Abstract
Upregulation of sphingosine kinase 1 (SPHK1) protein has been reported to be associated with a poor prognosis in a variety of malignant tumors. However, the role of SPHK1 in bladder cancer (BC) has not been thoroughly elucidated. The purpose of this study was to assess SPHK1 expression and to explore its contribution to BC. Real-time quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) was conducted to detect SPHK1 mRNA expression in 37 pairs of fresh-frozen BC tissues and corresponding noncancerous tissues. Results showed that SPHK1 mRNA expression level in BC tissues was significantly higher than that in corresponding noncancerous tissues. To investigate the association between SPHK1 protein expression and clinicopathological characteristics of BC, immunohistochemistry (IHC) was performed in 153 archived paraffin-embedded BC samples. Interestingly, high SPHK1 expression was significantly associated with histologic grade (P = 0.045) and tumor stage (P < 0.001) of patients with BC. The Kaplan–Meier survival curve showed that patients with high SPHK1 expression had significantly reduced overall 5-year survival rates (P < 0.001). Multivariate Cox regression analysis further suggested that the increased expression of SPHK1 was an independent poor prognostic factor for this disease. In conclusion, our data offer the convincing evidence for the first time that the increased expression of SPHK1 may be involved in the pathogenesis and progression of BC. SPHK1 might be a potential marker to predict the prognosis in BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer (BC) is an increasingly significant international public health problem. In the USA, BC is the second most common genitourinary malignant disease, with 69,250 new cases and 14,990 mortalities estimated in 2011 [1]. The incidence of BC increases with age, peaking between 50 and 70 years, and the disease is approximately three times more common in males than in females [2]. The established risk factors for bladder cancer include tobacco smoke, exposure to industry-related aromatic amines, and uptake of drugs such as phenacetin, chlornaphazine, and cyclophosphamide [3]. Exposure to these chemical carcinogens may lead to direct and indirect DNA damage, genome instability, and carcinogenesis [4]. However, the precise mechanism of BC development remains unclear.
The rate-limiting enzyme of sphingosine 1 phosphate (S1P) synthesis is sphingosine kinase (SPHK), which critically regulates the ceramide/sphingosine-S1P rheostat [5]. SPHK is a highly conserved lipid kinase. Two functional SPHK isoenzymes, sphingosine kinase 1 (SPHK1) and SPHK2, have been identified in mammalian cells and tissues [6–8]. Multiple lines of evidence indicate that SPHK1 is an oncogenic enzyme and that activation of SPHK1 is closely associated with antiapoptosis, transformation, proliferation, and survival of tumor cells [9–13]. Previous studies have shown that SPHK1 protein is overexpressed in many types of malignant tumors [9, 11–17]. In addition, Kalari et al. [18] reported that SPHK1 may play a positive and essential role in the growth and development of malignant mesothelioma. However, the expression patterns and involvement of SPHK1 in BC are still unclear. Therefore, the aim of this study was to investigate the clinical significance of SPHK1 expression in BC.
Materials and methods
Patients and tissues samples
This study was approved by the Research Ethics Committee of Bethune International Peace Hospital. Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
For qRT-PCR, 37 pairs of fresh BC and matched adjacent normal tissue specimens were collected from patients who underwent surgery between May 2008 and August 2008 in the Bethune International Peace Hospital. The fresh tissue specimens were immediately frozen in liquid nitrogen until use. For immunohistochemical assay, a total of 153 paraffin-embedded BC samples were collected from our hospital between July 2005 and January 2008. Patient characteristics are shown in Table 1. None of the patients recruited in this study had undergone preoperative chemotherapy or radiotherapy. The duration of follow-up was calculated from the date of surgery to death or last follow-up, and patients were excluded if they had incomplete medical records or inadequate follow-up.
Real-time quantitative reverse transcriptase–polymerase chain reaction
For mRNA quantitative real-time PCR (qRT-PCR) assay, total RNA was extracted from gastric cancer tissues and adjacent normal tissues using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. RNAse-free DNase I was used to eliminate DNA contamination. qRT-PCR was performed according to standard methods. Sequences of the real-time PCR primers were designed using the Primer Express Software Version 2.0 and sequences are as follows: SPHK1 forward primer 5′-CTTGCAGCTCTTCCGGAGTC-3′, SPHK1 reverse primer 5′-GCTCAGTGAGCATCAGCGTG-3′, and SPHK1 probe 5′-(FAM)CCCTTTTGGCTGAGGCTGAAATCTCC(TAMRA)-3′. Expression data were normalized to the geometric mean of housekeeping gene GAPDH to control the variability in expression levels (forward primer 5′-GACTCATGACCACAGTCCATGC-3′, reverse primer 5′-AGAGGCAGGGATGATGTTCT G-3′, and probe 5′-(FAM)CATCACTGCCACCCAGAAGACTGTG(TAMRA)-3′) and calculated as 2−[(Ct of SPHK1) − (Ct of GAPDH)], where Ct represents the threshold cycle for each transcript.
Immunohistochemistry
Immunohistochemistry was performed as described previously [19]. SPHK1 antibody (1:200 dilution, Abgent, CA) was used. Immunohistochemistry (IHC) results were evaluated and scored independently by two pathologists without knowledge of the clinicopathological outcomes of the patients. Semiquantitative estimation was made using a composite score obtained by multiplying the values of staining intensity and relative abundance of positive cells. Intensity was graded as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). The abundance of positive cells was graded from 0 to 4 (0, <5 % positive cells; 1, 5–25 %; 2, 26–50 %; 3, 51–75 %; 4, >75 %). For Kaplan–Meier survival analysis, a composite score greater than the median value was considered high expression and a composite score less than or equal to the median value was considered low expression.
Statistical analysis
A chi-square test was used to determine the association between SPHK1 expression and clinicopathological parameters. Kaplan–Meier analysis and log-rank tests were used to assess the survival rate and to compare differences in survival curves. Cox regression analysis was performed to assess the significance of multiple predictors of survival. Differences were considered significant at P < 0.05.
Results
Expression of SPHK1 in BC tissues by qRT-PCR
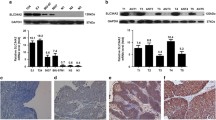
We examined SPHK1 protein expression in 37 pairs of BC tissues and the corresponding noncancerous tissues by qRT-PCR. As shown in Fig. 1, the increased SPHK1 mRNA expression in BC was observed in 29 of the 37 cases, suggesting that the mRNA level of SPHK1 was significantly higher in tumor tissues than in corresponding noncancerous tissues (3.54 ± 1.12 vs. 1.02 ± 0.43, P < 0.001).
Expression of SPHK1 in BC tissues by IHC
We further examined the expression of SPHK1 protein in 153 paraffin-embedded BC samples and 153 matched corresponding noncancerous samples by IHC analysis. We observed that 53.6 % (82/153) of the BC samples showed high SPHK1 expression. In comparison, the rate of high SPHK1 protein expression was 20.9 % (32/153) in adjacent noncancerous samples (Fig. 2). The protein expression level of SPHK1 was significantly higher in BC tissues than the level in adjacent noncancerous tissues (P < 0.001).
Correlation of SPHK1 expression with clinicopathological characteristics
The relationship between SPHK1 expression and different clinicopathological factors was shown in Table 1. Increased SPHK1 expression in BC was found to be associated with histologic grade (P = 0.045) and tumor stage (P < 0.001). However, no correlation was observed between SPHK1 expression and other clinicopathologic variables, such as age, gender, tumor size, and tumor number (all P > 0.05).
Relationship between SPHK1 expression and BC patients’ survival
The association between SPHK1 expression and survival of BC patients was investigated by Kaplan–Meier analysis and log-rank test. As shown in Fig. 3, BC patients with high SPHK1 expression tend to have shorter overall survival than those with low SPHK1 expression (log-rank test: P < 0.001).
Univariate analysis demonstrated that histologic grade, tumor stage, and SPHK1 expression were significantly associated with overall survival of BC patients (all P < 0.05, Table 2). No significant associations were found for age, gender, tumor size, tumor number, and patient outcome. Multivariate analysis using the Cox proportional hazards model for all variables that were significant in the univariate analysis showed that histologic grade, tumor stage, and SPHK1 expression were independent prognostic factors for patients with BC (all P < 0.05, Table 2).
Discussion
BC has diverse biological and functional characteristics. Conventional histopathological evaluation, such as tumor stage or grade and lymph node status and numerous biomarkers have been investigated as prognostic indicators of BC [20, 21]. However, none of the histological criteria or biomarkers reported to date have sufficient sensitivity and specificity for detecting the whole spectrum of bladder cancer diseases in a routine clinical practice [22]. The limited value of the established prognostic markers requires the analysis of new molecular parameters in predicting the prognosis and treatment of BC patients. Recently developed microarray technology has permitted the development of numerous cancer classifiers, identification of tumor subclasses, discovery of progression markers, and prediction of disease outcome in many types of cancer [23–25]. Molecular staging may provide more accurate predictions of patient outcome than is currently possible with histopathological staging. Also, molecular staging could improve the treatment of patients by allowing treatment to be tailored to the severity of the disease. Although considerable effort has been devoted to identifying a prognostic model of BC that can provide useful information about survival and treatment options at diagnosis, the ability to predict the survival of BC patients remains a major clinical challenge. Thus, there is a critical need for methods capable of assessing the prognosis of patients with BC [26].
The essential roles of SPHK1 in tumorigenesis have been revealed recently [9–18]. The upregulation of SPHK1 was reported in various types of human cancers [9, 11–17]. SPHK1 overexpression serves as a prognostic marker for judging the survival of patients with salivary gland carcinomas [27], head and neck carcinoma [28], gastric cancer [29], and human astrocytomas [30]. In addition, Rosa et al. [31] recently reported that SPHK1 overexpression contributes to cetuximab resistance in human colorectal cancer models.
To our knowledge, this is the first reported research on a large number of clinical samples looking at the predictive power of SPHK1 in BC and the correlation between SPHK1 expression and clinicopathologic features and prognosis of BC patients. In this study, we found that the levels of SPHK1 protein and mRNA were significantly higher in BC tissues compared with corresponding noncancerous tissues. Moreover, the overexpression of SPHK1 protein was significantly related to histologic grade and tumor stage. Furthermore, the results of Kaplan–Meier survival curves and Cox multivariate analysis indicated that overexpression of SPHK1 may be an independent predictor of poor clinical outcome and decreased survival.
In summary, our present study demonstrated that elevated SPHK1 expression levels were associated with the progression and poor prognosis in patients with BC, which indicates that SPHK1 may serve as a valuable prognosis marker in BC. However, the possible underlying mechanisms for its participation in tumor progression are still unclear; therefore, as the next step, we will make further research from cell signaling pathway in order to gain a better molecular mechanism understanding in this field.
References
Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi:10.3322/caac.20121.
Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi:10.1016/S0140-6736(09)60491-8.
Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect. 1997;105:875–82.
Franekova M, Halasova E, Bukovska E, Luptak J, Dobrota D. Gene polymorphisms in bladder cancer. Urol Oncol. 2008;26:1–8. doi:10.1016/j.urolonc.2006.10.011.
Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201.
Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol. 2002;71:493–511.
Olivera A, Spiegel S. Sphingosine kinase: a mediator of vital cellular functions. Prostaglandins Other Lipid Mediat. 2001;64:123–34.
Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–20. doi:10.1074/jbc.M002759200.
Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705.
Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–23.
Le Scolan E, Pchejetski D, Banno Y, Denis N, Mayeux P, Vainchenker W, et al. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106:1808–16. doi:10.1182/blood-2004-12-4832.
Akao Y, Banno Y, Nakagawa Y, Hasegawa N, Kim TJ, Murate T, et al. High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced up-regulation. Biochem Biophys Res Commun. 2006;342:1284–90.
Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–8. doi:10.1096/fj.05-4331fje.
Guan H, Liu L, Cai J, Liu J, Ye C, Li M, et al. Sphingosine kinase 1 is overexpressed and promotes proliferation in human thyroid cancer. Mol Endocrinol. 2011;25:1858–66. doi:10.1210/me.2011-1048.
Pan J, Tao YF, Zhou Z, Cao BR, Wu SY, Zhang YL, et al. An novel role of sphingosine kinase-1 (SPHK1) in the invasion and metastasis of esophageal carcinoma. J Transl Med. 2011;9:157. doi:10.1186/1479-5876-9-157.
Fuereder T, Hoeflmayer D, Jaeger-Lansky A, Rasin-Streden D, Strommer S, Fisker N, et al. Sphingosine kinase 1 is a relevant molecular target in gastric cancer. Anticancer Drugs. 2011;22:245–52.
Bao M, Chen Z, Xu Y, Zhao Y, Zha R, Huang S, et al. Sphingosine kinase 1 promotes tumour cell migration and invasion via the S1P/EDG1 axis in hepatocellular carcinoma. Liver Int. 2012;32:331–8. doi:10.1111/j.1478-3231.2011.02666.x.
Kalari S, Moolky N, Pendyala S, Berdyshev EV, Rolle C, Kanteti R, et al. Sphingosine kinase 1 is required for mesothelioma cell proliferation: role of histone acetylation. PLoS One. 2012;7:e45330. doi:10.1371/journal.pone.0045330.
Du ZM, Hu CF, Shao Q, Huang MY, Kou CW, Zhu XF, et al. Upregulation of caveolin-1 and CD147 expression in nasopharyngeal carcinoma enhanced tumor cell migration and correlated with poor prognosis of the patients. Int J Cancer. 2009;125(8):1832–41. doi:10.1002/ijc.24531.
Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS, Jeong P, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer. 2010;9:3. doi:10.1186/1476-4598-9-3.
Kim YK, Kim WJ. Epigenetic markers as promising prognosticators for bladder cancer. Int J Urol. 2009;16:17–22. doi:10.1111/j.1442-2042.2008.02143.x.
Habuchi T, Marberger M, Droller MJ, Hemstreet 3rd GP, Grossman HB, Schalken JA, et al. Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology. 2005;66:64–74. doi:10.1016/j.urology.2005.08.065.
Dyrskjøt L, Zieger K, Real FX, Malats N, Carrato A, Hurst C, et al. Gene expression signatures predict outcome in non-muscle-invasive bladder carcinoma: a multicenter validation study. Clin Cancer Res. 2007;13:3545–51. doi:10.1158/1078-0432.CCR-06-2940.
Takata R, Katagiri T, Kanehira M, Tsunoda T, Shuin T, Miki T, et al. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res. 2005;11:2625–36. doi:10.1158/1078-0432.CCR-04-1988.
Eschrich S, Yang I, Bloom G, Kwong KY, Boulware D, Cantor A, et al. Molecular staging for survival prediction of colorectal cancer patients. J Clin Oncol. 2005;23:3526–35. doi:10.1200/JCO.2005.00.695.
Kim WJ, Kim SK, Jeong P, Yun SJ, Cho IC, Kim IY, et al. A four-gene signature predicts disease progression in muscle invasive bladder cancer. Mol Med. 2011;17:478–85. doi:10.2119/molmed.2010.00274.
Liu G, Zheng H, Zhang Z, Wu Z, Xiong H, Li J, et al. Overexpression of sphingosine kinase 1 is associated with salivary gland carcinoma progression and might be a novel predictive marker for adjuvant therapy. BMC Cancer. 2010;10:495. doi:10.1186/1471-2407-10-495.
Facchinetti MM, Gandini NA, Fermento ME, Sterin-Speziale NB, Ji Y, Patel V, et al. The expression of sphingosine kinase-1 in head and neck carcinoma. Cells Tissues Organs. 2010;192:314–24. doi:10.1159/000318173.
Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–9. doi:10.1158/1078-0432.CCR-08-1158.
Li J, Guan HY, Gong LY, Song LB, Zhang N, Wu J, et al. Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival. Clin Cancer Res. 2008;14:6996–7003. doi:10.1158/1078-0432.CCR-08-0754.
Rosa R, Marciano R, Malapelle U, Formisano L, Nappi L, D’Amato C, et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res. 2013;19:138–47. doi:10.1158/1078-0432.CCR-12-1050.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Meng, XD., Zhou, ZS., Qiu, JH. et al. Increased SPHK1 expression is associated with poor prognosis in bladder cancer. Tumor Biol. 35, 2075–2080 (2014). https://doi.org/10.1007/s13277-013-1275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1275-0