Abstract
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer death worldwide. Cancer metastasis is a major obstacle in clinical cancer therapy. The mechanisms underlying the metastasis of HCC remain unclear. Glucose-regulated protein 94 (GRP94) is a key protein involved in mediating cancer progression, and it is highly expressed in HCC specimens. However, the role of GRP94 in cancer metastasis is unclear. A specific short hairpin RNA (shRNA) was employed to knock down GRP94 gene expression in HCC cell lines. Wound-healing migration, transwell migration, and invasion assays were performed to determine the migration and invasive ability of HCC cells. We demonstrated that silencing GRP94 inhibited HCC cell wound healing, migration, and invasion. Furthermore, our findings indicated that GRP94 knockdown might attenuate HCC cell metastasis by inhibiting CCT8/c-Jun/EMT signaling. Our study indicated that silencing GRP94 significantly reduced the migration and invasion abilities of HCC cells. Moreover, depleting GRP94 inhibited cell migration and invasion by downregulating CCT8/c-Jun signaling. Thus, our data suggest that the GRP94/CCT8/c-Jun/EMT signaling cascade might be a new therapeutic target for HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death in the world [1]. Persistent infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) is a major risk factor for the development of HCC [2]. Despite recent progress in therapy, the prognosis of HCC patients is still not optimistic. HCC is often diagnosed at late stages; thus, most patients have a limited number of therapeutic options. Clearly, there is an urgent need to identify useful biomarkers for the diagnosis and treatment of this aggressive cancer.
Glucose-regulated protein 94 (GRP94), an endoplasmic reticulum chaperone, plays a pivotal role in the regulation of cellular homeostasis [3]. GRP94 maintains calcium homeostasis and protects cancer cells from apoptosis [4]. Additionally, GRP94 was demonstrated to be involved in the Wnt-survivin pathway, which is critical in carcinogenesis [5]. Overexpression of GRP94 has been frequently found in several types of cancers, including breast, liver, lung, colorectal, gastric, pancreatic, and head and neck cancers [6–8]. The induction of GRP94 has been suggested to play critical roles in cell proliferation, metastasis, poor prognosis, drug resistance, and decreased survival [9–12]. GRP94 induction is associated with lymph node metastasis and carcinoma recurrence, and silencing of GRP94 reduced the migration and proliferation of breast cancer cells [13]. High GRP94 expression was associated with the pathogenesis, aggressive behavior, and prognosis of several cancer types [12]. However, the relationship between GRP94 and HCC progression and the exact function of GRP94 in the migration and invasion of HCC cells require further elucidation.
Chaperonin-containing TCP1 complex (CCT) proteins 1–8 are highly conserved molecular chaperones involved in promoting the correct folding of newly synthesized proteins or the refolding of misfolded proteins [14]. Moreover, CCT proteins play important roles in maintaining cellular proteostasis. They are implicated in the regulation of folding of cyclin E, cyclin B, and p21ras, indicating that they might be involved in the regulation of cell proliferation and cancer progression [15]. Depletion of CCT inhibited cell growth, altered cell morphology, and affected cell motility [16, 17]. Furthermore, CCT proteins have been suggested to be involved in the progression of several cancer types, including breast cancer, colorectal cancer, uterine sarcoma, and lung cancer [15, 18–21]. Recently, CCT8 overexpression was discovered in colon cancer, breast cancer, glioma, and HCC [22–25]. Although CCT8 overexpression was identified in HCC, the connection between CCT8 induction and the progression of HCC remains unclear.
We conducted the present study to investigate the role of GRP94 in the migration and invasion of HCC cells. We found that silencing of GRP94 caused decreases in cell migration and invasive ability. Exploring potential mechanisms, we found that CCT8 may be a key molecule in the GRP94-mediated migration and invasion of HCC cells. Taken together, our results demonstrate that targeting GRP94/CCT8 may offer a new therapeutic strategy for the treatment of HCC.
Materials and methods
Chemicals, reagents, and cell cultures
Triton X-100, Tris-HCl, puromycin, Trypan blue EDTA, ribonuclease-A, and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Co. (St. Louis, MO). The anti-GRP94 (MABT196) antibody was purchased from Millipore. The anti-CCT8 (GTX105725) antibody was purchased from GeneTex. Antibodies against JNK (9258), E-cadherin (3195), and N-cadherin (13116) were purchased from Cell Signaling Technology. The anti-p-JNK (ab4821) antibody was purchased from Abcam. Antibodies against vimentin (sc6260), fibronectin (sc9068), c-Jun (sc1694), c-Fos (sc-52), and GAPDH (sc32233) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). HepJ5 and Mahlavu cell were gifted from Dr. C.S Yang, National Taiwan University, and Dr. C. P. Hu, Veterans General Hospital, Taiwan [26–28]. The HCC cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL, Grand Island, NY, USA), which was adjusted to contain 2 mM l-glutamine, 1.5 g/l sodium bicarbonate, 10 % fetal bovine serum (Gibco BRL), and 2 % penicillin-streptomycin (10,000 U/ml penicillin and 10 mg/ml streptomycin). Cells were maintained in a 5 % CO2 humidified incubator at 37 °C as previously described [29, 30].
Generation of GRP94 knockdown in HCC cells
GRP94 knockdown (GRP94-KD) cells were generated as previously described [29–32]. HCC cells were transfected with GRP94-specific short hairpin RNA (shRNA) (National RNAi Core Facility, Academia Sinica, Taiwan) and the parental vector (pLKO.1 < −puro) and then selected with puromycin. The target sequence for the human GRP94 mRNA (NM_003299) gene was 5′-GCGAGACTCTTCAGCAACATA-3′. The MISSION non-target shRNA control vector (SHC002) was used as a scrambled control (Sigma Chemical Co.) [29–32]. The transfection protocol was performed as previously described [31–33]. Briefly, 1.5 × 105 cells were washed twice with phosphate-buffered saline (PBS) and mixed with 0.5 μg of plasmid. We applied one pulse for a duration of 20 ms under a fixed voltage of 1.4 kV on a Neon pipette-type microporator (Invitrogen Life Technologies, Grand Island, NY), and the stably transfected cell lines were selected using puromycin (1 mg/ml) as previously described [31–33].
Protein extraction and Western blot analysis
Protein abundance was determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot as previously described [29, 30]. The cells were washed with cold PBS and lysed using a cell lysis buffer containing protease inhibitors (Complete Protease Inhibitor Tablets, Boehringer Mannheim, Indianapolis, IN). Equal amounts of proteins were separated by 10 % SDS-PAGE under reducing conditions and electrotransferred onto PVDF membranes (Bio-Rad Laboratories), which were subsequently blotted using anti-target antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000). The blots were then visualized with enhanced chemiluminescence reagent (Amersham, Piscataway, NJ) and detected with VersaDoc 5000 (Bio-Rad Laboratories) [29, 30].
Wound-healing assay
The wound-healing assay was performed using the ibidi culture-inserts system. Briefly, 5 × 105 scrambled control or GRP94-KD HCC cells in 70 μl of DMEM containing 10 % fetal calf serum were seeded into ibidi cell culture inserts (ibidi GmbH, Inc., Munchen, Germany) in 35-mm dishes and incubated at 37 °C in 5 % CO2. After 24 h, the culture inserts were removed and added to the media. The cell-free gap was monitored under a time-lapse microscope (Lumascope Model ×500 video microscopy). The gap was analyzed with ImageJ software.
Migration assay
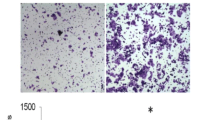
In vitro cell migration was assayed in a BD Falcon cell culture insert (BD Biosciences). Aliquots of 5 × 104 cells suspended in 500 μl of serum-free DMEM were seeded into the upper part of each chamber, and the lower compartments were filled with 1 ml of DMEM containing 10 % FCS. After incubation for 24 h at 37 °C in 5 % CO2, the non-migrating cells were mechanically removed from the upper surface of the membrane. The cells on the reverse side were stained with 0.1 % crystal violet and counted under a microscope at 100-fold magnification.
In vitro invasion assays
Cell motility was assessed using a 24-well BD BioCoat™ Matrigel Invasion Chamber (BD Biosciences). Scrambled control or GRP94-KD cells (1 × 105) suspended in 500 μl of serum-free DMEM were seeded into the upper compartments of each chamber, and the lower compartments were filled with 1 ml of DMEM containing 10 % fetal calf serum. After incubation for 24 h at 37 °C in 5 % CO2, the non-migrating cells were removed from the upper surface of the membrane by scrubbing. The cells on the reverse side were stained with 0.1 % crystal violet, and invading cells were counted under a microscope at ×100 magnification [29, 30].
Evaluation of migratory ability with the xCELLigence biosensor system
Experiments were performed using an RTCA DP instrument (ACEA Biosciences Inc. San Diego, CA, USA) that was placed in a 5 % CO2 humidified incubator maintained at 37 °C. Cell migration was assessed using specifically designed 16-well plates (CIM-plate 16, ACEA Biosciences Inc.) with 8-μm pores. These plates are similar to conventional transwell plates with microelectrodes located on the underside of the membrane of the upper chamber. After 10 % FCS medium was added to the lower chamber, cells were seeded into the upper chamber at 20,000 cells/well in serum-free medium. The CIM-plate 16 was monitored every 10 s for 40 min and once every hour thereafter. The data were analyzed using RTCA software 1.2 (supplied with the instrument).
Statistical analysis
All the collected data were expressed as the means ± standard deviation (SD) of at least three separate experiments. The data presented in some figures are from representative experiments and were quantitatively similar to the data from the replicate experiments. Statistical significance was determined with Student’s t tests (two-tailed) comparing two groups of data sets. The asterisks shown in the figures indicate significant differences between the experimental group and the corresponding control condition (P < 0.05; see figure legends).
Results
Silencing of GRP94 expression inhibited migration ability in wound-healing migration assays
Migration and invasion are key processes in cancer metastasis. To determine whether targeting GRP94 affects the migratory ability of HCC, we first performed the wound-healing assay using scrambled control and GRP94-KD HCC cells. A live imaging system was used to perform the wound-healing assay. As shown in Fig. 1, the gap area remained 31 % (scrambled control) vs. 47 % (GRP94-KD) in HepJ5 cells and 45 % (scrambled control) vs. 57 % (GRP94-KD) in Mahlavu cells after 12 h of observation. After 20 h, the wound was completely healed in the scrambled control cells, but a 25 % gap remained in the GRP94-KD HepJ5 cells and a 34 % gap remained in the GRP94-KD Mahlavu cells. These results indicate that silencing of GRP94 caused a decrease in wound-healing activity in HCC cells.
Silencing of GRP94 decreases wound-healing migratory ability in HCC cells. a GRP94-KD cells were generated, the expression levels of GRP94 in scrambled control and GRP94-KD cells were determined by Western blot, and b the quantified results were plotted. b, c The wound-healing migratory ability of scrambled control and GRP94-KD cells was analyzed as described in the “Materials and methods” section. Gaps were generated using the ibidi wound-healing migratory template. The wound-healing ability of scrambled control and GRP94-KD in b HepJ5 cells or c Mahlavu cells was monitored using time-lapse microscopy (Lumascope system). All experiments were repeated independently at least three times
Silencing of GRP94 expression inhibited cell migration and invasive ability in HCC cells
The transwell migration assay and biosensor system were used to determine the migration ability of HCC cells after silencing of GRP94. As shown in Fig. 2, migratory ability was reduced significantly in GRP94-KD HepJ5 cells compared to scrambled control cells (Fig. 2a). In Mahlavu cells, we also found the same tendency (Fig. 2a). The quantitative results of the transwell migration assays indicated that the number of migrated cells was reduced by approximately 53 % in GRP94-KD HepJ5 cells and 60 % in GRP94-KD Mahlavu cells compared to the scrambled control (Fig. 2b). These results indicated that silencing of GRP94 may cause a reduction in the migratory ability of HCC cells. To further examine the invasive ability of scrambled control and GRP94-KD cells, the invasion assay was performed. As shown in Fig. 3, the number of invasive cells in GRP94-KD cells was significantly lower than that in scrambled control cells (P < 0.01) for both HepJ5 and Mahlavu cells, indicating that GRP94 plays an important role in the invasive ability of HCC cells.
Suppression of GRP94 reduced the migratory and invasive ability of HCC cells. a, b The migratory ability of GRP94-KD and scrambled control HepJ5 cells was monitored using the transwell migration system, which showed a decrease in migration ability after knockdown of GRP94 expression in HepJ5 cells. c, d The real-time biosensor system demonstrated that GRP94-KD cells had less migratory ability than scrambled control cells. Quantitative results of the transwell migration assays are shown. The y-axis represents the number of migrated cells. All of the experiments were independently repeated at least three times. **P < 0.01
Depletion of GRP94 influenced the expression levels of EMT biomarkers. a The expression levels of EMT biomarkers (fibronectin, N-cadherin, E-cadherin, and vimentin) in scrambled control and GRP94-KD HepJ5 or Mahlavu cells were determined by Western blotting. b The band intensities were determined with ImageJ software. The y-axis represents relative protein levels (fold change compared with scrambled control). All of the experiments were independently repeated at least three times. **P < 0.01
Knockdown of GRP94 altered the expression patterns of EMT biomarkers
The epithelial-mesenchymal transition (EMT) is a crucial process in the carcinogenesis of different types of cancers. Thus, to dissect the mechanism by which knockdown of GRP94 suppresses metastasis of HCC, the expression levels of the EMT markers E-cadherin, N-cadherin, fibronectin, and vimentin were examined by Western blotting. As shown in Fig. 3, silencing of GRP94 dramatically decreased the expression levels of N-cadherin, fibronectin, and vimentin, and E-cadherin expression was stimulated after silencing GRP94. These results suggested that GRP94 may mediate cancer metastasis through the EMT pathway.
Silencing of GRP94 influences the MAPK pathway
Previous studies have indicated that the p38 and JNK pathways are involved in cancer metastasis [34, 35]. Therefore, JNK, c-Jun, c-Fos, and p38 were examined by Western blotting. As shown in Fig. 4, p-JNK expression levels were attenuated by 57 % in GRP94-KD cells. In addition, the levels of c-Jun were decreased by 53 % in GRP94-KD HepJ5 cells and 42 % in GRP94-KD Mahlavu cells (Fig. 4b). Interestingly, there was no change in JNK, c-Fos, or p38 levels after silencing GRP94 in HepJ5 and Mahlavu cells (Fig. 4a, b). These results indicate that the reduced migratory ability in GRP94-silenced cells may be due to the influence of AP-1 and the JNK pathway.
Depletion of GRP94 influenced the expression levels of EMT biomarkers. The expression levels of a p38, b p-JNK, JNK, c-JUN, and c-Fos in scrambled control and GRP94-KD HepJ5 or Mahlavu cells were determined by Western blotting. The intensities of the images were quantified with ImageJ. c The y-axis represents relative protein levels (fold change compared with scrambled control). All of the experiments were independently repeated at least three times. **P < 0.01
CCT8 may act downstream of GRP94 to mediate the migratory ability of HCC cells
CCT8 is a molecular chaperone, and its overexpression has been reported in different cancers, including HCC [22–25]. Our results showed that depletion of GRP94 decreased CCT8 expression by 45 % in HepJ5 and 43 % in Mahlavu cells (Fig. 5a). To assess the potential functional relevance of CCT8 and GRP94, we transiently overexpressed CCT8 in GRP94-KD HepJ5 cells to determine whether the inhibition of migration could be reversed. As shown in Fig. 5b, CCT8 expression increased 48 h after transfection. In wound-healing and transwell migration assays, we found an increase in the migratory ability of CCT8-overexpressing GRP94-KD cells (Fig. 5c). These results indicate that GRP94 may modulate migratory ability via CCT8. In a further examination of the levels of the p-JNK, JNK, c-Jun, and EMT biomarkers, we found increased levels of p-JNK, JNK, c-Jun, fibronectin, and N-cadherin after overexpression of CCT8 in GRP94-KD cells (Fig. 6). In contrast, the levels of E-cadherin were reduced in CCT8-overexpressing GRP94-KD cells. These results are consistent with the increase in migratory ability after CCT8 overexpression in GRP94-KD cells (Fig. 6). Interestingly, the vimentin levels were similar in vector control and CCT8-overexpressing GRP94-KD cells.
GRP94 regulated metastasis through CCT8. The expression levels of CCT8 in scrambled control and GRP94-KD HepJ5 or Mahlavu cells were determined by Western blotting. The band intensities were quantified with ImageJ. The y-axis represents the relative protein levels (fold change compared with scrambled control). b The level of CCT8 increased after the transient transfection of CCT8-overexpressing plasmids into GRP94-KD HepJ5 cells. c The wound-healing migration assay was performed in GRP94-KD/vector and GRP94/CCT8 cells. All of the experiments were independently repeated at least three times. **P < 0.01
Overexpression of CCT8 reversed migratory molecules. The expression levels of the EMT biomarkers p-JNK, JNK, c-Jun, CCT8, and GRP94 in scrambled control/vector, GRP94-KD/vector, and GRP94-KD/CCT8-overexpressing (GRP94-KD/CCT8) HepJ5 cells were determined by Western blotting. b The band intensities were quantified with ImageJ. The y-axis represents the relative protein levels (fold change compared to scrambled control). Asterisk indicates a comparison to scrambled control/vector; number sign indicates a comparison to GRP94-KD/vector. All of the experiments were repeated independently at least three times. **P < 0.01; #P < 0.05; ##P < 0.01
Discussion
HCC is one of the most common malignancies and is also the leading cause of cancer-related death in the world [36]. The diagnosis and treatment of HCC remain challenging [36]. Thus, the identification of novel signaling pathways involved in HCC progression would help provide potential opportunities for treatment of this deadly disease. In our previous study, we demonstrated that GRP94 was overexpressed in HCC cell lines (e.g., HepJ5 and Mahlavu cells) compared to a normal liver cell line (THLE2) [37]. Depletion of GRP94 significantly inhibited wound healing, migration, and invasion in both HepJ5 and Mahlavu cells. Furthermore, GRP94 silencing might attenuate HCC cell metastasis through the inhibition of the CCT8/c-Jun/EMT pathways. These results indicate that the depletion of GRP94 inhibits HCC cell migration and invasion, and it may serve as a prognostic marker.
We also identified that silencing GRP94 altered the expression of EMT biomarkers. Recent evidence indicates that the EMT process plays a key role in tumor cell invasion and metastasis [38], and the reduction of E-cadherin levels in tumor cells is a defining feature of EMT [39]. Clinical observations have also demonstrated that the absence of E-cadherin expression is associated with poor survival in liver cancer patients [40]. Moreover, the activation of EMT-related transcription factors, such as snail, slug, twist-2, and zeb-2, has been found to induce chemoresistance in liver cancer cells [41]. In the EMT process, GRP94 enhances the disruption of cell adhesion, increases cell motility, and facilitates the transition to the mesenchymal phenotype in liver cancer cell lines by decreasing E-cadherin and β-catenin localization at cell-cell boundaries. Fibronectin and vimentin are two important mesenchymal markers [42, 43]. Several studies have demonstrated that fibronectin is expressed by multiple types of cells, and it plays important roles in cell adhesion, migration, growth, and differentiation [44]. High fibronectin expression is also an indicator of poor prognosis in liver cancer patients whose cancers have higher migratory ability and display elevated expression of fibronectin and vimentin [45].
Elevated GRP94 expression is suggested to be a novel molecular hallmark of malignant cancer [9, 12, 46]. Higher expression of GRP94 was observed in HBV-infected liver cancer patients compared to uninfected patients, which is consistent with our previous study showing that the HBV protein promotes GRP94 expression through NF-κB activation [47]. In addition, the HCV envelope protein (HCV E2) blocks apoptosis induced by HCV infection through overproduction of GRP94 [48]. Stimulation of GRP94 resulted in inhibition of apoptosis, thus maintaining prolonged infection states [48]. Depletion of GRP94 expression promotes apoptosis in pancreatic cancer cells [49]. Several specific inhibitors of GRP94 have been identified [50, 51], and one of them reduces the viability of ERBB2-overexpressing breast cancer cells and multiple myeloma cells in vitro [5, 50]. It would be worthwhile to investigate whether elevated expression of GRP94 contributes to the intrinsic anti-apoptotic, drug-resistant, and potently regenerative properties of liver cancer, which are the major obstacles to the development of effective therapies.
Our study indicated that GRP94 knockdown significantly inhibited the migration and invasion of HCC cells. Furthermore, our results demonstrated that the expression levels of GRP94/CCT8/c-Jun signaling are positively correlated with migration and metastasis of HCC cancer cell lines. CCT8, an essential subunit of the CCT complex, is postulated to be involved in ATPase activity [52]. CCT8 was suggested to be involved in modulating the proliferation of HCC cells [22]. CCT activity is required for cell cycle progression and the organization and integrity of the actin- and tubulin-based cytoskeletal systems [17]. CCT8 was found to be associated with cancer progression in breast cancer, colon cancer, and HCC [22, 53, 54]. Numerous studies have confirmed that the induction of CCT subunits contributed to cancer malignancy. However, the biological functions of CCT8 and its involvement in HCC progression require further elucidation. Our study first showed that targeting GRP94 may cause downregulation of CCT8 in HCC cells and may suppress their migration and invasive abilities. CCT8 overexpression could rescue defects in migration and invasion ability in GRP94-deficient cells. Therefore, targeting GRP94/CCT8 in HCC cells might exert beneficial effects on the inhibition of HCC migration and invasion.
c-Jun, one of the components of the AP-1 protein complex, has pro-oncogenic function and regulates the expression of genes involved in cell proliferation and neoplastic transformation [55]. Induction of AP-1 was discovered in both HCC and chronic hepatitis [56]. Among the AP-1 proteins, c-Jun was suggested to play a crucial role in the regulation of cell proliferation and the initiation stage of liver carcinogenesis [57]. Additionally, c-Jun can directly regulate EMT-related genes [58]. However, further evidence of the involvement of c-Jun in HCC progression is still lacking. In this study, we demonstrated that GRP94 knockdown inhibited migration and invasion of liver cancer cells, possibly by downregulating the CCT8/c-Jun/EMT pathway. These results not only demonstrate that the GRP94/CCT8/c-Jun/EMT pathway is a significant oncogenic signaling cascade in HCC but also suggest that GRP94 antagonists may be used in targeted therapy of liver cancer.
In summary, we previously demonstrated elevated GRP94 expression in HCC cell lines and tissues. Our investigation revealed that downregulation of GRP94 resulted in significantly reduced migration and invasion abilities of HCC cancer cells. Furthermore, silencing GRP94 inhibited cell migration and invasion through downregulation of CCT8 signaling. We suggested that GRP94/CCT8/c-Jun signaling was positively correlated with HCC cell migration and invasion abilities. Most importantly, our results indicate that the GRP94/CCT8/c-Jun/EMT signaling cascade might be a promising therapeutic target for HCC.
Abbreviations
- GRP94:
-
Glucose-regulated protein 94
- HCC:
-
Hepatocellular carcinoma
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Tornesello ML, Buonaguro L, Tatangelo F, Botti G, Izzo F, Buonaguro FM. Mutations in tp53, ctnnb1 and pik3ca genes in hepatocellular carcinoma associated with hepatitis b and hepatitis c virus infections. Genomics. 2013;102:74–83.
Liu B, Staron M, Hong F, Wu BX, Sun S, Morales C, et al. Essential roles of grp94 in gut homeostasis via chaperoning canonical wnt pathway. Proc Natl Acad Sci U S A. 2013;110:6877–82.
Reddy RK, Lu J, Lee AS. The endoplasmic reticulum chaperone glycoprotein grp94 with ca(2+)-binding and antiapoptotic properties is a novel proteolytic target of calpain during etoposide-induced apoptosis. J Biol Chem. 1999;274:28476–83.
Hua Y, White-Gilbertson S, Kellner J, Rachidi S, Usmani SZ, Chiosis G, et al. Molecular chaperone gp96 is a novel therapeutic target of multiple myeloma. Clin Cancer Res. 2013;19:6242–51.
Lin CY, Lin TY, Wang HM, Huang SF, Fan KH, Liao CT, et al. Gp96 is over-expressed in oral cavity cancer and is a poor prognostic indicator for patients receiving radiotherapy. Radiat Oncol. 2011;6:136.
Wang XP, Liu GZ, Song AL, Chen RF, Li HY, Liu Y. Expression and significance of heat shock protein 70 and glucose-regulated protein 94 in human esophageal carcinoma. World J Gastroenterol. 2005;11:429–32.
Wang HX, Liu YF, Yang SJ, Duan CG, Wang YX, Zhao J, et al. Expression of hsp70 grp94 and igg in human lung carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24:447–9.
Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, et al. Overexpression of grp78 and grp94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–9.
Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, et al. Expression of heat shock proteins (hsp27, hsp60, hsp70, hsp90, grp78, grp94) in hepatitis b virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol. 2005;11:2072–9.
Hodorova I, Rybarova S, Solar P, Vecanova J, Prokopcakova L, Bohus P, et al. Gp96 and its different expression in breast carcinomas. Neoplasma. 2008;55:31–5.
Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14:263–76.
Dejeans N, Glorieux C, Guenin S, Beck R, Sid B, Rousseau R, et al. Overexpression of grp94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: implications for tumor recurrence. Free Radic Biol Med. 2012;52:993–1002.
Gomez-Puertas P, Martin-Benito J, Carrascosa JL, Willison KR, Valpuesta JM. The substrate recognition mechanisms in chaperonins. J Mol Recognit. 2004;17:85–94.
Chen L, Zhang Z, Qiu J, Zhang L, Luo X, Jang J. Chaperonin cct-mediated aib1 folding promotes the growth of eralpha-positive breast cancer cells on hard substrates. PLoS One. 2014;9:e96085.
Thulasiraman V, Yang CF, Frydman J. In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J. 1999;18:85–95.
Grantham J, Brackley KI, Willison KR. Substantial cct activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp Cell Res. 2006;312:2309–24.
Coghlin C, Carpenter B, Dundas SR, Lawrie LC, Telfer C, Murray GI. Characterization and over-expression of chaperonin t-complex proteins in colorectal cancer. J Pathol. 2006;210:351–7.
Qian-Lin Z, Ting-Feng W, Qi-Feng C, Min-Hua Z, Ai-Guo L. Inhibition of cytosolic chaperonin cctzeta-1 expression depletes proliferation of colorectal carcinoma in vitro. J Surg Oncol. 2010;102:419–23.
Lin YF, Tsai WP, Liu HG, Liang PH. Intracellular beta-tubulin/chaperonin containing tcp1-beta complex serves as a novel chemotherapeutic target against drug-resistant tumors. Cancer Res. 2009;69:6879–88.
LLeonart ME, Vidal F, Gallardo D, Diaz-Fuertes M, Rojo F, Cuatrecasas M, et al. New p53 related genes in human tumors: significant downregulation in colon and lung carcinomas. Oncol Rep. 2006;16:603–8.
Huang X, Wang X, Cheng C, Cai J, He S, Wang H, et al. Chaperonin containing tcp1, subunit 8 (cct8) is upregulated in hepatocellular carcinoma and promotes hcc proliferation. APMIS. 2014;122:1070–9.
Yokota S, Yamamoto Y, Shimizu K, Momoi H, Kamikawa T, Yamaoka Y, et al. Increased expression of cytosolic chaperonin cct in human hepatocellular and colonic carcinoma. Cell Stress Chaperones. 2001;6:345–50.
Yokota S, Yanagi H, Yura T, Kubota H. Cytosolic chaperonin is up-regulated during cell growth. Preferential expression and binding to tubulin at g(1)/s transition through early s phase. J Biol Chem. 1999;274:37070–8.
Qiu X, He X, Huang Q, Liu X, Sun G, Guo J, et al. Overexpression of cct8 and its significance for tumor cell proliferation, migration and invasion in glioma. Pathol Res Pract. 2015;211:717–25.
Huang YH, Lin KH, Chen HC, Chang ML, Hsu CW, Lai MW, et al. Identification of postoperative prognostic microrna predictors in hepatocellular carcinoma. PLoS One. 2012;7:e37188.
Hsu ML, Chen SW, Lin KH, Liao SK, Chang KS. Cytokine regulation of hiv-1 ltr transactivation in human hepatocellular carcinoma cell lines. Cancer Lett. 1995;94:41–8.
Wang RC, Huang CY, Pan TL, Chen WY, Ho CT, Liu TZ, et al. Proteomic characterization of annexin l (anx1) and heat shock protein 27 (hsp27) as biomarkers for invasive hepatocellular carcinoma cells. PLoS One. 2015;10:e0139232.
Chiou JF, Tai CJ, Huang MT, Wei PL, Wang YH, An J, et al. Glucose-regulated protein 78 is a novel contributor to acquisition of resistance to sorafenib in hepatocellular carcinoma. Ann Surg Oncol. 2010;17:603–12.
Chang YJ, Chiu CC, Wu CH, An J, Wu CC, Liu TZ, et al. Glucose-regulated protein 78 (grp78) silencing enhances cell migration but does not influence cell proliferation in hepatocellular carcinoma. Ann Surg Oncol. 2010;17:1703–9.
Wang SK, Liang PH, Astronomo RD, Hsu TL, Hsieh SL, Burton DR, et al. Targeting the carbohydrates on hiv-1: interaction of oligomannose dendrons with human monoclonal antibody 2g12 and dc-sign. Proc Natl Acad Sci U S A. 2008;105:3690–5.
Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, et al. Membrane nanotubes physically connect t cells over long distances presenting a novel route for hiv-1 transmission. Nat Cell Biol. 2008;10:211–9.
Wei PL, Chang YJ, Ho YS, Lee CH, Yang YY, An J, et al. Tobacco-specific carcinogen enhances colon cancer cell migration through alpha7-nicotinic acetylcholine receptor. Ann Surg. 2009;249:978–85.
Ji J, Jia S, Jia Y, Ji K, Hargest R, Jiang WG. Wisp-2 in human gastric cancer and its potential metastatic suppressor role in gastric cancer cells mediated by jnk and plc-gamma pathways. Br J Cancer. 2015;113:921–33.
Wu MZ, Chen SF, Nieh S, Benner C, Ger LP, Jan CI, et al. Hypoxia drives breast tumor malignancy through a tet-tnfalpha-p38-mapk signaling axis. Cancer Res. 2015;75:3912–24.
Behne T, Copur MS. Biomarkers for hepatocellular carcinoma. Int J Hepatol. 2012;2012:859076.
Huang CY, Batzorig U, Cheng WL, Huang MT, Chen WY, Wei PL, et al. Glucose-regulated protein 94 mediates cancer progression via akt and enos in hepatocellular carcinoma. Tumour Biol. 2015. doi:10.1007/s13277-015-4254-9.
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54.
Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–81.
Ju BL, Chen YB, Zhang WY, Yu CH, Zhu DQ, Jin J. Mir-145 regulates chemoresistance in hepatocellular carcinoma via epithelial mesenchymal transition. Cell Mol Biol (Noisy-le-grand). 2015;61:12–6.
Sokolowski KM, Koprowski S, Kunnimalaiyaan S, Balamulurgan M, Gamblin C, Kunnimalaiyaan M. Potential molecular targeted therapeutics: role of pi3-k/akt/mtor inhibition in cancer. Anti Cancer Agents Med Chem. 2015;16:29–37.
Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904.
Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–9.
Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3.
Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–83.
Chhabra S, Jain S, Wallace C, Hong F, Liu B. High expression of endoplasmic reticulum chaperone grp94 is a novel molecular hallmark of malignant plasma cells in multiple myeloma. J Hematol Oncol. 2015;8:77.
Fan H, Yan X, Zhang Y, Zhang X, Gao Y, Xu Y, et al. Increased expression of gp96 by hbx-induced nf-kappab activation feedback enhances hepatitis b virus production. PLoS One. 2013;8:e65588.
Lee SH, Song R, Lee MN, Kim CS, Lee H, Kong YY, et al. A molecular chaperone glucose-regulated protein 94 blocks apoptosis induced by virus infection. Hepatology. 2008;47:854–66.
Pan Z, Erkan M, Streit S, Friess H, Kleeff J. Silencing of grp94 expression promotes apoptosis in pancreatic cancer cells. Int J Oncol. 2009;35:823–8.
Patel PD, Yan P, Seidler PM, Patel HJ, Sun W, Yang C, et al. Paralog-selective hsp90 inhibitors define tumor-specific regulation of her2. Nat Chem Biol. 2013;9:677–84.
Duerfeldt AS, Peterson LB, Maynard JC, Ng CL, Eletto D, Ostrovsky O, et al. Development of a grp94 inhibitor. J Am Chem Soc. 2012;134:9796–804.
Kubota H, Hynes G, Willison K. The eighth cct gene, cctq, encoding the theta subunit of the cytosolic chaperonin containing tcp-1. Gene. 1995;154:231–6.
Shaw PG, Chaerkady R, Wang T, Vasilatos S, Huang Y, Van Houten B, et al. Integrated proteomic and metabolic analysis of breast cancer progression. PLoS One. 2013;8:e76220.
Seiden-Long IM, Brown KR, Shih W, Wigle DA, Radulovich N, Jurisica I, et al. Transcriptional targets of hepatocyte growth factor signaling and ki-ras oncogene activation in colorectal cancer. Oncogene. 2006;25:91–102.
Maeda S, Karin M. Oncogene at last--c-jun promotes liver cancer in mice. Cancer Cell. 2003;3:102–4.
Liu P, Kimmoun E, Legrand A, Sauvanet A, Degott C, Lardeux B, et al. Activation of nf-kappa b, ap-1 and stat transcription factors is a frequent and early event in human hepatocellular carcinomas. J Hepatol. 2002;37:63–71.
Shaulian E, Karin M. Ap-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–136.
Liu J, Han Q, Peng T, Peng M, Wei B, Li D, et al. The oncogene c-jun impedes somatic cell reprogramming. Nat Cell Biol. 2015;17:856–67.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology, ROC (NSC101-2314-B-038-030-MY2 and MOST103-2314-B-038-039), and Taipei Medical University–Shuang Ho Hospital (102TMU-SHH-14).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Po-Li Wei and Chien-Yu Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wei, PL., Huang, CY., Tai, CJ. et al. Glucose-regulated protein 94 mediates metastasis by CCT8 and the JNK pathway in hepatocellular carcinoma. Tumor Biol. 37, 8219–8227 (2016). https://doi.org/10.1007/s13277-015-4669-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4669-3