Abstract
Colorectal cancer (CRC) is one of the most common cancers worldwide. Sphingosine kinase 1 (SphK1), which phosphorylates sphingosine to sphingosine-1-phosphate (S1P), is overexpressed in various types of cancers and may act as an oncogene in tumorigenesis. However, little is known about the role of SphK1 in CRC patients. We studied the expression of SphK1 in 85 cases of CRC tissues by immunohistochemistry, qRT-PCR, and western blot. We also evaluated the effect of SphK1 on cell proliferation and invasion by MTT and transwell invasion assay. SphK1 is overexpressed in CRC tissues and cell lines, and upregulation of SphK1 correlated significantly with the following parameters: lymph node metastasis, liver metastasis, and advanced TNM stage. SphK1 knockdown results in inhibition of cancer cell proliferation. Inhibition of CRC cell migration and invasion is also evident through reversal of EMT by increases in E-cadherin expression and decreases in vimentin expression. In conclusion, SphK1 is associated with the proliferation and invasiveness of CRC cells and the SphK1 gene may contribute to a novel therapeutic approach against CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) is the third common form of cancer in males and the second in females; it also remains the third leading cause of cancer-related death [1]. The development of CRC is a complex process determined by many factors which promoted tumor progression. In its early stages, CRC is curable by radical surgical and adjuvant therapies. Nevertheless, treatment in many patients is often precluded by metastasis, and the overall survival rate remains unfavorable [2–4]. Therefore, it is of urgent need to elucidate the potential mechanism that mediates the initiation and progression of CRC for this disease diagnosis and treatment.
Sphingosine kinase 1 (SphK1) is a lipid kinase that converts sphingosine to sphingosine-1-phosphate (S1P) by phosphorylation [5–8]. High SphK1 expression has been associated with a wide range of cellular processes in a variety of cancer cells. However, little is known about the molecular significance of SphK1 in CRC. In this study, we demonstrated that SphK1 expression is upregulated in CRC. Inhibition of SphK1 supresses CRC cell proliferation, migration, and invasion in vitro, indicating that SphK1 may serve as a novel therapeutic target for CRC.
Materials and methods
Tissue samples
A total of 85 CRC tissue sections and adjacent tissues of cancer were collected from the first Affiliated Hospital of Sun Yat-Sen University. The clinicopathologic characteristics of the CRC patients were recorded. All tissue sections were confirmed by the original histopathological and clinical diagnosis. None of our study patients had received preoperative chemotherapy and/or radiotherapy. All these samples were formalin-fixed and paraffin-embedded. Besides, freshly frozen tissue samples were available and snap-frozen in liquid nitrogen immediately after surgery and stored at −80 °C until use. This study was approved by the legislation and ethical boards of the first Affiliated Hospital of Sun Yat-Sen University. All subjects or their caregivers have written informed consent.
Cell culture and transfection
Human colorectal cancer cell lines HCT116, SW480, SW620, LoVo, CaCo-2, and HT-29 were purchased from the American Type Culture Collection (Manassas, VA, USA). SW620 and SW480 were cultured by Leibovitz’s L-15 supplemented with 10 % fetal bovine serum (FBS) (Sigma-Aldrich, St Louis, MO, USA); Caco-2 and HT-29 were maintained in RPMI1640 with 10 % FBS, and LoVo was cultured by F-12K with 10 % FBS.
At the time of transfection, cells were seeded on six-well plates at 2 × 105 cells per well. Small interfering RNA (siRNA) was used to knock down endogenous SphK1 gene expression in CRC cells. Cells were transfected with SphK1-specific siRNA or negative control siRNA (Qiagen, Valencia, CA, USA) using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) for 48 h. SphK1 knockdown (SphK1-KO) was checked by RT-PCR and western blotting.
Immunohistochemistry
Paraffin sections from clinical specimens were deparaffinized in xylene and rehydrated in a descending ethanol series (100, 95, 90, 80, and 70 % ethanol) and double-distilled water according to standard protocols. Endogenous peroxidase activity was blocked with 0.3 % hydrogen peroxide for 10 min. After antigen retrieval by microwaving, the sections were incubated with SphK1 antibody (Abcam, dilution 1:200) overnight at 4 °C. After phosphate-buffered saline washing, the tissue sections were incubated with the biotinylated secondary antibody and streptavidin-horseradish peroxidase complex, each for 20 min at room temperature. Diaminobenzidine was used as the chromogen, and tissue sections were counterstained with hematoxylin and then viewed under a bright-field microscope. Samples incubated with PBS instead of the primary antibody served as negative controls.
Evaluation of staining
The stained slides were evaluated independently by two investigators who were unaware of the clinical parameters. Breast tissue was used a positive control for SphK1. Semiquantitative scoring of intensity (0, no staining; 1, weak staining; 2, strong staining) and fraction of positive cancer cells (0, no staining; 1, less than half; 2, more than half) was undertaken [9]. Combining intensity and percentage staining resulted in the final staining score (0–6). Final staining scores of 0–3 and 4–6 were, respectively, considered to be low and high expressions.
Real-time reverse transcription PCR
Expressions of SphK1 in CRC tissues and paired adjacent normal tissues were detected. Total RNA was prepared by Qiazol extraction (Qiagen) and reverse-transcribed into cDNA with the Transcriptor cDNA Synthesis System (Roche, Indianapolis, IN, USA). Real-time qPCR was performed with SYBR Green Master mix system (Roche). The primers were as follows: for human SphK1, forward 5′-CTTGCAGCTCTTCCGGAGTC-3′ and reverse 5′-GCTCAGTGAGCATCAGCGTG-3′; for human GAPDH, forward 5′-GACTCATGACCACAGTCCATGC-3′ and reverse 5′-AGAGGCAGGGATGATGTTCTG-3′. The relative expression of SphK1 was calculated and normalized using the 2−ΔΔCt method relative to GAPDH. Independent experiments were done in triplicate.
Western blot
The cells or tissues were lysed using modified radioimmunoprecipitation assay buffer (50 mM Tris–HCl (pH 7.4), 1 % NP-40, 0.25 % sodium deoxycholate, 150 mM NaCl, 1 mM ethylene diamine tetraacetic acid (EDTA), protease inhibitor cocktail complete). Amounts of total protein extracts were determined using BCA assay and samples were stored at −80 °C until use. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Probing and detection of specific proteins was performed with enhanced chemiluminescence after antibody binding. The following antibodies were used: anti-SphK1 (1:1000; Abcam), anti-E-cadherin (1:1000; Abcam), anti-Vimentin (1:1000; Abcam), and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were then incubated with a horseradish peroxidase-conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO, USA). The proteins were detected by an enhanced chemiluminescence detection system.
Cell viability assay
Cell viability was determined using a 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously [10].
Transwell cell invasion assay
In vitro invasion assay was determined using Matrigel invasion chambers (BD Bioscience). Living cells transfected with SphK1 siRNA or negative control siRNA were seeded into inserts at 5.0 × 104 per insert in serum-free medium and then transferred to wells filled with the culture medium containing 10 % FBS as a chemoattractant. After 24 h of incubation, noninvading cells on the top of the membrane were removed with a cotton swab. The migrated cells on the underside of the filter membrane were fixed and stained with 0.1 % crystal violet. The number of migrated cells on the membrane was counted in five randomly selected microscopic fields and photographed. The protocol used for the invasion assay was the same as that used for the migration assay, except that the transwell insert was coated with Matrigel (BD Biosciences, Heidelberg, Germany).
Statistical analysis
SPSS version 11.5 was used for all analyses. For continuous variables, data are expressed as mean ± standard deviation (SD). The difference of SphK1 levels between tumor tissue and normal colon mucosa was evaluated using Student’s t test. Significance is displayed as *P < 0.05, **P < 0.01, or ***P < 0.001.
Results
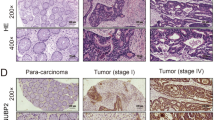
The PCR and western blot data demonstrated that SphK1 mRNA and protein were expressed in 85 pairs of human CRC and adjacent normal colon mucosa tissue samples. The results demonstrated that SphK1 mRNA was overexpressed in CRC samples compared with normal tissue (Fig. 1a), whereas 78.82 % (67 out of 85) cancer samples expressed higher SphK1 protein level than adjacent normal tissue samples (Fig. 1b). Then, we investigate the expression of nuclear SphK1 protein in 85 CRC tissue samples by IHC analysis (Fig. 2a, b). Nuclear SphK1 expression was significantly higher in tumor tissues than in nontumor tissues (P < 0.001). Overexpression of nuclear SphK1 was observed in 65 of the 85 patients (76.47 %).
Quantitative RT-PCR analysis was used to determine the levels of SphK1 mRNA in CRC cell lines. Results show that SphK1 was highly expressed in SW480 and HCT116 (Fig. 3a), so SW480 and HCT116 cells were selected for SphK1 gene silencing to study the effect of SphK1 knockdown on oncogenic phenotypes. Our result showed that the level of SphK1 protein was remarkably reduced in SW480/si or HCT116/si cells compared with those transfected with control siRNA (Fig. 3b).
Therefore, we investigate the effect of inhibition of SphK1 on CRC cell proliferation and invasion, we evaluated cell growth and invasion activity using SW480/si or HCT116/si knockdown SphK1 cell. MTT assay showed that proliferation rate of cells treated with SphK1-specific siRNA was significantly decreased compared to cells transfected with control vector or parental cells. As shown in Fig. 4a, b, cell growth curve analysis results indicated that knockdown of SphK1 in HCT116/si or SW480/si cells significantly inhibited cell proliferation compared with control cells (P < 0.001). These data suggested that siRNA mediated knockdown of SphK1 could lead to growth suppression of CRC cells. Transwell cell migration/invasion assay results indicated that downregulation of SphK1 in HCT116 and SW480 cells significantly decreased invasive activity compared with control cells (Fig. 5). Therefore, siRNA-mediated knockdown of SphK1 could significantly inhibit the ability of invasion of CRC in vitro.
To investigate the underlying mechanisms by which SphK1 promotes CRC cell proliferation and invasion, western blotting was performed to show expression of EMT-relevant markers, and the results indicated that knockdown of SphK1 in HCT116 and SW480 significantly decreased the expression of vimentin compared with control cells, while E-cadherin expression was significantly increased, indicating that SphK1 is involved in the EMT process in CRC (Fig. 3b).
Discussion
CRC remains a major public health problem worldwide [11]. Greater knowledge of the molecular mechanisms underlying the development of this deadly neoplasm is required. In recent years, the clinical significance of SphK1 has gained more and more attention. SphK1 overexpression in tumor cells has been shown to be an independent prognostic factor in several types of tumors [12–16]. However, the molecular mechanism by which SphK1 exerts its activity in CRC progression and aggressiveness remains poorly understood.
CRC is a complex neoplasm which results from several factors such as genetic alteration, chromosomal instability, and activity of growth factor pathways. In order to identify the pathological roles of SphK1 in human cancers, we evaluated the status of SphK1 expression in CRC tissues and cell lines. In this study, we detected the level of SphK1 in a series of CRC tissues and adjacent normal mucosa tissues. Our result showed that SphK1 has clinical significance and plays a functional role in human CRC. SphK1 is frequently increased in CRC tissues both at the transcriptional level and at the translational level, suggesting that abnormal SphK1 expression plays key roles in colorectal tumorigenesis. In addition, the levels of SphK1 were greatly elevated in all human CRC cell lines but expression levels of SphK1 varied among them. The expression of SphK1 was highest in SW480 and HCT116 cell lines.
Our study showed that knocked down SphK1 suppressed tumor cell proliferation in CRC cells. The MTT assay showed that SphK1 siRNA significantly reduced the proliferation rate of HCT116 and SW480 cells compared with the control siRNA-transfected cells. SphK1 appears to aggravate cell invasiveness and migration. In our study, we found that downregulation of SphK1 could inhibit CRC cell migration and invasion, indicating that SphK1 contributes to tumor progression and suggesting that SphK1 could be a useful target for the treatment of CRC.
The mechanism by which SphK1 exerts its invasive and metastatic activity remains unclear. The possible mechanism of the role of SphK1 in inducing invasion of CRC is that EMT is involved in tumor progress. EMT is a process implicated in the conversion of early stage tumors to invasive malignancies that downregulates epithelial gene expression and upregulates mesenchymal gene expression [17, 18]. Induction of EMT will result in weakened intercellular adhesion and enhanced cell motility, thereby allowing tumor cells to metastasize. Loss or disruption of tight junctions and E-cadherin/β-catenin complexes at cell boundaries via upregulation of E-box repressors such as ZEB-1, ZEB-2, Snail, and Twist1 is one of the hallmarks of the EMT. SphK1 may promote cancer cell metastasis through the EMT process [19–23]. In our study, inhibition of SphK1 expression is associated with significant upregulation of E-cadherin, and expression of vimentin was reduced in the SphK1-siRNA HCT116 and SW480 cells.
In summary, this study provided evidence for the clinical significance of overexpressed SphK1 in patients with CRC. Our findings indicate that targeting SphK1 might provide a new therapeutic modality for the treatment of CRC.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141:67–75.
Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2012;143:844–57.
Toiyama Y, Hur K, Tanaka K, Inoue Y, Kusunoki M, Boland CR. Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer. Ann Surg. 2014;259(4):735–43.
Shimada H, Tanaka K, Endou I, Ichikawa Y. Treatment for colorectal liver metastases: a review. Langenbecks Arch Surg. 2009;394:973–83.
Zhang H, Wang Q, Zhao Q, Di W. MiR-124 inhibits the migration and invasion of ovarian cancer cells by targeting SphK1. J Ovarian Res. 2013;6(1):84.
Yang YL, Ji C, Cheng L, He L, Lu CC, Wang R, et al. Sphingosine kinase-1 inhibition sensitizes curcumin-induced growth inhibition and apoptosis in ovarian cancer cells. Cancer Sci. 2012;103(8):1538–45.
Santulli P, Marcellin L, Noël JC, Borghese B, Fayt I, Vaiman D, et al. Sphingosine pathway deregulation in endometriotic tissues. Fertil Steril. 2012;97(4):904–11.
Datta A, Loo SY, Huang B, Wong L, Tan SS, Tan TZ, et al. SPHK1 regulates proliferation and survival responses in triple-negative breast cancer. Oncotarget. 2014;5(15):5920–33.
Wang Y, Tang Q, Li M, Jiang S, Wang X. MicroRNA-375 inhibits colorectal cancer growth by targeting PIK3CA. Biochem Biophys Res Commun. 2014;444:199–204.
Van Cutsem E, Nordlinger B, Adam R, K€ohne CH, Pozzo C, Poston G. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–21.
Meng XD, Zhou ZS, Qiu JH, Shen WH, Wu Q, Xiao J. Increased SPHK1 expression is associated with poor prognosis in bladder cancer. Tumour Biol. 2014;35(3):2075–80.
Lu Z, Zhang W, Gao S, Jiang Q, Xiao Z, Ye L, et al. MiR-506 suppresses liver cancer angiogenesis through targeting sphingosine kinase 1 (SPHK1) mRNA. Biochem Biophys Res Commun. 2015;468(1–2):8–13.
Shi J, He YY, Sun JX, Guo WX, Li N, Xue J, et al. The impact of sphingosine kinase 1 on the prognosis of hepatocellular carcinoma patients with portal vein tumor thrombus. Ann Hepatol. 2015;14(2):198–206.
Lufrano M, Jacob A, Zhou M, Wang P. Sphingosine kinase-1 mediates endotoxemia-induced hyperinflammation in aged animals. Mol Med Rep. 2013;8(2):645–9.
Karimian G, Buist-Homan M, Schmidt M, Tietge UJ, de Boer JF, Klappe K, et al. Sphingosine kinase-1 inhibition protects primary rat hepatocytes against bile salt-induced apoptosis. Biochim Biophys Acta. 2013;1832(12):1922–9.
Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003.
Liotta LA, Steeq PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–36.
Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, et al. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol Cancer Ther. 2009;8(4):809–20.
Vettorazzi S, Bode C, Dejager L, Frappart L, Shelest E, Klaßen C, et al. Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SphK1. Nat Commun. 2015;6:7796.
Zhu L, Wang Z, Lin Y, Chen Z, Liu H, Chen Y, et al. Sphingosine kinase 1 enhances the invasion and migration of non-small cell lung cancer cells via the AKT pathway. Oncol Rep. 2015;33(3):1257–63.
Ader I, Gstalder C, Bouquerel P, Golzio M, Andrieu G, Zalvidea S, et al. Neutralizing S1P inhibits intratumoral hypoxia, induces vascular remodelling and sensitizes to chemotherapy in prostate cancer. Oncotarget. 2015;6(15):13803–21.
Cho SY, Cho S, Park E, Kim B, Sohn EJ, Oh B, et al. Coumestrol suppresses hypoxia inducible factor 1α by inhibiting ROS mediated sphingosine kinase 1 in hypoxic PC-3 prostate cancer cells. Bioorg Med Chem Lett. 2014;24(11):2560–4.
Acknowledgments
This work is supported by the Science and Technology Planning Project of Guangdong Province (2013B021800284)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Long J and Xie Y are co-first authors.
Rights and permissions
About this article
Cite this article
Long, J., Xie, Y., Yin, J. et al. SphK1 promotes tumor cell migration and invasion in colorectal cancer. Tumor Biol. 37, 6831–6836 (2016). https://doi.org/10.1007/s13277-015-4542-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4542-4