Abstract
Early diagnosis of intraperitoneal metastasis is a pivot for survival of patients with serous epithelial ovarian cancers (SEOC). However, to date, there is lack of efficient molecular biomarker for early metastasis of SEOC. Here, we found that the expression of chloride intracellular channel 1 (CLIC1) is highly correlative with intraperitoneal metastasis. There is very low expression of CLIC1 in normal ovaries (NO), benign ovarian tumor (BOT), and primary ovarian cancer without metastasis (POCNM); but its expression is remarkably high in primary ovarian cancer with metastasis (POCM) omentum and peritoneal metastasis. Furthermore, for clinic prediction of intraperitoneal metastasis of SEOC, the sensitivity and specificity of CLIC1 overexpression were 97.4 and 88.1 %, respectively. Collectively, CLIC1 may be a potential sensitive and specific molecular biomarker for early diagnose for SEOC metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian carcinoma accounts for high lethality in women malignancies worldwide [1]. Serous epithelial ovarian cancers (SEOC) account for almost 80 % of all epithelial ovarian cancers [2]. Due to lack of effective tools for early detection, most patients with SEOC present with intraperitoneal metastasis at diagnosis. The prognosis of SEOC is usually poor with 5-year survival rate around 50 % [3]. Although sophisticated surgery and new chemotherapy regimens appear in the recent years, the 5-year survival rate for patients with SEOC did not improved significantly during the last two decades [4]. Failure of SEOC treatment is largely due to widely intraperitoneal metastasis, an important prognostic index for SEOC patients in clinical setting [3]. Therefore, it is necessary to identify certain effective biomarker that can help in predicting intraperitoneal metastasis of patients with SEOC.
Recently, chloride intracellular channel 1 (CLIC1) was proposed as a novel potential prognostic factor in gastric and gallbladder cancer, which was significantly upregulated in 67.9 and 62.7 % of cancer patients [5, 6]. Other studies have shown that CLIC1 is highly expressed in tumors, such as colon, liver, lung, and nasopharyngeal [7–9], suggesting that this protein may have important function in the malignant transformation of cancer cells.
To date, there have been no published reports evaluating the relationship between CLIC1 and intraperitoneal metastasis in SEOC. Here, we demonstrated that CLIC1 protein level is highly correlated with malignancy in SEOC and that it could be a novel biomarker for prediction of intraperitoneal metastasis in SEOC.
Materials and methods
Patients and treatment
Total 120 cases of SEOC patients were selected for our study following the approval of Institutional Review Board, The Fourth Hospital of Harbin Medical University. The criteria for selection include : (1) confirmed diagnosis of SEOC by pathological examination; (2) complete clinical records; (3) no serious complications or other malignant diseases; (4) absence of prior treatment for cancer; (5) already had cytoreductive surgery. All selected patients were informed about the illness and consent with the treatment.
Quantitative PCR
Total RNA was extracted from human tissues using the RNeasy Plus Mini Kit (74134, Qiagen) and then converted into cDNAs using the High Capacity cDNA Reverse Transcription Kit (4368814, Applied Biosystems) following the manufacturer’s instruction. Quantitative PCR (qRT-PCR) was performed with a CFX96 (Bio-Rad) using the RT2 SYBR Green (330500, SA Biosciences). The primer sequences for CLIC1 were: 5′-AGAGGCTGAATG AG CTGGAG-3′ (forward) and 5′- TGGCAATTTTGGTCCATTTT-3′ (reverse). All values were normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) abundance. Data were presented as the average of triplicates ± SD.
Western blotting analysis
Frozen samples of BOT (18 cases) and SEOC (40 cases) were homogenized in lyses buffer consisting of 1 % Triton X-100 in phosphate-buffered saline (PBS) supplemented with protease inhibitor cocktail (Roche) and then incubated on ice for 30 min. After that, the mixture was centrifuged at 13,200g for 15 min at 4 °C and collected the supernatant. Sixty microgram of protein extracts was loaded onto 15 % SDS polyacrylamide gel electrophoresis and transferred onto a PVDF membrane (Millipore Company, USA). The blots were blocked with blocking buffer (pH 7.6) containing 5 % nonfat dry milk for 1 h and then incubated with rabbit polyclonal anti-human CLIC1 antibody (1:500) (sc-81873, Santa Cruz Biotechnology, Inc) overnight at 4 °C. Immunoreactive proteins were stained using a chemiluminescence’s detection system. Membranes were stripped with stripping buffer for 1 h and re-probed with human β-actin antibody (Abcam, Cambridge, MA) as an internal control [2, 3].
Immunofluorescence
For immunofluorescent staining, antibodies against the following antigens used CLIC1 antibody (1:100). Secondary antibodies were purchased from Molecular Probes and Vector Laboratories. Confocal microscopy images were taken with a Zeiss LSM 700 microscope and evaluated using the ZEN2010 software. For mean fluorescence intensity measurements, confocal microscopy images were analyzed with ImageJ. Single CLIC1-positive cells were selected using the ellipsoid selection tool and analyzed for mean fluorescence intensity of CLIC1 expression (Alexa 594) by using the histogram tool (only the value of the red channel was used). Slides were observed using a Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss MicroImaging; Thornwood, NY), and images were captured using Openlab3 software (Improvision, Lexington, MA). For tissue, 5-μm serial sections were cut from paraffin, followed by the same blocking/antibody protocol for cells as listed above.
Semi-quantitative analysis of CLIC1 staining
Scoring of CLIC1 in EOC samples via immunofluorescence (IF) staining follows the methods previously published [2, 3]. All of the IF staining samples from SEOC patients were evaluated by experienced two pathologists independently.
Statistical analysis
The chi-square test or Fisher’s exact test was use to identify statistical differences in the clinicopathologic variable analysis. Univariate and multivariate logistic regression with covariate adjustment were use to assess the association of CLIC1 overexpression with SEOC metastasis. A two-sided P < 0.05 was considered as statistically significant. Statistical analysis was carried out using SAS software (version 9.1.3, SAS Institute, Cary, NC).
Results
Demographic characteristics of SEOC patients
The demographic and clinical data about SEOC patients are shown in Table 1. Among the 120 patients with SEOC, the percentage of FIGO stages I and II and III and IV is 35 and 65 %, respectively. Moreover, there were 110 cases with serum CA-125 level greater than 35 (91.67 %) and 81 (67.5 %) cases with ascites volume more than 100 ml. Intraperitoneal and lymph node metastasis was observed in 78 and 19 patients, respectively. In addition, the distribution of histopathological differentiation of 120 SEOC patients from grade 1 to 3 is 31 (25.8 %), 34 (28.3 %), and 55 (45.9 %), respectively.
CLIC1 expression is highly correlative with malignancy
In present study, we detected that CLIC1 mRNA level was significantly increased in primary ovarian cancer with metastasis (POCM), omentum, and peritoneal metastasis as compared to benign ovarian tumor (BOT) and primary ovarian cancer with no metastasis (POCNM) via qRT-PCR (Fig. 1a). Consistently, the protein level of CLIC1 was significantly higher in POCM, omentum, and peritoneal metastasis as compared to BOT and POCNM (Fig. 1b, c).
a qRT-PCR assay of CLIC1 levels in benign ovarian tumor (BOT) and serous ovarian carcinoma (SEOC) tissues. GAPDH serves as an internal control. b Western blotting analysis of CLIC1 expression in normal ovaries and ovarian carcinoma tissues. Protein samples obtained from frozen benign ovarian tumor (BOT) and serous ovarian carcinomas (SEOC) were analyzed by SDS-PAGE followed by immunoblotting with antibody against CLIC1. The levels of β-actin were used as an internal control. c Histogram of pooled data from fresh benign ovarian tumor (BOT, N = 18), serous ovarian carcinomas, and intraperitoneal metastasis (N = 40). The expression of CLIC1 was increased both in ovarian carcinomas compared with fresh BOT. Each of the protein samples was repeated for three times. (ns, P > 0.05; *P < 0.05; ***P < 0.001)
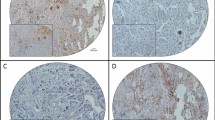
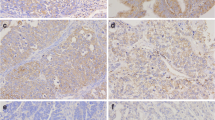
Further analyses by immunofluorescent staining revealed that CLIC1 was hardly detectable in normal ovaries and BOT (Fig. 2a, b), while strong staining of CLIC1 was detected in most SEOC samples (Fig. 2a, b). Interestingly, we noticed that among 120 cases of SEOC, POCM (97.4 %) tumors have relatively high level of CLIC1 (Table 1 and Fig. 2b), suggesting CLIC1 level may positively correlate to SEOC intraperitoneal metastasis. CLIC1 level was significantly higher in late stages (III and IV) than early stages (I and II) of SEOC samples (Table 1). Furthermore, the results indicated that CLIC1 overexpression correlated positively with ascites volume and negatively with the degree of histopathological differentiation (Table 1).
a Representative examples showing high and low CLIC1 expression (positivity + intensity). Upper left panel: normal ovaries, score: 0 + 0; Upper right panel: benign ovarian tumors, score 0 + 0. Middle left panel: shows representative staining patterns of low CLIC1 expression in primary ovarian cancer with not metastasis (POCNM), score: 1 + 0. Middle right panel: shows representative staining patterns of high CLIC1 expression in primary ovarian cancer with metastasis (POCM), score: 2 + 2. Lower left panel: shows representative staining patterns of high CLIC1 expression in omentum metastasis (OM), score: 2 + 2. Lower right panel: shows representative staining patterns of high CLIC1 expression in peritoneal metastasis (PM), score: 2 + 2 (×200 original magnification). b Statistical analysis of CLIC1 stain positively in normal ovaries (N = 22), BOT (N = 18), POCNM (N = 42), PONM (N = 78), OM (N = 78), and PM (N = 78) (*P < 0.05; **P < 0.01; ***P < 0.001)
CLIC1 is a potential sensitive and specific biomarker for prediction of intraperitoneal metastasis in SEOC
To assess the association between high level of CLIC1 protein and intraperitoneal metastasis, we first analyzed the traditional clinicopathological variability about intraperitoneal metastasis. Intraperitoneal metastasis was highly correlative with the stage of FIGO (P < 0.0001) and the presence of ascites P < 0.0001), respectively. To evaluate the independent correlation between CLIC1 expression and intraperitoneal metastasis, statistically significant factors (age, ascites, and CLIC1 expression) are used to adjust the multivariate logistic regression model. The results showed that high level of CLIC1 was independently correlative to intraperitoneal metastasis (OR 37.96; 95 % CI 4.75, 293.22; P < 0.001) (Table 2). For intraperitoneal metastasis, the sensitivity and specificity of CLIC1 overexpression were 97.4 and 88.1 %, respectively.
Discussion
CLIC1 is a novel identified member of a chloride channel protein family, which includes CLIC1 to 6 and p64 et al. [10]. To date, more and more documents demonstrated that CLIC1 is critical for many physiological processes and pathology, such as in cell cycle, proliferation, and migration [11, 12]. Furthermore, CLIC1 was reported to initiate and promote tumor development [5, 6, 13].
However, the associations between CLIC1 level and malignancy in SEOC have not been reported. In this study, we analyzed CLIC1 expression in 120 patients with SEOC by Immunofluorescence and Western blotting and found that high expression of CLIC1 can be detected in SEOC among 81 patients. In NO, CLIC1 cannot be detected; and its expression level is very low in BOT and POCNM. However, it can be highly expressed in POCM. CLIC1 expression is also a negative correlation with histopathological differentiation.
Immunohistochemistry results revealed that among 81 patients with CLIC1 high expression, 78 (96.3 %) presented intraperitoneal metastasis, suggested that CLIC1 expression is highly correlated with metastasis in SEOC. For intraperitoneal metastasis, the sensitivity and specificity of CLIC1 overexpression were 97.4 % (76/78) and 88.1 % (37/42), respectively (Table 1). Therefore, CLIC1 could be a new potential predictor of intraperitoneal metastasis of SEOC.
In conclusion, CLIC1 expression is highly correlated to malignancy in SEOC, and it could be a highly sensitive and specific biomarker for the diagnosis of intraperitoneal metastasis in SEOC.
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics. CA Cancer J Clin. 2005;55:10–30.
Yin M, Xu Y, Lou G, Hou Y, Meng F, Zhang H, et al. LAPTM4B overexpression is a novel predictor of epithelial ovarian carcinoma metastasis. Int J Cancer. 2011;129(3):629–35.
Yin M, Li C, Li X, Lou G, Miao B, Liu X, et al. Over-expression of LAPTM4B is associated with poor prognosis and chemotherapy resistance in stages III and IV epithelial ovarian cancer. J Surg Oncol. 2011;104(1):29–36.
Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–38.
Ding Q, Li M, Wu X, Zhang L, Wu W, Ding Q, et al. CLIC1 overexpression is associated with poor prognosis in gallbladder cancer. Tumour Biol. 2014. doi:10.1007/s13277-014-2606-5.
Chen CD, Wang CS, Huang YH, Chien KY, Liang Y, Chen WJ. Overexpression of CLIC1 in human gastric carcinoma and its clinicopathological significance. Proteomics. 2007;7(1):155–67.
Petrova DT, Asif AR, Armstrong VW, Dimova I, Toshev S, Yaramov N, et al. Expression of chloride intracellular channel protein 1 (CLIC1) and tumor protein D52 (TPD52) as potential biomarkers for colorectal cancer. Clin Biochem. 2008;41(14–15):1224–36.
Kim W, Oe Lim S, Kim JS, Ryu YH, Byeon JY, Kim HJ, et al. Comparison of proteome between hepatitis B virus-and hepatitis C virus-associated hepatocellular carcinoma. Clin Cancer Res. 2003;9(15):5493–500.
Wang W, Xu X, Wang W, Shao W, Li L, Yin W. The expression and clinical significance of CLIC1 and HSP27 in lung adenocarcinoma. Tumour Biol. 2011;32(6):1199–208.
Tulk BM, Kapadia S, Edwards JC. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am J Physiol Cell Physiol. 2002;282:1103–12.
Sun HJ, Bahk YY, Choi YR, Shim JH, Han SH, Lee JW. A proteomic analysis during serial subculture and osteogenic differentiation of human mesenchymal stem cell. J Orthop Res. 2006;24(11):2059–71.
Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Warton K, Musgrove EA, et al. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol. 2000;529(Pt 3):541–52.
Tian Y, Guan Y, Jia Y, Meng Q, Yang J. Chloride intracellular channel 1 regulates prostate cancer cell proliferation and migration through the MAPK/ERK pathway. Cancer Biother Radiopharm. 2014;29(8):339–44.
Acknowledgments
The authors thank all the people and patients who had predicated in this study.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Mingzhu Yin is a joint first author and the two authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ye, Y., Yin, M., Huang, B. et al. CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumor Biol. 36, 4175–4179 (2015). https://doi.org/10.1007/s13277-015-3052-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3052-8