Abstract
The identification of prognostic factors in patients with renal cell carcinoma (RCC) represents an area of increasing interest. In this retrospective study, we evaluated the prognostic role of carbonic anhydrase-IX, ezrin, and neuropilin in metastatic RCC patients. The expression of several biomarkers were measured by immunohistochemistry (IHC) in 45 patients with advanced stage RCC treated with second-line tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor (VEGF) after failure of interferon-alpha between January 2007 and June 2012. Kaplan-Meier curves and log-rank tests were used for analysis of progression-free survival (PFS) and overall survival (OS), and a multivariate Cox proportional hazard model was employed to identify factors with an independent effect on the survival. Age, ezrin and neuropilin-2 overexpression were found to be statistically significant factors (P < 0.05) for PFS in the univariate analysis. Ezrin and neuropilin-2 overexpression, hemoglobin and albumin level were statistically significant factors (P < 0.05) for OS in the univariate analysis. Multivariate analysis revealed that low expression of ezrin and neuropilin-2 was an independent prognostic factor for PFS and OS. The median PFS was 4 months for patients overexpressing neuropilin-2 versus 11 months for those with lower expression of neuropilin-2 (p = 0.033). The median OS was longer in patients with low levels of neuropilin-2 expression (26 months) compared to patients overexpressing neuropilin-2 (13 months) (p = 0.023). Increased expression of ezrin was associated with poor prognosis in patients treated with TKIs targeting VEGF (PFS, 3 vs 7 months; p = 0.012). High ezrin expression was associated with shorter OS (p = 0.009). This is the first study in the literature showing that neuropilin-2 and ezrin are related with prognosis in patients with advanced RCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Each year, approximately 57,000 patients are diagnosed with renal or upper urinary system cancer in the USA, resulting in more than 12,900 deaths [1]. These tumors account for nearly 3 % of all adult cancers, with a male-to-female ratio of 1.6:1. Renal cell carcinoma (RCC) occurs mostly between 50 to 70 years of age. RCC is not a single entity, but rather a collection of different types of tumors, each derived from the various parts of the nephron possessing distinct genetic characteristics and tumor biology [2–5]. Metastatic RCC is relatively resistant to treatment. Antitumoral activity of conventional cytotoxic chemotherapy is minimal in patients with RCC [6]. Interferon-alpha (IFN-α) or interleukin-2 (IL-2)-based cytokine therapy had objective response rates of only 15–30 % with severe side effects [7]. Better understanding of the molecular biology of RCC has led to identification of many molecular pathways which can be targeted for the treatment of patients with advanced stage/metastatic RCC. As a result, RCC treatment modalities have been changed dramatically in recent years. Thus, currently novel therapies directed at the inhibition of these targets are at the forefront of RCC treatment [8]. Targeted antiangiogenic agents including axitinib, sunitinib, sorafenib, pazopanib, bevacizumab (in combination with IFN-α), temsirolimus, and everolimus have been approved by the FDA for use in patients with advanced stage RCC.
Molecules related to the underlying biology of RCC have been investigated as potential biomarkers for prediction of therapeutic benefit. Unfortunately, there are currently no predictive biomarkers that can help in the selection of systemic therapy in metastatic disease, and most data are retrospective in nature. Carbonic anhydrase IX (CA IX) may play a crucial part in cell proliferation and adhesion and malignant cell invasion [9].
Immunohistochemical analysis of CA-IX expression has been investigated as a diagnostic and a prognostic marker for clear cell RCC previously [10, 11]. Another marker called “ezrin” serves as an intermediate between the cell membrane and the actin cytoskeleton. It plays a key role in the control of cell morphology. Ezrin regulates cell-cell and cell- matrix adhesions by interacting with adhesion molecules E-cadherin and beta-catenin and has an important role in the regulation of adhesive and invasive behaviors of cancer cells and also in the tumor progression and metastasis [12].
Neuropilin-2 (Nrp-2) is a non-tyrosine kinase glycoprotein which has been characterized as a neuronal receptor for certain members of the collapsin/semaphorin family. Nrp-2 has an extensive distribution into tissues including tumor-derived cells and endothelial cells. Nrp-2 found in embryonic neurons is considered to be responsible for the regulation of growth of axons and guiding axon growth via binding to certain members of the semaphorin family. Studies in mouse embryos suggested a role of Nrp-1 in the angiogenesis and vasculogenesis. Inhibition of Nrp-2 in the colorectal carcinoma cells resulted in reduced cell growth, invasion, and a 60 to 80 % regression in hepatic metastases [13].
In the present study, we aimed to determine the effect of immunohistochemical staining of ezrin, carbonic anhydrase IX (CA IX), and neuropilin-2 on the prognosis of patients diagnosed with metastatic RCC who were treated with TKIs between January 2007 and June 2012.
Materials and methods
Patient population
Data were obtained in 53 consecutive patients with metastatic RCC who were treated with tyrosine kinase inhibitors (TKI) in the Department of Oncology at the University of Gazi between January 2007 and June 2012. Eight patients were excluded from the analysis because of unavailable tissue for accurate reevaluation (n = 4), incomplete records (n = 2), or administration of therapy outside the study institution (n = 2). For each patient, treatment modalities, clinical course, laboratory and imaging findings, presence of metastasis, and disease status were recorded.
The relationship between immunohistochemical staining of ezrin, carbonic anhydrase-IX, and neuropilin-2 and response to treatment with TKIs was investigated in patients with metastatic RCC. For the purposes of this study, progression-free survival (PFS) was defined as time from the start of treatment with TKIs to clinical and/or radiological evidence of disease progression and overall survival (OS) was defined as the time from the date of metastatic RCC diagnosis to the last follow-up visit date or death of the patient.
The study enrolled 45 patients who showed disease progression or who had intolerance to IFN-α during first-line IFN-α therapy. All of the patients had metastatic RCC treated with an anti-VEGF-targeted therapy (sunitinib,sorafenib, or pazopanib) as their second-line anti-VEGF agent. Patients with intermediate-risk group according to the Memorial Sloan-Kettering Cancer Center (MSKCC) included in the study.
Statistical analysis
Data analysis was conducted using Statistical Package for the Social Sciences (SPSS) 17.0 software package. Descriptive analyses were used for demographic data and Kaplan-Meier test for survival analysis. OS and PFS were estimated using Kaplan-Meier method, and a log-rank test was used for comparison of study groups for survival. Multivariate analyses were performed using a Cox regression analysis. A p value of <0.05 was considered significant for statistical analyses.
Immunohistochemical examination
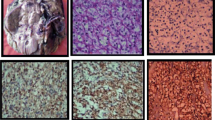
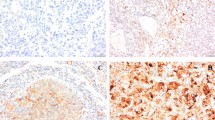
Hematoxylin-eosin (H&E)-stained tumor sections of the patients were re-examined by an uropathologist and immunohistochemical staining for ezrin, neuropilin-2, and carbonic anhydrase IX was performed on the 4-micron sections from corresponding paraffin blocks using the standard streptavidin-biotin-peroxidase procedure. Sections underwent heat-induced epitope retrieval in 0.01 M sodium citrate buffer at pH 6.0 for 20 min. As primary antibodies, carbonic anhydrase-IX Ab (rabbit polyclonal antibody, Clone H-120, Santa Cruz Biotechnology, at 1/200 dilution), ezrin antibody (ezrin Ab-1, Thermo Scientific, Clone 3C12 mouse polyclonal antibody, MS-661-P1, at 1/200 dilution), and neuropilin-2 antibody (polyclonal NRP2 ab, Sigma-Aldrich, Atlas antibodies, at 1/200 dilution) were used. For each staining session, clear cell RCC for carbonic anhydrase-IX, lung parenchyma for ezrin, and placental tissue for neuropilin-2 were used as positive controls.
The pathology slides were reviewed by an expert urologic pathologist who had no knowledge of patients’ treatment and outcomes. Tumors were scored positive for ezrin-carbonic anhydrase IX and neuropilin-2 if tumor cells showed definite nuclear and/or membranous staining and negative if tumor nuclei and cell membrane had no immunoreactivity. Staining intensity (weak, 1 point; moderate, 2 points; strong, 3 points) and percentage groups of positive tumor cells (≤25 %, 1 point; 26–50 %, 2 points; 51–75 %, 3 points; >75 %, 4 points) were multiplied to achieve a score between 1 and 12. Subsequently, patients were divided into two groups (underexpressing or overexpressing) based on their median values for each stain (Figs. 1, 2, and 3).
Results
Characteristics of patients
This study enrolled a total of 45 patients diagnosed with metastatic RCC who had disease progression or who didn’t tolerate first-line interferon-α (IFN-α) treatment during follow-up visits. Median age of the patients was 58 years (range, 34–80) and 24.4 % were female. Demographic, clinical, and pathological characteristics of patients are shown in Table 1.
In all RCC tissue samples, positive staining signals of CA-IX were detected in 16 (35.6 %) with low immunoreactivity and in 29 (64.4 %) with high immunoreactivity, respectively. Of the 45 immunostained RCC specimens, 23 (51 %) showed low expression, and 22 (49 %) showed high expression level of neuropilin-2. Twenty-two of 45 cases (49 %) was considered as ezrin-low group, while 23 of 45 cases (51 %) was considered as ezrin-high group.
The median follow-up was 15 months (range, 1–61) and 34 patients (75.6 %) were died. Median OS and median PFS were 17 months (95 % CI 9–24) and 6 months (95 % CI 3–8), respectively.
Prognostic factors for PFS and OS
Univariate analysis
Eight independent predictive factors that influenced PFS and OS were evaluated (Tables 2 and 3). As shown by the univariate analysis, statistically significant predictive factors for PFS were age at diagnosis and overexpression of ezrin and neuropilin (Table 2). Univariate analysis revealed that hemoglobin value, albumin level, and ezrin and neuropilin overexpression were significantly related to overall survival in patients with metastatic RCC (Table 3).
A multivariate analysis of factors influencing PFS and OS
In univariate analysis factors that reached a p value below 0.05 entered into the multivariate model. In multivariate analysis age (p = 0.022) and overexpression of ezrin (p = 0.012) and neuropilin (p = 0.033) were remained significant factors influencing PFS (Table 4) (Fig. 4). Statistically, independent prognostic factors for overall survival were overexpression of ezrin (p = 0.009) and neuropilin (p = 0.023) (Fig. 5).
Discussion
In the present study, we investigated the effect of different biomarkers on prognosis in metastatic RCC. To the best of our knowledge, this is the first study in the literature which simultaneously analyze three biomarkers namely ezrin neuropilin-2 and carbonic anhydrase IX in metastatic RCC. As a result, we have demonstrated that ezrin and neuropilin-2 has prognostic implications in RCC. As far as we know, these data are novel and first in literature.
In recent years, promising results have been obtained in the treatment of metastatic RCC with the introduction of targeted agents owing to molecular advances. Inevitably, increased use of targeted molecular therapies in the clinical practice in parallel with rapid developments in the field of tumor biology required novel biomarkers in order to identify patient groups who would most benefit from such therapies. A predictive biomarker would not only be helpful in identification of patient groups who would benefit from a therapy but also would prevent exposure of patients to ineffective and toxic therapies by defining a tumor group with “de novo” resistance. When designing the study, we aimed to investigate the prognostic significance of expressions of carbonic anhydrase-IX, ezrin, and neuropilin for metastatic RCC patients who were receiving TKIs.
Currently, a multitude of clinical and analytical factors are considered to affect prognosis in metastatic RCC patients as shown by studies. Of these factors, performance status, histological type and grade of the tumor, number of metastases, disease-free interval, hemoglobin, serum calcium, and lactate dehydrogenase levels are the most extensively studied and widely recognized. The primary goal of individualized metastatic RCC therapy is to provide sufficiently reliable prognostic and predictive markers to the clinicians. Here, the specific purpose should be identification of biological markers with the ability to distinguish patient subgroups who would most benefit from a particular therapy.
Various molecular markers serve as independent prognostic factors for RCC and provide important information on the tumor biology [14–17]. One of these factors, CA IX is regulated by Von Hippel-Lindau (VHL) gene and expressed by most clear cell RCC [10, 18, 11]. Immune staining of CA IX may serve as a marker for response to systemic therapy in patients with advanced stage disease [18–20]. In the present study, we did not identify an effect of underexpression or overexpression of CA IX on PFS and OS in patients with metastatic RCC who were treated with a TKI and showed progression following cytokine therapy. In the recently published TARGET study [21], there was not a statistically significant difference in median PFS (5.5 vs 5.4 months, p = 0.97) between patients with high or low levels of CA IX expression. In the present study, median PFS and median OS were 6 and 20 months for the patient group with a high level of CA IX expression respectively (p = 0.310 ). In the low expression group, PFS was 7 months and the OS was 17 months (p = 0.998).
Currently, ezrin is considered to be involved in the development of various types of cancer including malignant soft tissue sarcomas and esophageal, pancreatic, gastric, endometrial, ovary, and breast cancer [22–27]. Zhang et al. have shown that ezrin expression was an unfavorable prognostic factor for both survival and disease-free interval in patients with non-small cell lung cancer [28].
In patients with hepatocellular cancer, recurrence of the disease right after resection of the tumor was found to be associated with ezrin expression. Additionally, ezrin expression is related to the expression of cytokeratin 19 (CK 19) which is a marker for progenitor cells in these patients. Measurement of ezrin expression is considered for potential use in identification of increased risks among patients due to recurrence of the disease.
Ezrin is synthesized by the glomerular and tubular epithelial cells of the kidney. To our best knowledge, there are no studies which investigated the prognostic significance of ezrin in metastatic RCC patients receiving TKIs. In the present study, increased expression of ezrin was found to be an independent prognostic factor for both PFS and OS. As an independent prognostic factor, overexpression of ezrin might become a novel molecular target in patients receiving TKIs.
Nrp-2 acts a coreceptor for vascular endothelial growth factor (VEGF)-D and has a critical role in the metastasis to lymph nodes in several types of cancer including papillary thyroid cancer [29–31]. It would not be surprising to target antiangiogenic approaches for cancers, given the dependency of RCC on angiogenesis and unavailability of effective forms of systemic therapy. Aberrant activation of additional signaling pathways may contribute to altered kinetics of the cell cycle in the RCC and may provide an excellent target for therapeutic interventions. Neuropilins are overexpressed in various tumors including melanoma, glioblastoma, leukemia and lymphoma and carcinomas (e.g., pancreatic, prostate, breast, colon, and renal). Generally, neuropilin expression is clinically associated with more aggressive tumor behavior. Indeed, expression of Nrp-1 and Nrp-2 is associated with poor prognosis independent of well-known prognostic factors [32]. In this study, we aimed to demonstrate the prognostic significance of neuropilin expression in metastatic RCC patients. Both PFS and OS were significantly reduced in patients with neuropilin overexpression compared to those with lower levels of expression (PFS 4 vs 11 months, p = 0.033, and OS 13 vs 26 months, p = 0.023). No studies have been conducted to demonstrate the prognostic significance of Nrp-2 for metastatic RCC. It would be interesting to investigate molecular agents targeting both Nrp-2 and VEGF for the treatment of metastatic RCC in future studies.
More importantly, these studies would represent a major step forward in the field of targeted therapy which is tremendously important for oncology and encourage further molecular and clinical studies to identify reliable biomarkers suitable for use in the clinic and to provide increased benefit from therapies targeting angiogenesis with selection of appropriate patients.
Our study has a number of limitations. Firstly, bias in patient selection and data collection which is typical for a retrospective study and particularly differences in characteristics of patients selected for histopathological examination were shortcomings of the study. Secondly, it was difficult to ascertain the presence or absence of an interaction because of the small sample size. Thirdly, the results of this study cannot be extrapolated to all patients receiving treatment with other TKIs because most of the study patients were treated with sunitinib.
In conclusion, the prognosis of metastatic RCC is still poor despite rapid advances in the field of tumor biology and a need for novel biomarkers has emerged to identify patient groups who would most benefit from the treatment following the introduction of molecular targeted therapies into our daily clinical practice. CA IX expression measured in the tissue does not seem to have an effect on PFS or OS among metastatic RCC patients treated with TKIs. Overexpression of ezrin has an adverse independent effect on both PFS and OS in metastatic RCC patients treated with TKIs. Neuropilin overexpression has a negative independent effect on PFS and OS in metastatic RCC patients receiving TKIs. New molecular models are needed for making decisions on treatment with targeted agents giving consideration to clinical factors as well as molecular characteristics of the tumor which affect the prognosis. Prospective studies in a larger number of patients are needed to demonstrate such molecular models in metastatic RCC. Our study is the first to demonstrate the independent prognostic significance of ezrin and neuropilin-2 expressions on OS and PFS histopathologically in addition to clinical factors.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59:225–29.
Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163–72.
Cohen HT, McGovern FJ. Medical progress: renal-cell carcinoma. N Engl J Med. 2005;353:2477–90.
Novick AC. Kidney cancer: past, present and future. Urol Oncol. 2007;25:188–95.
Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–32.
Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163(2):408–17.
Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19(2):148–54.
Rini B. Vascular endothelial growth factor targeted therapy in renal cell carcinoma: current status and future directions. Clin Cancer Res. 2007;13(4):1098–106.
Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–19.
Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–11.
Leibovich BC, Sheinin Y, Lohse CM, Thompson RH, Cheville JC, Zavada J, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25(30):4757–64.
Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999;112(18):3081–90.
Gray MJ, Van Buren G, Dallas NA, Xia L, Wang X, Yang AD, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100:109–20.
Bui MH, Zisman A, Pantuck AJ, Han KR, Wieder J, Belldegrun AS. Prognostic factors and molecular markers for renal cell carcinoma. Expert Rev Anticancer Ther. 2001;1(4):565–75.
Han KR, Pantuck AJ, Belldegrun AS. Basic biology and clinical behavior of renal cell carcinoma. Kidney Cancer. 2003; 67-89.
Crispen PL, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED. Predicting disease progression after nephrectomy for localized renal cell carcinoma: the utility of prognostic models and molecular biomarkers. Cancer. 2008;113:450–60.
Parker AS, Leibovich BC, Lohse CM, Sheinin Y, Kuntz SM, Eckel-Passow JE, et al. Development and evaluation of BioScore: a biomarker panel to enhance prognostic algorithms for clear cell renal cell carcinoma. Cancer. 2009;115(10):2092–103.
Bui MH, Visapaa H, Seligson D, Kim H, Han KR, Huang Y, et al. Prognostic value of carbonic anhydrate IX and Ki67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171:2461–66.
Atkins M, Regan M, McDermott D, Mier J, Stanbridge E, Youmans A, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–21.
Cho D, Signoretti S, Dabora S, Regan M, Seeley A, Mariotti M, et al. Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2007;5:379–85.
Choueiri TK, Cheng S, Qu AQ, Pastorek J, Atkins MB, Signoretti S. Carbonic anhydrase IX as potential biomarker of efficacy in metastatic clear-cell renal cell carcinoma patients receiving sorafenib or placebo: Analysis from the treatment approaches in renal cancer global evaluaion trial (TARGET).Urol Oncol. 2012
Weng WH, Ahlén J, Aström K, Lui WO, Larsson C. Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue esarcomas. Clin Cancer Res. 2005;11:6198–204.
Zeng H, Xu L, Xiao D, Zhang H, Wu X, Zheng R, et al. Altered expression of ezrin in esophageal squamous cell carcinoma. J Histochem Cytochem. 2006;54:889–96.
Bal N, Yildirim S, Nursal TZ, Bolat F, Kayaselcuk F. Association of ezrin expression in intestinal and diffuse gastric carcinoma with clinicopathological parameters and tumor type. World J Gastroenterol. 2007;13:3726–729.
Köbel M, Langhammer T, Hüttelmaier S, Schmitt WD, Kriese K, Dittmer J, et al. Ezrin expression is related to poor prognosis in FIGO stage I endometrial carcinomas. Mod Pathol. 2006;19:581–87.
Köbel M, Gradhand E, Zeng K, Schmitt WD, Kriese K, Lantzsch T, et al. Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int J Gynecol Pathol. 2006;25:121–30.
Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005;7:365–73.
Zhang XQ, Chen GP, Wu T, Yan JP, Zhou JY. Expression and clinical significance of ezin in non-small-cell lung cancer. Clin Lung Cancer. 2012;13(3):196–204.
Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–83.
Yasuoka H, Nakamura Y, Zuo H, Tang W, Takamura Y, Miyauchi A, et al. VEGF-D expression and lymph vessels play an important role for lymph node metastasis in papillary thyroid carcinoma. Mod Pathol. 2005;18:1127–33.
Nakamura Y, Yasuoka H, Zuo H, Takamura Y, Miyauchi A, Nakamura M, et al. Nitric oxide in papillary thyroid carcinoma: induction of vascular endothelial growth factor d and correlation with lymph node metastasis. J Clin Endocrinol Metab. 2006;91:1582–85.
Ghosh S, Sullivan CA, Zerkowski MP, Molinaro AM, Rimm DL, Camp RL, et al. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol. 2008;39:1835–43.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cetin, B., Gonul, I.I., Buyukberber, S. et al. The impact of immunohistochemical staining with ezrin-carbonic anhydrase IX and neuropilin-2 on prognosis in patients with metastatic renal cell cancer receiving tyrosine kinase inhibitors. Tumor Biol. 36, 8471–8478 (2015). https://doi.org/10.1007/s13277-015-3589-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3589-6