta. 2015;1849(7):836–44.
Lozier AM, Rich ME, Grawe AP, Peck AS, Zhao P, Chang AT, et al. Targeting ornithine decarboxylase reverses the LIN28/Let-7 axis and inhibits glycolytic metabolism in neuroblastoma. Oncotarget. 2015;6(1):196–206.
Paz EA, LaFleur B, Gerner EW. Polyamines are oncometabolites that regulate the LIN28/let-7 pathway in colorectal cancer cells. Mol Carcinog. 2014;53 Suppl 1:E96–106.
Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20(1):65–71.
Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 microRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356(2 Pt A):156–64.
Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010;42(7):626–30.
Shyh-Chang N, Zhu H. Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155(4):778–92.
Frost RJ, Olson EN. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc Natl Acad Sci U S A. 2011;108(52):21075–80.
Cargnello M, Tcherkezian J, Roux PP. The expanding role of mTOR in cancer cell growth and proliferation. Mutagenesis. 2015;30(2):169–76.
Tsialikas J, Romer-Seibert J. LIN28: roles and regulation in development and beyond. Development. 2015;142(14):2397–404.
Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94.
Dang CV. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found Symp Proc. 2007;4:35–53.
Ma X, Li C, Sun L, Huang D, Li T, He X, et al. Lin28/let-7 axis regulates aerobic glycolysis and cancer progression via PDK1. Nat Commun. 2014;5:5212.
Jin J, Jing W, Lei XX, Feng C, Peng S, Boris-Lawrie K, et al. Evidence that Lin28 stimulates translation by recruiting RNA helicase A to polysomes. Nucleic Acids Res. 2011;39(9):3724–34.
Qiu C, Ma Y, Wang J, Peng S, Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2010;38(4):1240–8.
Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15(3):357–61.
Spence T, Perotti C, Sin-Chan P, Picard D, Wu W, Singh A, et al. A novel C19MC amplified cell line links Lin28/let-7 to mTOR signaling in embryonal tumor with multilayered rosettes. Neuro Oncol. 2014;16(1):62–71.
van der Pluijm G. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone. 2011;48(1):37–43.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Wang SS, Jiang J, Liang XH, Tang YL. Links between cancer stem cells and epithelial-mesenchymal transition. Onco Targets Ther. 2015;8:2973–80.
Biddle A, Mackenzie IC. Cancer stem cells and EMT in carcinoma. Cancer Metastasis Rev. 2012. doi:10.1007/s10555-012-9345-0 [doi]
Guo L, Chen C, Shi M, Wang F, Chen X, Diao D, et al. Stat3-coordinated Lin-28-let-7-HMGA2 and miR-200-ZEB1 circuits initiate and maintain oncostatin M-driven epithelial-mesenchymal transition. Oncogene. 2013;32(45):5272–82.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
Chien CS, Wang ML, Chu PY, Chang YL, Liu WH, Yu CC, et al. Lin28B/Let-7 regulates expression of Oct4 and Sox2 and reprograms oral squamous cell carcinoma cells to a stem-like state. Cancer Res. 2015;75(12):2553–65.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67.
Jolly MK, Jia D, Boareto M, Mani SA, Pienta KJ, Ben-Jacob E, et al. Coupling the modules of EMT and stemness: a tunable ‘stemness window’ model. Oncotarget. 2015;6(28):25161–74.
Chang CJ, Hsu CC, Chang CH, Tsai LL, Chang YC, Lu SW, et al. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol Rep. 2011;26(4):1003–10.
Li Y, VandenBoom 2nd TG, Kong D, Wang Z, Ali S, Philip PA, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69(16):6704–12.
Wang YC, Chen YL, Yuan RH, Pan HW, Yang WC, Hsu HC, et al. Lin-28B expression promotes transformation and invasion in human hepatocellular carcinoma. Carcinogenesis. 2010;31(9):1516–22.
Oh JS, Kim JJ, Byun JY, Kim IA. Lin28-let7 modulates radiosensitivity of human cancer cells with activation of K-Ras. Int J Radiat Oncol Biol Phys. 2010;76(1):5–8.
Teng R, Hu Y, Zhou J, Seifer B, Chen Y, Shen J, et al. Overexpression of Lin28 decreases the chemosensitivity of gastric cancer cells to oxaliplatin, paclitaxel, doxorubicin, and fluorouracil in part via microRNA-107. PLoS One. 2015;10(12):e0143716.
Lv K, Liu L, Wang L, Yu J, Liu X, Cheng Y, et al. Lin28 mediates paclitaxel resistance by modulating p21, Rb and Let-7a miRNA in breast cancer cells. PLoS One. 2012;7(7):e40008.
Tian N, Han Z, Li Z, Zhou M, Fan C. Lin28/let-7/Bcl-xL pathway: the underlying mechanism of drug resistance in Hep3B cells. Oncol Rep. 2014;32(3):1050–6.
Yang X, Cai H, Liang Y, Chen L, Wang X, Si R, et al. Inhibition of c-Myc by let-7b mimic reverses mutidrug resistance in gastric cancer cells. Oncol Rep. 2015;33(4):1723–30.
Nadiminty N, Tummala R, Lou W, Zhu Y, Shi XB, Zou JX, et al. MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth. PLoS One. 2012;7(3), e32832.
Nadiminty N, Tummala R, Lou W, Zhu Y, Zhang J, Chen X, et al. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem. 2012;287(2):1527–37.
Tummala R, Nadiminty N, Lou W, Zhu Y, Gandour-Edwards R, Chen HW, et al. Lin28 promotes growth of prostate cancer cells and activates the androgen receptor. Am J Pathol. 2013;183(1):288–95.
Proverbs-Singh T, Feldman JL, Morris MJ, Autio KA, Traina TA. Targeting the androgen receptor in prostate and breast cancer: several new agents in development. Endocr Relat Cancer. 2015;22(3):R87–106.
Wang Q, Zhou J, Guo J, Teng R, Shen J, Huang Y, et al. Lin28 promotes Her2 expression and Lin28/Her2 predicts poorer survival in gastric cancer. Tumour Biol. 2014;35(11):11513–21.
Feng C, Neumeister V, Ma W, Xu J, Lu L, Bordeaux J, et al. Lin28 regulates HER2 and promotes malignancy through multiple mechanisms. Cell Cycle. 2012;11(13):2486–94.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Zhao, Q., Deng, K. et al. Lin28: an emerging important oncogene connecting several aspects of cancer. Tumor Biol. 37, 2841–2848 (2016). https://doi.org/10.1007/s13277-015-4759-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4759-2