Abstract
Liver is the organ responsible for hematopoiesis during fetal life, which is also a target organ of metastasis for several cancers. In order to recognize the hepatic metastatic changes, obtain a better grasp of cancer prevention, treatment, and inhibition mode of hepatic metastasis progression, we investigate the changes and transformation of normal hepatic niche cells to metastatic niche ones in this review. On the other hand, since metastatic diseases alter the liver function, the changes in a number of cancers that metastasize to the liver have also been reviewed. Relevant English-language literature was searched and retrieved from PubMed (1994–2014) using the following keywords: hepatic stem cell niche, hepatic metastatic niche, chemokine, and microRNAs (miRNAs). Also, over 86 published studies were investigated, and bioinformatics analysis of differentially expressed miRNAs in hepatic cancer and metastasis was performed. Metastasis is developed in several stages with specific changes and mechanisms in each stage. Recognition of these changes would lead to detection of new biomarkers and clinical targets involved in specific stages of liver metastasis. Investigation of the hepatic stem cell niche, development of metastasis in liver tissue, as well as changes in chemokines and miRNAs in metastatic hepatic niche can significantly contribute to faster detection of liver metastasis progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis is the main cause of death in the majority of cancers, which is different in terms of type and severity in various cancer types [1]. Liver is often the target organ of metastasis. Liver tissue contains stem cells with a high regeneration potential maintaining homeostasis of liver [2]. Due to endocrine and exocrine functions, the liver requires an environment in which stem cells are able to proliferate and differentiate. Normal stem cells in this specific environment (known as hepatic niche) are transformed to liver cancer cells under the influence of pathophysiologic factors like abnormal liver regeneration and inflammation. Subsequently, the balance between proliferation and differentiation of hepatic niche cells is disrupted, providing an appropriate ground for cancer development and liver metastasis [2].
Tumor metastasis is not only dependent upon the characteristics as well as invasion and migration potential of cancer cells but is also related to the interaction between tumor and metastatic niche cells [3]. Increased or decreased expression of microRNA (miRNA) molecules (as oncogenes) is a factor enhancing tumor growth, which can reprogram the normal liver cells to phenotype of a cancer cell [3]. Studying the profile of miRNA expression in normal stem cells and cancer cells would result in the identification of target genes of these miRNA molecules in cancer cells. Recognition of these target genes as biomarkers can be useful to prevent the development of metastasis [4]. Evaluation of signaling pathways and comparison of changes in their components between normal and metastatic niches can significantly contribute to identification of therapeutic agents for prevention of cancer progression and metastasis [5]. In most cases, metastasis is diagnosed in the final stages when few therapeutic measures can be taken for the patient. Since liver is the organ playing an important role during hematopoiesis in fetal life, investigation of drug metabolism and detoxification, characteristics of hepatic stem cell niche, and development of liver metastasis can be helpful in faster detection of metastasis progression [6]. Therefore, normal hepatic niche and the changes in metastatic hepatic niche will be dealt with in this review paper. Afterward, the cancers that metastasize to liver tissue and miRNAs involved in liver metastasis will be reviewed.
Hepatic stem cell niche
Liver is the site of hematopoiesis during the fetal life. Thus, it requires a specific environment to be able to regulate self-renewal, proliferation, differentiation, and apoptosis of hematopoietic cells [7]. This environment, known as hepatic niche, is made up of different cells directly involved in maintaining the homeostasis of stem cells [8]. Studies suggest that the liver has multiple niches containing heterogeneous populations of progenitor and supportive cells. Due to presence of several hepatic stem cell niches, activation of cells in a particular niche is dependent upon the mechanism and site of insult. In order to identify the characteristics of hepatic stem cell niches, further studies are needed, which require knowledge of specific markers of the liver stem cells [9, 10].
Hepatic niche cells maintain the balance between self-renewal and differentiation capacity of niche stem cells through direct contact with supportive cells such as stellate cells and myofibroblasts [2]. Bipotential liver cells are among the niche cells capable of self-renewal and differentiation to hepatocytes and cholangiocytes [11]. Few specific markers have been introduced to detect the population of hepatic progenitor cells. Delta-like homolog 1 (DLK-1) and α-fetoprotein have been recognized as surface markers of liver progenitor cells, which are normally expressed during liver development [12]. Stellate cells are an important component of hepatic niche, which are increased during liver development [13]. These cells secrete important hematopoietic growth factors such as erythropoietin and regulate hematopoiesis during the fetal period through contact with hematopoietic cells via secretion of stromal-derived factor 1 (SDF1) [14, 15]. They are also involved in maintenance, expansion, and development of hematopoietic stem cells and subsequent formation of blood cells in hepatic niche via secretion of cytokines such as interleukin-1 (IL-1), IL-6, and stem cell factor (SCF). In fact, stellate cells play their role in the liver in a niche similar to bone marrow (BM) hematopoietic stem cell niche [16].
During liver development, macrophages in the hematopoietic environment bind erythroblasts and Jagged-1 via expression of vascular cell adhesion molecule 1 (VCAM-1). Jagged-1 is a ligand of Notch signaling pathway involved in the regulation of hematopoiesis [17, 18]. Kuepfer cells among fetal liver macrophage populations are involved in the regulation of liver erythropoiesis. In general, knowing the role of any population of cells in hematopoiesis regulation and liver differentiation demands further studies [19].
Signaling pathways are among the factors regulating the function of hepatic niche cells. Wnt/β-catenin signaling pathway in hepatic niche plays an important role in cell division during fetal liver development as well as regeneration following liver damage. Regulation of increased proliferation rate of hepatic progenitor cells and stellate cells is another function of this signaling pathway [20]. Notch signaling is another signaling pathway involved in differentiation regulation of hepatoblasts and tubule formation in distinct stages during fetal liver development. In addition to the role of this signaling pathway in liver development during fetal life, activation of it after birth leads to differentiation of bile ducts [21]. Notch-1 and Notch-3 are receptors of Notch signaling pathway expressed in quiescent stellate cells. Decreased and increased expression of Notch-1 and Notch-3 is observed during transdifferentiation of hepatic stellate cells to myofibroblasts, respectively [22]. In addition, HNF1B expression in hepatic niche is controlled by this signaling pathway. HNF1B is a transcription factor playing a role in biliary specification and hepatic lineage commitment [23]. In general, as an important signaling pathway in biology of liver, Notch signaling is involved in a wide range of liver functions, including expression of hepatic transcription factors, differentiation, fate determination, and liver regeneration [22].
Hepatic metastatic niche
Despite the importance of hepatic stem cell niche in maintaining liver homeostasis, it can be affected with pathophysiological changes. Ectopic liver regeneration, inflammation, and fibrosis can alter the hepatic niche and provide for its malignancy [2]. Most tumors are lethal when they expand from their original site to other organs of the body, a phenomenon known as metastasis. Studies show that there are four successive stages in liver metastasis. During microvascular phase, cancer cells infiltrate into the liver. This phase includes mechanisms to cease intravascular death of cancer cells and cause their survival in a specific site in hepatic microcirculation [24]. During intralobular micrometastasis phase, cancer cells are activated, and immune response against the tumor is inhibited in the liver. The third phase is angiogenic lobular micrometastasis followed by lobular growth of liver metastasis, which is the clinical phase of metastasis [24].
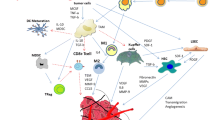
Infiltration into lymphatic system or blood circulation is a prerequisite for metastasis of cancer cells in the secondary organ to cause damage and clinical symptoms [25]. Figure 1 shows a schema of the role of hepatic niche components in transformation of pre-metastatic niche to metastatic hepatic niche. Cancer cells take advantage of protease enzymes to infiltrate into vessels or the lymphatic system. In normal conditions, tissue inhibitor of metalloproteinases (TIMP-1) inhibits the function of proteases, but it would cause development of metastasis via activating the protease function [26]. A high level of TIMP-1 causes liver to be immunocompromised via SDF-1 binding to its receptor, providing ideal conditions for cancer cells and local inflammation of the liver [27]. Proliferation induction and anti-apoptosis effect are other contributions of TIMP-1 to development of cancer. In other words, TIMP-1 leads to a pre-metastatic niche to expand liver metastases [28]. This niche is rich in BM myeloid cells that play a role in development of liver metastasis [29]. Hypoxia is another factor stimulating gene expression in solid tumors during metastasis. Expression of transcription factors is changed in hypoxic conditions. Hypoxia-inducible factor-1 (Hif-1) is an important transcription factor subject to overexpression under hypoxic conditions, which is associated with an increased risk of metastasis and can therefore be considered as a factor of tumor progression [30]. Hepatocyte growth factor (HGF) and its receptor (Met) are activated under hypoxic conditions and have promigratory and proinvasive functions during development of liver metastasis [31]. Met has been introduced as a downstream target of Hif-1 [32]. There are reports suggesting that increased expression of TIMP-1 potentiates the activity of hepatic growth factor and its receptor. With regard to the relationship between TIMP-1, Hif-1, and HGF/Met, it can be assumed that increased expression of TIMP-1 (as an inhibitor of matrix metalloproteinase) causes enhanced liver metastasis by increasing HGF/Met-dependent induction of Hif-1 signaling [33]. Epithelial-mesenchymal transition (EMT) is an important event in tumor invasion, and reduced expression of E-cadherin is considered as a sign of EMT and cancer incidence [34]. Given the role of E-cadherin in adhesion cell junctions, the absence of its expression is associated with the occurrence of metastasis in hepatocellular carcinoma. On the other hand, Twist transcription factor plays an important role in EMT induction as well as inhibition of E-cadherin expression. Overexpression of Twist has been associated with the likelihood of liver metastases via induction of EMT changes (Fig. 1).
a Schema of the role of hepatic niche components in transformation of pre-metastatic niche to metastatic hepatic niche. EMT epithelial to mesenchymal transition, HIF-1 hypoxia inducible factor-1, HGF hepatocyte growth factor, TNF-α tumor necrosis factor-alpha, TIMP-1 tissue inhibitor of metalloproteinases-1, MMP 9 matrix metalloproteinase 9, IL-1 interleukin 1, SDF-1 stromal-derived factor 1, CXCL1 chemokine (C-X-C) ligand 1
Malignant liver tissue includes a set of heterogeneous cells that play an important role in the formation and growth of tumor. This heterogeneous collection of cells can be called hepatic cancer stem cell (HCSC) [35]. Metastasis to liver depends on the interaction between cancer cells and metastatic niche [36]. Studies have shown that following infiltration of cancer cells into the liver, they will change the hepatic niche cells and lead to proinflammatory events. In fact, malignant liver tissue is a function of disturbed proliferation and interaction of liver niche cells [23]. In this regard, Kuepfer cells in hepatic niche trigger the release of tumor necrosis factor alpha (TNF-α) and IL-1. In addition, Kuepfer cells play a significant role in increased binding of tumor cells to liver cells. Release of inflammatory cytokines and increasing cell adhesion molecules in liver sinusoids by Kuepfer cells results in development of metastasis [37]. In metastatic niche, chemokines play a significant role in the interaction between tumor cells and target tissue, such that the secondary tissue as target of metastasis can provide signals for homing of malignant cells via expression of chemoattractant molecules [38]. In other words, ligand/chemokine receptor combination in niche of metastasis target organ plays an important role in tissue metastasis [38]. Overexpression of vascular endothelial cell adhesion molecules such as P-selectin and E-selectin causes extravasation of tumor cells to hepatic parenchyma and triggers a signaling pathway leading to diapedesis and invasion of cancer cells to liver parenchyma. It could be argued that binding of cancer cells to sinusoidal endothelial E-selectin is associated with metastatic liver, and overexpression of E-selectin as a proinflammatory response may signal the onset and progression of liver metastasis [39–41]. Interaction between hepatic niche cells and cancer cells is important in generation of pre-metastatic hepatic niche. In this regard, neutrophils form a component of liver niche capable of increasing liver metastasis depending on their ability to bind cancer cells. They can also be involved in the formation of pre-metastatic hepatic niche. There is a significant correlation between increased neutrophil count and increasing production of CXCL1 chemokine [42, 43]. The role of neutrophils in acceleration of liver metastasis is a function of their ability to produce matrix degradation proteins such as matrix metalloproteinase 9 (MMP-9), which causes the development of tumor and migration of tumor cells [44, 45]. Moreover, given the role of MMP-9 in mobilization of hematopoietic stem cells in BM niche, MMP-9 overexpression in liver tumor cells could be used as marker for increased mobilization of tumor cells as well as increased risk of liver metastases [46]. In addition, changing expression of some of these proteins results in poor prognosis of cancer. Therefore, a dual role can be assumed for neutrophils in formation of metastases in the liver (Fig. 1).
Signaling pathways of metastatic hepatic niche also play an important role in maintaining the phenotype of liver cancer cells. Mutation in components of these pathways (such as Wnt signaling) can lead to self-renewal and stemness of these cancer cells [40]. In this respect, over activity of Wnt/β-catenin signaling pathway in liver stem cells will increase proliferation and carcinogenesis of liver tissue [47]. Loss of polarity in epithelial cells and development of mesenchymal phenotype is a marker of tumor progression and metastasis, an event known as EMT [48]. Ras and transforming growth factor-β (TGF-β) signaling pathways are known as important molecular markers of transformation and metastasis. Studies suggest that Ras/Raf/MAPK signaling can lead to unlimited growth, invasion, and metastasis by itself [48].
Metastasis to liver
Lung cancer
In lung cancer, liver metastases are often asymptomatic, but changing gene expression and chemotactic factors play a significant role in the spread of this cancer to liver. In this context, increased expression of insulin-like growth factor receptor type 1 shows the extension of lung cancer to the liver, which is followed by changing expression of extracellular matrix proteins such as types 3 and 7 collagen [49]. Binding of type 7 collagen to integrin α2 indicates liver metastasis in lung cancer [49]. Macrophage-stimulating protein is a chemotactic factor accelerating metastasis of lung cancer to liver not only through increased migration of endothelial cells but also via enhancement of angiogenesis [50]. Extracellular matrix proteins play an important role in pre-metastatic and metastatic niches. In this regard, fibronectin is involved in the attachment of cancer cells to pre-metastatic lung niche [35]. Tenascin C (TnC) is an extracellular matrix molecule and a factor of lung metastases [51]. The expression of this molecule is affected with metastasis suppressor miRNAs, including miR-335, which is considered as a negative regulator of TnC [52]. Studies suggest that TnC is associated with relapse likelihood of lung cancer. TnC blocking does not affect primary tumor growth but reduces the incidence of lung metastases [52]. The interaction of signaling pathways and their associated genes is important in the incidence of lung metastasis. In this regard, it has been reported that leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) and musashi homolog 1 (MSI1) are the genes respectively expressed by Wnt and Notch signaling pathways, which are essential for metastasis in lung [53]. There is a close relationship between TnC, signaling pathways, and their associated genes in development of metastatic lung niche. The role of TnC in interaction with these signaling pathways is played by LGR5 expression in response to Wnt ligand and Notch signaling dependent inhibition of MSI1 by JAK/STAT signaling. In other words, TnC is of particular importance in the induction of lung metastasis during interaction with Notch and Wnt signaling pathways [52].
Colon cancer
Nearly 20–25 % of patients with colon cancer show liver metastasis upon diagnosis of the disease [1]. Increased E-selectin expression in colon cancer is an indication of liver metastasis, which plays a role in the induction and progression of liver metastasis via cell-cell junctions [54]. Colon cancer cells can bind liver endothelium through the interaction of selectin ligands on their surface with selectin on endothelial cells of the liver. This may indicate a relationship between cancer cells and niche factors in development of metastasis [52]. Several chemokines and their receptors have been reported to be involved in metastasis of colon cancer to liver [18]. CXCR4 is the receptor for CXCL12, and a high expression of it has been associated with increased risk of liver metastases. CXCR3 stimulates the invasion of metastatic colon cells to liver, and CXCR6 is involved in increasing recruitment of cancer cells in metastasis of colon to liver [18]. Changes in gene expression profiles can also be used as a clinical marker for diagnosis of metastatic colon cancer. Studies have reported the close relationship between simultaneous expression of fibroblast growth factor genes and their receptors in colon cancer patients with clinical and pathological factors, particularly during metastasis [55]. Fibroblast growth factor receptor 1 (FGFR-1) gene shows a higher level of expression in colon cancer patients with liver metastases compared to patients who do not show signs of liver metastasis. In other words, high expression of FGFR-1 can be used as a clinical marker for metastatic liver in patients with colon cancer [55]. PRDX4, CKS2, and MAGED are among the genes showing a high expression level in metastatic colon cancer to the liver [56]. As an important EMT event, reduced expression of E-cadherin is associated with liver metastasis risk. In fact, studies show that E-cadherin plays an important role in the induction of secondary tumors in the liver [57]. Changes in expression of tight junction proteins have been reported in colon cancer, among which increased expression of claudin-1 (CL-1) has prognostic significance. Studies suggest that increased expression of CL-1 leads to cellular transformation, colon cancer invasion, and subsequent metastasis to liver [58]. In general, gene expression profiles of cancers showing metastasis in secondary stages of the disease are different from cancers without metastasis. These changes in gene expression can significantly contribute to faster identification of tumor progress stages in metastatic tumor tissue [56].
Pancreatic cancer
Metastasis is an indicator of poor prognosis in pancreatic cancer. Therefore, understanding the biology and incidence of metastasis is of great importance in assessment of clinical and therapeutic targets [59]. Evaluation of pancreatic gene profile has indicated that downregulation of plasminogen activator inhibitor-1 (PAI-1), a serine protease inhibitor, leads to invasion of pancreatic cancer cells and metastasis to the liver [59]. In addition, high expression of vascular endothelial growth factor (VEGF) is associated with the risk of liver metastasis in pancreatic cancer, with an emphasis on migration of pancreatic cancer cells and development of malignancy by VEGF in this regard [60]. The role of VEGF in induction of liver metastasis in pancreatic cancer is mediated by JAK/STAT signaling pathway. There is evidence that STAT3 regulates the incidence of liver metastasis via VEGF expression. In fact, pancreatic cancer cells activate STAT3 and subsequently increase VEGF expression through overexpression of growth factors and cytokines such as IL-6 and TGF-β. In other words, STAT3 may be considered as an important transcription factor in tumor progression and metastasis [61]. Expression of VEGF is affected by BM hematopoietic progenitors, and VEGF receptor is involved in homing of cancer cells to pre-metastatic sites [35]. Another factor stimulating the expression of VEGF in pancreatic cancer and subsequent metastasis to liver is hypoxia in the niche of cancer cells, in which Hif-1 plays a significant role by activating VEGF gene promoter followed by increased VEGF expression [62]. Interaction between chemokine receptors in cancer cells and the specific chemokines in target tissue can be considered as another reason for metastasis [63]. Interaction between SDF-1/CXCR4 is important since high expression of SDF-1 in liver tissue leads to migration of its specific receptor of CXCR4 from pancreatic cancer cells to target organ and the associated risk of liver metastases. In fact, it can be stated that CXCR4 inhibitor may be a therapeutic strategy to prevent the interaction between the receptor and its specific ligand in liver tissue, which will eventually prevent the incidence of metastasis [64]. The study of cancer stem cells (CSCs) in metastatic liver is of particular importance. For example, pancreatic cancer stem cells are rich in CD133 marker. A population of these cells expressing CXCR4 has been detected, which can lead to liver metastasis as invasive cells [52]. In general, the study of genes and signaling pathways, specific interactions between ligands and receptors involved in cancer, CSCs as well as their relationship with metastasis incidence can lead to identification of their potential therapeutic targets.
MiRNA network in hepatic metastatic niche
MicroRNAs are small non-coding RNA molecules involved in the regulation of gene expression, cell proliferation, and differentiation [65]. MiRNAs play important roles in the regulation of liver function during liver development, including determination of the fate of hepatocytes and their differentiation. Let-7f is a miRNA molecule, which has been recognized as a negative regulator of liver differentiation [66]. MiRNA dysfunction is closely associated with the development of cancer. In addition to the role of miRNAs in cancer cells, there are reports on the involvement of miRNA molecules in liver stem cells. As miRNAs are involved in the regulation of gene expression, reports suggest that these RNA molecules are capable of transforming normal liver cells to cancer cells [40]. Study of miRNA profiles leads to the identification of their target genes, which promote metastasis progression conditions including migration, invasion, proliferation, and angiogenesis. Altered expression of these molecules plays a significant role in the development of liver metastasis [40]. In addition to miRNAs involved in liver metastasis, changing expression of them has been reported in cancers that metastasize to liver tissue in secondary stages (Table 1). Studying the altered expression of miRNAs in metastatic hepatic niche can elucidate their tumor suppressor or oncogene role to use these molecules for therapeutic strategies [67]. Recognition of mechanisms associated with regulatory miRs in liver metastasis from lung tissue requires further research in this field. For example, regulatory effect of crk on miR-126 changes the metastatic characteristics of lung cancer cells, including adhesion, migration, and invasion [68]. On the other hand, decreased expression of miR-126 would result in metastasis recurrence [68], and ectopic expression of it would inhibit lung colonization of liver cancer cells [69]. Thus, it is suggested that miR-126 can be used as a marker of prognosis in liver carcinoma. In addition, miR-29c is a tumor suppressor miR in lung metastasis, inhibiting the metastatic features of lung cancer cells via inhibition of target genes such as matrix metalloproteinase 2 (MMP2) and integrin β1 [70]; however, identification of secondary regulatory effects of this miR in liver tissue requires further investigations.
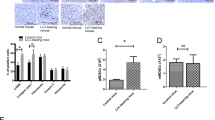
In addition, overall, seven publications were retrieved on various miRNAs involved in hepatic cancer and metastasis. We then divided the selected miRNAs into two subgroups based on the regulation profile, including upregulated and downregulated groups. Among selected miRNAs and published genes, almost all the selected genes were among the strongly validated genes for selected miRNAs except NFҡB gene for miR-143 (Table 2).
Considering qPCR results on expression profiles of various miRNAs, only has-mir-122 among the selected miRNAs was highly expressed in the liver (Fig. 2).
Based on the regulatory network in the upregulated group, there are strong evidences for the interaction between miR-181a and miR-143 with WIF1 and NFҡB genes, respectively (Fig. 3). Hence, in the downregulated group, miR-122 and miR-145 showed strong evidence of association with the selected gene (Fig. 3).
Discussion and future perspectives
Liver metastasis involves complicated cellular and molecular changes. Understanding the role of signaling pathways in hepatic niche cells and recognizing their role in accelerating the formation of liver metastasis could introduce these signaling pathways as therapeutic models for treatment of malignant liver tumors [40]. Investigation of changes in miRNA molecules as important regulators of gene expression together with their target genes can indicate their tumor suppressor or oncogene roles as markers of prognosis or diagnosis of metastatic liver [71]. Furthermore, studies on components of pre-metastatic and metastatic niches indicates that despite the focus of therapeutic investigations on metastatic cancer cells, stromal niche of these cancer cells can also be considered as a proper therapeutic target [52]. For example, targeting TnC in lung cancer or CD133+ CXCR4+ cancer stem cells in pancreatic cancer could be considered as therapeutic anti-metastatic strategies due to their role in the induction of metastatic liver. In addition, design of antibodies against metastatic factors may contribute to therapeutic approaches [52, 78]. The use of antibodies in clinical stages is important, such that the use of monoclonal antibodies can reduce the risk of metastasis. These antibodies inhibit the expression of glycoprotein receptors on cancer cells, which leads to inhibition of cancer cell binding to hepatocytes [79]. On the other hand, it has been reported that high expression of chemokines and their receptors can be a sign of cancer and metastasis. For example, CXCR4 expression in primary tumors can be associated with a high risk of recurrence, metastasis, and survival. Therefore, the expression of chemokine/receptor axis can be a biomarker of aggressive and metastatic cancer [64].
References
Zhang Y, Davis C, Ryan J, Janney C, Peña MMO. Development and characterization of a reliable mouse model of colorectal cancer metastasis to the liver. Clin Exp Metastasis. 2013;30(7):903–18.
Greenbaum LE, Wells RG. The role of stem cells in liver repair and fibrosis. Int J Biochem Cell Biol. 2011;43(2):222–9.
Xia M, Hu M. The role of microRNA in tumor invasion and metastasis. J Cancer Mol. 2010;5(2):33–9.
Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs—the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9(4):293–302.
Katoonizadeh A, Poustchi H. Adult hepatic progenitor cell niche: how it affects the progenitor cell fate. Middle East J Digestive Dis. 2014;6(2):57–64.
Clark AM, Wheeler SE, Taylor DP, Pillai VC, Young CL, Prantil-Baun R, et al. A microphysiological system model of therapy for liver micrometastases. Exp Biol Med. 2014;239(9):1170–9.
Payushina OV. Hematopoietic microenvironment in the fetal liver: roles of different cell populations. ISRN Cell Biology. 2012;2012:1–7.
Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2008;1782(2):61–74.
Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47(6):1994–2002.
Petersen B, Shupe T. Location is everything: the liver stem cell niche. Hepatology. 2008;47(6):1810–2.
Kamiya A, Kakinuma S, Yamazaki Y, Nakauchi H. Enrichment and clonal culture of progenitor cells during mouse postnatal liver development in mice. Gastroenterology. 2009;137(3):1114–26.
Vestentoft PS. Development and molecular composition of the hepatic progenitor cell niche. Danish Med J. 2013;60(5):B4640-B.
Villeneuve J, Pelluard-Nehme F, Combe C, Carles D, Chaponnier C, Ripoche J, et al. Immunohistochemical study of the phenotypic change of the mesenchymal cells during portal tract maturation in normal and fibrous (ductal plate malformation) fetal liver. Comp Hepatol. 2009;8(5):1–12.
Kiassov AP, Van Eyken P, van Pelt JF, Depla E, Fevery J, Desmet VJ, et al. Desmin expressing nonhematopoietic liver cells during rat liver development: an immunohistochemical and morphometric study. Differentiation. 1995;59(4):253–8.
Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Laboratory Investig J Tech Methods Pathol. 1994;70(4):511–6.
Kordes C, Sawitza I, Götze S, Häussinger D. Hepatic stellate cells support hematopoiesis and are liver-resident mesenchymal stem cells. Cell Physiol Biochem. 2013;31(2–3):290–304.
Li D, Wang G-Y, Liu Z-F, Shi Y-X, Zhang H, Bai Z-L. Macrophage-associated erythropoiesis and lymphocytopoiesis in mouse fetal liver: ultrastructural and ISH analysis. Cell Biol Int. 2004;28(6):457–61.
Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Høyer-Hansen G, et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013;73(7):2031–43.
Isern J, Fraser ST, He Z, Baron MH. The fetal liver is a niche for maturation of primitive erythroid cells. Proc Natl Acad Sci. 2008;105(18):6662–7.
Lee WB, Erm SK, Kim KY, Becker RP. Emperipolesis of erythroblasts within kupffer cells during hepatic hemopoiesis in human fetus. Anat Rec. 1999;256(2):158–64.
Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127(6):1775–86.
Morell CM, Strazzabosco M. Notch signaling and new therapeutic options in liver disease. J Hepatol. 2014;60(4):885–90.
Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136(10):1727–39.
Vidal-Vanaclocha F. The Tumor microenvironment at different stages of hepatic metastasis. In: Brodt P, editor . 1st ed. Liver metastasis: biology and clinical management; 2011.
Seubert B, Grünwald B, Kobuch J, Cui H, Schelter F, Schaten S, et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015;61(1):238–48.
Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S, et al. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67(18):8615–23.
Tanaka M, Itoh T, Tanimizu N, Miyajima A. Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J Biochem. 2011;149(3):231–9.
Chirco R, Liu X-W, Jung K-K, Kim H-RC. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25(1):99–113.
Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–93.
Dachs G, Tozer G. Hypoxia modulated gene expression: angiogenesis, metastasis and therapeutic exploitation. Eur J Cancer. 2000;36(13):1649–60.
Benvenuti S, Comoglio PM. The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol. 2007;213(2):316–25.
Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3(4):347–61.
Schelter F, Halbgewachs B, Bäumler P, Neu C, Görlach A, Schrötzlmair F, et al. Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by hypoxia-inducible factor-1α. Clin Exp Metastasis. 2011;28(2):91–9.
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12(18):5369–76.
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7.
Ma S, Chan KW, Hu L, Lee TKW, Wo JYH, Ng IOL, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–56.
Gassmann P, Hemping-Bovenkerk A, Mees ST, Haier J. Metastatic tumor cell arrest in the liver–lumen occlusion and specific adhesion are not exclusive. Int J Color Dis. 2009;24(7):851–8.
Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26(3–4):453–67.
Khatib A-M, Auguste P, Fallavollita L, Wang N, Samani A, Kontogiannea M, et al. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am J Pathol. 2005;167(3):749–59.
Auguste P, Fallavollita L, Wang N, Burnier J, Bikfalvi A, Brodt P. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol. 2007;170(5):1781–92.
Khatib A-M, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59(6):1356–61.
Yamamoto M, Kikuchi H, Ohta M, Kawabata T, Hiramatsu Y, Kondo K, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68(23):9754–62.
Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, et al. Neutrophils promote liver metastasis via mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72(16):3919–27.
Fox-Robichaud A, Kubes P. Molecular mechanisms of tumor necrosis factor α-stimulated leukocyte recruitment into the murine hepatic circulation. Hepatology. 2000;31(5):1123–7.
Noël A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor–host interface. Semin Cell Dev Biol. 2008;19(1):52–60.
Shi Y, Xu T, Li LP, Chen XP. Over-expression of VEGF and MMP-9 in residual tumor cells of hepatocellular carcinoma after embolization with lipidol. J Huazhong Univ Sci Technolog Med Sci. 2013;33:90–5.
Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123(5):1911–8.
Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, et al. Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis dissection of ras signaling pathways. J Cell Biol. 2002;156(2):299–314.
Burnier J, Wang N, Michel R, Hassanain M, Li S, Lu Y, et al. Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene. 2011;30(35):3766–83.
Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40(4):558–66.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–24.
Irmisch A, Huelsken J. Metastasis: new insights into organ-specific extravasation and metastatic niches. Exp Cell Res. 2013;319(11):1604–10.
Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17(7):867–74.
Ye C, Kiriyama K, Mistuoka C, Kannagi R, Ito K, Watanabe T, et al. Expression of E-selectin on endothelial cells of small veins in human colorectal cancer. Int J Cancer. 1995;61(4):455–60.
Sato T, Oshima T, Yoshihara K, Yamamoto N, Yamada R, Nagano Y, et al. Overexpression of the fibroblast growth factor receptor-1 gene correlates with liver metastasis in colorectal cancer. Oncol Rep. 2009;21(1):211–6.
Li M, Lin Y-M, Hasegawa S, Shimokawa T, Murata K, Kameyama M, et al. Genes associated with liver metastasis of colon cancer, identified by genome-wide cDNA microarray. Int J Oncol. 2004;24(2):305–12.
Osada T, Sakamoto M, Ino Y, Iwamatsu A, Matsuno Y, Muto T, et al. E‐cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 1996;24(6):1460–7.
Kinugasa T, Akagi Y, Ochi T, Tanaka N, Kawahara A, Ishibashi Y, et al. Increased claudin-1 protein expression in hepatic metastatic lesions of colorectal cancer. Anticancer Res. 2012;32(6):2309–14.
Niedergethmann M, Alves F, Neff J, Heidrich B, Aramin N, Li L, et al. Gene expression profiling of liver metastases and tumour invasion in pancreatic cancer using an orthotopic SCID mouse model. Br J Cancer. 2007;97(10):1432–40.
Shi Q, Le X, Abbruzzese JL, Peng Z, Qian C-N, Tang H, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61(10):4143–54.
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22(3):319–29.
Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270(22):13333–40.
Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14(3):181–5.
Saur D, Seidler B, Schneider G, Algül H, Beck R, Senekowitsch–Schmidtke R, et al. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129(4):1237–50.
Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6(60):1–7.
Davoodian N, Lotfi AS, Soleimani M, Mola SJ, Arjmand S. Let-7f microRNA negatively regulates hepatic differentiation of human adipose tissue-derived stem cells. J Physiol Biochem. 2014;70(3):781–9.
Qi W, Liang W, Jiang H, Waye MM. The function of miRNA in hepatic cancer stem cell. BioMed Res Int. 2013;2013:1–9.
Chen H, Miao R, Fan J, Han Z, Wu J, Qiu G, et al. Decreased expression of miR-126 correlates with metastatic recurrence of hepatocellular carcinoma. Clin Exp Metastasis. 2013;30(5):651–8.
Crawford M, Brawner E, Batte K, Yu L, Hunter M, Otterson G, et al. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem Biophys Res Commun. 2008;373(4):607–12.
Wang H, Zhu Y, Zhao M, Wu C, Zhang P, Tang L, et al. miRNA-29c suppresses lung cancer cell adhesion to extracellular matrix and metastasis by targeting integrin beta1 and matrix metalloproteinase2 (MMP2). PloS one. PLoS One. 2013 6;8(8):e70192
Ji D, Chen Z, Li M, Zhan T, Yao Y, Zhang Z, et al. MicroRNA-181a promotes tumor growth and liver metastasis in colorectal cancer by targeting the tumor suppressor WIF-1. Mol Cancer. 2014;13(1):86.
Tsai WC, Hsu PWC, Lai TC, Chau GY, Lin CW, Chen CM, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49(5):1571–82.
Zhang X, Liu S, Hu T, Liu S, He Y, Sun S. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology. 2009;50(2):490–9.
Yao J, Liang L, Huang S, Ding J, Tan N, Zhao Y, et al. MicroRNA-30d promotes tumor invasion and metastasis by targeting Galphai2 in hepatocellular carcinoma. Hepatology. 2010;51(3):846–56.
Liang L, Wong CM, Ying Q, Fan DNY, Huang S, Ding J, et al. MicroRNA-125b suppressesed human liver cancer cell proliferation and metastasis by directly targeting oncogene LIN28B2. Hepatology. 2010;52(5):1731–40.
Kahlert C, Klupp F, Brand K, Lasitschka F, Diederichs S, Kirchberg J, et al. Invasion front-specific expression and prognostic significance of microRNA in colorectal liver metastases. Cancer Sci. 2011;102(10):1799–807.
Zhang Y, He X, Liu Y, Ye Y, Zhang H, He P, et al. MicroRNA-320a inhibits tumor invasion by targeting neuropilin 1 and is associated with liver metastasis in colorectal cancer. Oncol Rep. 2012;27(3):685–94.
Kyutoku M, Taniyama Y, Katsuragi N, Shimizu H, Kunugiza Y, Iekushi K, et al. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med. 2011;28(2):181–6.
Wang J, Fallavollita L, Brodt P. Inhibition of experimental hepatic metastasis by a monoclonal antibody that blocks tumor-hepatocyte interaction. J Immunother. 1994;16(4):294–302.
Acknowledgments
We wish to thank all our colleagues in Shafa Hospital and Allied Health Sciences School, Ahvaz Jundishapur University of Medical Sciences.
Authors’ contributions
N.S. conceived the manuscript and revised it, Sh.A., A.A., M.Sh., and M.S. wrote the manuscript, prepared the figures and tables. F.R. performed the bioinformatic analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Highlights
Evaluation of the changes in hepatic pre-metastatic and metastatic niches
Factors involved in the formation of hepatic pre-metastatic and metastatic niches
Investigation of how cancer cells metastasize to the liver
Knowledge of tumor suppressor or oncogene function of miRNA molecules in hepatic metastatic niche
Rights and permissions
About this article
Cite this article
Azizidoost, S., Ahmadzadeh, A., Rahim, F. et al. Hepatic metastatic niche: from normal to pre-metastatic and metastatic niche. Tumor Biol. 37, 1493–1503 (2016). https://doi.org/10.1007/s13277-015-4557-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4557-x