Abstract
Tissue inhibitor metalloproteinase-1 (TIMP-1) is clinically associated with a poor prognosis for various cancers, but the roles of TIMP-1 in lung cancer metastasis are controversial. Our previous secretomic study revealed that TIMP-1 is highly abundant in high invasiveness cells of lung adenocarcinoma. In the current study, TIMP-1 abundances in primary lung adenocarcinoma tissues, as revealed by immunohistochemistry, are significantly higher in patients with lymph invasion and distant metastasis than in those without. Receiver operating characteristic curve analyses suggest 73.7 and 86.2 % accuracy to separate patients with lymph node and distant metastasis and those without, respectively. Moreover, we demonstrate that the expression level of TIMP-1 positively associates with cell mobility, invasiveness, and metastatic colonization. Most notably, the novel mechanism in which TIMP-1 facilitates metastatic colonization through the mediation of pericellular polyFN1 assembly was revealed. In summary, this study presents novel functions of TIMP-1 in promoting cancer metastasis and suggests TIMP-1 is a potential tissue biomarker for lymph invasion and distant metastasis of lung adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most serious threats to human health worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 85 % of all cases. Among the histological types of NSCLC, lung adenocarcinoma is the most common. Metastasis and recurrence are the leading cause of death in patients with lung cancer. About 90 % of lung cancer patients die from tumor metastasis. Overall, less than 10 % of people with primary lung cancer survive 5 years after diagnosis. However, 5-year survival rates can be as high as 35–40 % for those who have localized lung cancer removed in its early stage [1]. Thus, an in-depth study of molecular mechanisms in lung cancer invasion and metastasis is of immediate importance.
A family of tissue inhibitors known as metalloproteinase (TIMP) are extracellular proteins with four identified members (TIMP-1, TIMP-2, TIMP-3, and TIMP-4) to date. TIMP-1 is highly expressed by glandular cells in reproductive systems and is also known as fibroblast collagenase inhibitor and erythroid-potentiating activity (EPA) protein [2–4]. It is a 28.5-kDa glycoprotein and one of the endogenous inhibitors of matrix metalloproteinases (MMPs), the latter being enzymes involved in degradation of the extracellular matrix (ECM) and can promote cancer progression [5]. Given the role of MMPs in degrading matrices and thus promoting metastasis, TIMP-1 has been deemed a suppressor of tumor malignancy [6, 7]. TIMP-1 has been studied for its clinical expression in tissue specimens and plasma. There are many published results that have shown that higher expression levels of TIMP-1 appear in advanced or during aggressive stages of cancer and are associated with low chances of survival among patients with breast, gastric, colorectal, prostate, endometrial cancer, as well as NSCLC [8–13]. Interestingly, high expression levels of TIMP-1 could inhibit cancer progression, since TIMP-1 would be expected to inhibit MMP-facilitated breakdown of the ECM and basement membranes. However, the clinical results demonstrate distinct findings. One explanation for the high levels of TIMP-1 observed in the most aggressive tumors is that TIMP-1 may carry several MMP-independent functions that facilitate cancer cell progression [14, 15]. For instance, TIMP-1 could inhibit apoptosis by stimulating the Akt, FAK/PIK-3, or MAPK signaling pathway [16, 17] and regulate cell proliferation [18–20]. Additionally, the number of F-actin stress fibers and focal adhesions will be reduced as TIMP-1 causes both FAK (pY397) and paxillin to dephosphorylate. Likewise, phosphorylation of FAK will be reduced and cell migration will be inhibited when PTEN expression is stimulated by TIMP-1 [18]. More evidences have been studied that verify TIMP-1 could promote angiogenesis, invasion, and metastasis [19–23]. The role of TIMP-1 in cancer progression is very perplexing. These MMP-independent functions of TIMP-1 may be directly involved in the spread of cancer cells and eventually lead to the death of cancer patients.
In lung cancer, TIMP-1 has been shown as a potential prognostic marker in NSCLC tissue specimens [12, 24]. However, the functions of TIPM-1 in lung adenocarcinoma metastasis are still not fully confirmed. According to the previous findings of our secretome proteomics data, we found that TIMP-1 was up-regulated in a high invasiveness cell line (CL1-5) compared to a low invasiveness cell line (CL1-0) [25, 26]. In this study, the clinical examination and in vitro and in vivo functional assays were utilized to investigate the functions of TIMP-1 in lung adenocarcinoma metastasis. The results showed that the expression levels of TIMP-1 were significantly increased in patients with lymph node and/or distant metastasis. Moreover, functional assays affirmed that TIMP-1 could positively influence cell migration, invasion, and colonization, but not proliferation.
Materials and methods
Cell lines and culture
The poorly differentiated adenocarcinoma cell lines CL1-0 and CL1-5 were provided by Dr. P.-C. Yang (Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan, R.O.C) [27] and cultured at 37 °C with 5 % CO2 in RPMI 1640 media supplemented with 10 % fetal bovine serum (FBS) (Gibco BRL, Gaithersburg, MD), 1 % penicillin (Invitrogen, Carlsbad, CA), and 2 g/L NaHCO3. CL1-0 and CL1-5 cells showed low and high invasiveness, respectively, according to previous research in which the migration protein marker, vimentin, matrigel invasion assays, and lung colonization assays were used [27]. The samples of cell lysate and conditioned medium (CM) were collected according to the previous study [25].
Western blot
SDS-PAGE (12 % gel) was used to separate the CM and cell extract samples (30–50 μg). When transferring the proteins to a PVDF membrane, iBlot (Invitrogen, Carlsbad, CA) was used in accordance with the manufacturer’s protocol. The membrane was placed in a 5 % nonfat milk solution for 1 h at room temperature. Then, anti-TIMP-1 antibody (Epitomics, Burlingame, CA, USA) at 1:1,000 dilution was probed on the membranes overnight (4 °C). The membranes were then washed with Tris-buffered saline and Tween-20 (TBST) three times before being incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG) for 1 h at room temperature (Sigma-Aldrich, St. Louis, MO). Before the membranes were developed using enhanced chemiluminescence detection, they were washed with TBST five more times.
Tissue microarray and immunohistochemistry
Patients were selected if lung adenocarcinoma was their primary diagnosis, they had no prior history of cancer, and they received surgery as their first treatment. The testing samples were obtained from their surgery. The lung adenocarcinoma tissue samples were provided by the tissue bank of National Cheng Kung University Hospital from 2003 to 2009. All of the patients who provided tumor tissue specimens gave written informed consent in accordance with the rules and requirements of National Cheng Kung University Hospital’s ethics committee. The tissue microarray (TMA) slides were purchased from the tissue bank of hospitals. Each tissue specimen was sampled in replicate by sampling tumor tissue cores (1.0 mm in diameter). Immunohistochemistry (IHC) was performed on 4-μm-thick formalin-fixed paraffin-embedded sections. Rabbit anti-human TIMP-1 polyclonal antibody (1:100 dilution, Spring bioscience) was used as the primary antibody. The procedures were done with the Bond-Max Automated IHC stainer (Leica Biosystems Newcastle Ltd, Australia) according to the following protocol. Tissues were deparaffinized with xylene and pre-treated with the Epitope Retrieval Solution 1 (citrate buffer, pH 8.5) at 100 °C for 30 min, followed by hydroperoxide blocking for 5 min using the Bond Polymer Refine Detection Kit (Leica Biosystems Newcastle Ltd, UK). The primary antibody was incubated at room temperature for 30 min. Subsequently, tissues were incubated with polymer at room temperature for 8 min then developed with 3,3′-diaminobenzidine chromogen for 15 min. Counterstaining was carried out with hematoxylin. Image analysis of TIMP-1-positive cells was further quantified using the TissueFaxs microscopy system with the HistoQuest software module (TissueGnostics, Vienna, Austria).
TIMP-1 knockdown and overexpression
The siRNA of TIMP-1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The siRNA transfection process was performed in accordance with the manufacturer’s instructions. CL1-5 cells (3 × 105) were seeded in a six-well plate with 2 mL antibiotic-free RPMI 1640 with 10 % FBS for over 24 h. A half microgram of TIMP-1 siRNA or scramble control siRNA was mixed with 6 μL of siRNA transfection reagent and 100 μL of siRNA transfection media. The mixture was carefully overlaid onto cells for 6 h before replacing with RPMI 1640 with 10 % FBS. TIMP-1 overexpression in CL1-0 cells was achieved by transient transfection with TIMP-1 plasmids (human gene BC000866, OriGene Technologies, Inc., Rockville, MD, USA) using TurboFect transfection reagent following the manufacturer’s instructions (Thermo Fisher Scientific Inc., Huntsville, AL, USA). Briefly, 70 % confluent CL1-0 cells seeded in six-well plates were transfected with 4 μg of plasmid DNA or control plasmid DNA mixed with 12 μL of TurboFect transfection reagent in a 100 μL serum-free medium. The mixture was overlaid onto the cells in RPMI 1640 with 10 % FBS.
Cell growth test
When evaluating the direct effect of TIMP-1 knockdown and overexpression on cancer cell growth in vitro, cell counting and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (thiazolyl blue, MTT) assay were performed. In the cell counting experiment, 3 × 105 cells were seeded in a six-well plate and cells were harvested after 24 h of siRNA or plasmid DNA treatments. Trypan blue was used for viable cell counting. For the MTT assay, a 96-well plate was used, and the cells were seeded at a concentration of 1 × 103 cells per well. The cells were incubated for 24 h before being treated with siRNA or plasmid DNA. After 24 h, 2 μL of MTT (10 mg/mL) was added to each well and incubated for 3 h at 37 °C. The cells were lysed with 100 μL of dimethyl sulfoxide. Microplate photometers measured the absorbance at 595 nm (Thermo Fisher Scientific Inc., Waltham, MA, USA). The cell growth of each group was monitored every 24 h for 72 h.
Cell wound healing, transwell migration, and matrigel invasion assays
Cells were seeded on a plate with an ibidi Culture-Insert (Applied BioPhysics, Inc., Troy, NY, USA), which was removed after 12 h for initiation of the cell wound healing assay. Images of the gap were taken at 0, 12, and 24 h at the same position. Cell transwell migration was performed in BD Falcon multi-well insert systems with 8 μm pores (BD Biosciences, San Jose, CA, USA). Cells (1 × 105) seeded in the upper wells in serum-free medium were induced to migrate to lower chambers in which completed medium with 10 % FBS was filled. After 24 h, the cell counts in lower chambers were collected from six different fields per filter after methanol fixation and Giemsa staining. For invasion assays, BD Falcon multiwell insert systems with 8 μm pores were coated with 2 mg/mL BD Matrigel basement membrane matrix (BD Biosciences, San Jose, CA, USA) before seeding cells (1 × 105). The following steps were the same as those of the transwell migration assay.
Lung colonization
CL1-5 cells transfected with scramble or TIMP-1 siRNA were incubated in suspension for 2 h in 20 % FBS media followed by a 20-min treatment of myelin basic protein (MBP) and soluble truncated dipeptidyl peptidase IV (DPP IV) [28]. The cell suspensions (2 × 106 cells in 200 μL RPMI 1640 base medium) were subsequently injected into the lateral tail vein of 8-week-old nude mice, which were sacrificed after 8 weeks to remove and fix the lungs with 3.7 % formalin. Five mice in each group were used, and the representative lung tumors were embedded in paraffin, sectioned into 4-mm layers, and stained with hematoxylin and eosin (H&E) for histologic analysis.
Pericellular fibronectin assembly and flow cytometry
Pericellular fibronectin assembly was induced by incubating trypsinized cells in suspension with 20 % FBS media for 2 h at 37 °C [4]. After being washed with PBS, cells were incubated with FN1 antibody (Sigma-Aldrich, St. Louis, MO, USA), diluted 1:600 in PBS-1 % BSA for 1 h at 4 °C, followed by fluorescein isothiocyanate-conjugated secondary antibody in PBS with 1 % BSA for 1 h at 4 °C, and fixed in 2 % paraformaldehyde in PBS. Fluorescence-activated cell sorting (FACS) was performed to quantify polymeric FN (polyFN) on the cell surface using Coulter Epics Profile (FACSCalibur, BD Biosciences, San Jose, CA, USA). Non-immune serum was used in this assay as a negative control, replacing the primary antibody, to evaluate the nonspecific fluorescence signal.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL). A statistically significant p value less than 0.05 was required for the two-tailed tests. All experiments were performed in biological triplicate, and the results are shown as the mean ± S.D. The nonparametric Mann–Whitney U test was employed to analyze all of the differences between groups.
Results
Clinical expression levels of TIMP-1 in lung adenocarcinoma tissues
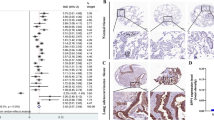
TIMP-1 was found up-regulated in the advanced stages of various kinds of cancer tissues. Here, TIMP-1 expression levels in lung adenocarcinoma and its associations with clinical features were investigated. Table 1 exhibits the TIMP-1 IHC results concerning the correlation of several clinical pathologic factors with the expression level of TIMP-1 among 85 tissue specimens. No significant correlations were found among age, sex, or the tumor size. The expression levels of TIMP-1 were significantly correlated with lymph node metastasis and distant metastasis (Table 1 and Fig. 1a).
TIMP-1 abundance in tissue is associated with the metastasis of primary lung adenocarcinoma. a Immunohistochemical staining of TIMP-1 in tissue sections from lung adenocarcinoma patients of indicated stages. b, c ROC curve analyses of TIMP-1 as a lymph invasion and distant metastasis biomarker, respectively
TIMP-1 expression levels were highly increased in patients with lymph node metastasis and distant metastasis compared to patients without. Receiver operating characteristic (ROC) curve analyses were used to evaluate the separate efficacy of patients with or without lymph node or distant metastasis. When evaluating lymph node metastasis, the area under the ROC curve (AUC) was determined to be 73.7 % (95 % CI, 0.62–0.84) for TIMP-1 (Fig. 1b). When the cutoff point gray value was chosen as 28.3, sensitivity and specificity values were 71.4 and 75.7 %, respectively. To assess if TIMP-1 can serve as a distant metastasis biomarker, the AUC was determined to be 86.2 % (95 % CI, 0.52–0.92) with 75.0 % sensitivity and 63.6 % specificity, when a cutoff of TIMP-1 gray value was set at 32.67 (Fig. 1c).
TIMP-1 mediates lung adenocarcinoma cancer cell migration and invasion in vitro
In order to investigate whether TIMP-1 could influence cell behavior in metastasis processes, TIMP-1 protein was either specifically knocked down in the high invasiveness cell line (CL1-5) by siRNA (Fig. 2a) or over-expressed by transient transfection with TIMP-1 plasmid in the low invasiveness cell line (CL1-0) (Fig. 3a). The protein level was validated in both cell lysate and conditioned media (CM) using western blots; since TIMP-1 is a secretory protein, the protein was abundant in CM but barely detectable in cell lysates. We found that knocking down TIMP-1 significantly impaired the cell wound healing process in CL1-5 cells (Fig. 2b), while TIMP-1 overexpression enhanced the efficiency of wound closure in CL1-0 cells (Fig. 3b). Likewise, TIMP-1 siRNA reduced the number of migrating CL1-5 cells by 62.3 % (Fig. 2c), and overexpression of TIMP-1 increased CL1-0 migration by 1.75-fold in the transwell migration assay (Fig. 3c). We further assessed the invasive potential of TIMP-1 knockdown or over-expressed cells and found that TIMP-1 knockdown inhibited CL1-5 cell invasion through matrigels by 90.9 % (Fig. 2d), and exogenous TIMP-1 expression increased CL1-0 invasion by 3.2-fold (Fig. 3d). In addition, cell counting and MTT assay data revealed that TIMP-1 did not influence cell growth/number as the confounding factor when evaluating cell migration and invasion assays (Figs. 2e, f and 3e, f). Taken together, these cell-based results suggest that TIMP-1 expression is sufficient and necessary to promote the migration and invasion of lung adenocarcinoma cells.
TIMP-1 knockdown in CL1-5 cells impaired cell mobility and invasiveness. a TIMP-1 siRNA substantially reduced TIMP-1 abundance in the cell extract (CE) and conditioned media (CM) as shown by western blotting with indicated antibodies. Approximately, 55 and 87 % of TIMP-1 levels were knocked down in CE and CM, respectively. Equal amounts of each CM sample with coomassie blue staining were used as loading controls. b TIMP-1 siRNA prohibited CL1-5 cell migration in the cell wound healing assay. c, d TIMP-1 siRNA significantly reduced the number of transwell migrated and invaded cells, respectively. e MTT assay. f Trypan blue cell counting. No significant differences were shown between scramble siRNA and TIMP-1 siRNA groups from 24 to 72 h. N.S. means no significance
TIMP-1 overexpression in CL1-0 cells up-regulates cell mobility and invasiveness. a Transient transfection with TIMP-1 plasmids in CL1-0 cells substantially enhanced TIMP-1 protein abundance in the cell extract (CE) and conditioned media (CM), as shown by western blotting with indicated antibodies. The expression level of TIMP-1 exceeded that of the control by approximately 79 % in CE and the level in CM is apparent after over-expression. Equal amounts of each CM sample with coomassie blue staining were used as loading controls. b Exogenous TIMP-1 expression elicited CL1-0 cell migration in the cell wound healing assay. c, d Exogenous TIMP-1 expression provoked transwell migrated and invaded CL1-0 cells, respectively. e MTT assay. f Trypan blue cell counting. No significant differences were shown between scramble siRNA and TIMP-1 siRNA groups from 24 to 72 h. N.S. means no significance
Down-regulation of TIMP-1 expression inhibited metastatic colonization in vivo
To further identify the role of TIMP-1 in the metastasis of lung adenocarcinoma in vivo, an experimental metastasis model was further applied to determine if TIMP-1 participates in tumor colonization. The size of colonized lungs for the group in which TIMP-1 synthesis was knocked down in CL1-5 cells by siRNA was significantly reduced as compared to that of scramble siRNA-treated CL1-5 (Fig. 4a, left). For each excised lung, pulmonary tumor nodules (>0.5 mm) revealed by hematoxylin and eosin stains were counted. The average number of pulmonary nodules from five animals injected with scramble siRNA- or TIMP-1 siRNA-treated CL1-5 cells was 539.4 and 204.2, respectively (p < 0.05) (Fig. 4a, right). The H&E staining of the tissue sections further confirmed the reduction of cancerous cells by TIMP-1 knockdown (Fig. 4b).
TIMP-1 facilitates metastatic colonization. a (Left) The size of colonized lungs was reduced when TIMP-1 siRNA was applied to CL1-5 cells before being intravenously injected into mice. (Right) The number of visible nodules in excised lungs was decreased by TIMP-1 siRNA treatment. b H&E staining of the represented lung sections. c Cells labeled by immunofluorescence staining of fibronectin was subjected to microscope imaging (left) and flow cytometry (right), showing that fibronectin assembly on the surface of CL1-5 cells was reduced by TIMP-1 siRNA
Metastatic colonization was mediated by pericellular polymeric FN assembly on suspended CL1-5 lung adenocarcinoma cells
Malignant cancer cells acquire the ability to reside in the secondary site as a metastatic tumor. According to our previous findings, pericellular polyFN is required for suspended CL1-5 cells to colonize the lungs. They do this by adhering to DPP IV on the cell surface of lung capillary endothelia to complete colonization [25]. We assumed that TIMP-1 might facilitate colonization through the regulation of pericellular polyFN assembly. Suspended CL1-5 cells treated with scramble siRNA were capable of assembling globular polyFn on the surface as shown by immunofluorescence staining and flow cytometry (Fig. 4c, upper panel). However, TIMP-1 siRNA substantially reduced the population of cells that assembled pericellular Fn from 76.14 to 22.80 % (Fig. 4c, lower panel). In summary, these findings imply that it is likely TIMP-1 plays a role in facilitating metastatic colonization through the coordination of cell surface polyFn assembly.
TIMP-1 positively modulates MMP-2 expression in invasive cells
To understand whether TIMP-1 is involved in the MMP-dependent pathway of invasive cells, the MMP-2 and MMP-9 protein levels in CM were studied as TIMP-1 knocked down CL1-5. The MMP-2 and MMP-9 protein levels were inspected in our own cell model. As shown in Fig. 5a, compared to CL1-0, higher levels of TIMP-1 and MMP-2 were discovered in CL1-5 CM, but not MMP-9. Once the TIMP-1 was knocked down in CL1-5, the MMP-2 level was significantly down-regulated (Fig. 5b).
TIMP-1 is involved in MMP-2 up-regulation within CL1-5 cells but not MMP-9. a Western blot data showed that MMP-2 had higher levels in CL1-5 than CL1-0 CM. b The MMP-2 level was significantly reduced in CL1-5 CM while TIMP-1 was knocked down. Equal amounts of each CM sample with coomassie blue staining were used as loading controls
Discussion
Cancer metastasis is a convoluted process. During metastatic progression, tumor cells go through several critical steps, including uncontrolled growth in the primary site, local invasion, intravasation, survival in circulation, extravasation, and eventually colonize, survive, and thrive in the secondary organs of distant tissues [29]. Unfortunately, the mechanisms that drive these metastatic processes are still being explored. Therefore, the effective diagnosis and treatments for lung adenocarcinoma metastasis are restricted by our limited knowledge, which leads further ineffective prognoses and high mortality rates among lung adenocarcinoma patients.
TIMP-1 is a naturally occurring inhibitor of the MMPs involved in tissue invasion, angiogenesis, cell growth, and apoptosis [30–34]. It has generally been considered a negative regulator of cancer metastasis [35–38]. As its name suggests, one of TIMP-1’s major functions is inhibiting cancer cell migration and invasion abilities by proteolysis of matrix metalloproteinases, which are capable of degrading all kinds of extracellular matrix proteins to promote cell invasion, such as MMP-2 and MMP-9 [31, 39]. Previous studies revealed that the up-regulation of TIMP-1 could reduce MMP-9 activation and therefore suppress tumor invasiveness and metastatic potential in breast, colon, and lung cancer [31, 40, 41]. However, there is increasing evidence that suggests TIMP-1 is multifunctional [5, 22, 42]. It plays a role not only as an inhibitor but also a promoter in metastasis. For instance, research has found that TIMP-1 has both MMP-dependent and MMP-independent pathways to differentially regulate metastasis processes [17, 18, 20, 31, 39]. Also, the balance between TIMP and MMP is a critical factor in triggering different steps of metastasis [20]. Clinical significance also exists in that higher TIMP-1 levels reflect advanced stages in various cancers [8–13, 40, 43]. Moreover, the presence of TIMP-1 creates a negative effect on the body as increased levels have been associated with poorer responses to chemotherapy and lower survival rates [44–48].
In previous research, a high concentration of TIMP-1 secreted by cancer cells has been connected with malignant progression in several cancer cell lines [22, 49–51]. Additionally, a previous study illustrated that TIMP-1 promotes cancer progression and the accumulation of cancer-associated fibroblasts that in turn provide a pro-tumor microenvironment [52]. TIMP-1 significantly reduced apoptosis induced by promyelocytic leukemia zinc finger protein (PLZF) in cervical carcinoma cells [53]. TIMP-1 can elicit an HIF-1-induced stress response and lead to increased metastasis [54]. TIMP-1 promotes liver metastasis in two independent tumor models by inducing the hepatocyte growth factor (HGF) signaling pathway and via the expression of several metastasis-associated genes, including HGF and HGF-activating proteases [22]. John and coworkers found that a matrix-bound adhesive glycoprotein, thrombospondin-1 (TSP-1), is not only capable of stimulating MMPs involvement in tumor invasion but also its corresponding inhibitor, TIMP-1, thereby promoting cancer invasion in both human breast and prostate cancer cell lines. Hence, they had proposed that the critical balance between proteases and their inhibitors could be a key determinant in cancer metastasis [20]. Interestingly, our previous findings in secretome analyses also explain that TSP-1, MMP-2, and TIMP-1 were all up-regulated in high invasiveness cells (CL1-5) in comparison to low invasiveness cells (CL1-0). Our current data indicates that MMP-2 levels were reduced while TIMP-1 was knocked down in CL1-5 (Fig. 5). Besides, the data showed that the migration and invasion abilities of high invasiveness CL1-5 cells were suppressed while TIMP-1 was knocked down. Likewise, the migration and invasion abilities of low invasiveness CL1-0 cells were promoted while TIMP-1 was over-expressed. The data suggested that the balance between TIMP-1 and MMP-2 levels could be a critical factor in promoting cell migration and invasion which means that high TIMP-1 levels could stimulate the secretion of MMP-2.
In vivo experiments showed that colonization levels were significantly decreased when TIMP-1 was knocked down in CL1-5 cells. We also found a possible mechanism that explains how TIMP-1 influences colonization within the lungs. According to our previous findings, when invasive cells CL1-5 were introduced into the systemic circulation of immunocompromised nude mice via intravenous injection, these cells colonized in the lungs after 8 weeks. Isolated lungs derived from CL1-5-treated mice were significantly larger in size compared to those from CL1-0-treated animals [25, 27]. In addition, the assembly levels of the metastasis-promoting pericellular fibronectin (FN1), which facilitates colonization of lung capillary endothelia by adhering to the cell surface receptor DPP IV, were higher on the surfaces of suspended CL1-5 cells than on those of the CL1-0 cells. In line with this finding, polyFN1 assembly and the lung colonization of suspended CL1-5 cells were inhibited when endogenous TIMP-1 protein was knocked down using siRNA. The results suggested that TIMP-1 is a required factor for cellular activities to enable their colonization in distant organs throughout supporting pericellular polyFN1 assembly in lung adenocarcinoma. According to our previous research, using siRNA to knock down endogenous A1AT protein would inhibit FN1 assembly and lung colonization of suspended CL1-5 cells [25]. In line with this finding, A1AT and TIMP-1 could be involved in the same mechanism as two co-functioning factors that regulate polyFN1 assembly. This mechanism might be triggered by phosphorylated protein kinase c epsilon (PRKCE) [25, 55] and MMP2 could be the downstream target of TIMP-1. The possible mechanisms need to be further validated.
In the clinical research regarding TIMP-1 expression, some studies suggested that TIMP-1 can be applied to the screening of prognostic markers in various cancers, such as breast, colorectal, gastric, and lung cancers [40, 43, 47, 56]. According to the results, TIMP-1 expression levels were significantly higher in the stages with lymph invasion or distant metastasis in tissue or plasma samples. In addition, the survival rates decreased among patients with higher TIMP-1 expression levels. Interestingly, a lot of studies concerning the mechanism support the idea that TIMP-1 is an inhibitor of invasion or metastasis in cancer progression. Logically, its expression levels might be suppressed when invasion or metastasis happen. However, a lot of clinical research found that TIMP-1 oppositely increased its expression levels. These evidences might imply that TIMP-1 is not only a protease inhibitor; it could also have other significant roles in cancer metastasis. In our tissue microarray data, TIMP-1 abundances were significantly increased in patients with lymph node and/or distant metastasis. Additionally, the ROC curve analyses showed that AUC values were 73.7 and 86.2 % between patients with or without lymph node and/or distant metastasis, respectively. The expression levels of TIMP-1 may provide useful information to assist doctors in the diagnosis of patients and further evaluate whether they have lymph node and/or distant metastasis.
In summary, although current research asserts that TIMP-1 was negatively associated with invasion and metastasis capacity in various cancers, more and more evidences support that TIMP-1 is multifunctional. TIMP-1 can implicate into MMP-dependent or MMP-independent pathways to mediate different functions and stages in metastasis. In our clinical data, the higher TIMP-1 levels indicated that lung adenocarcinoma patients developed lymph node or distant metastasis. Furthermore, our results regarding molecular functions showed that the down-regulation of TIMP-1 inhibits cell migration, invasion, and metastatic colonization in lung adenocarcinoma. In addition, the novel findings of TIMP-1’s function in facilitating surface polyFN assembly and tumor colonization further provide a strong basis to argue that TIMP-1 acts as a promoter in tumor malignancy.
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol. 2010;28:1248–50.
Carmichael DF, Sommer A, Thompson RC, Anderson DC, Smith CG, Welgus HG, et al. Primary structure and cDNA cloning of human fibroblast collagenase inhibitor. Proc Natl Acad Sci U S A. 1986;83:2407–11.
Docherty AJ, Lyons A, Smith BJ, Wright EM, Stephens PE, Harris TJ, et al. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985;318:66–9.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74.
Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42–51.
Johnsen M, Lund LR, Romer J, Almholt K, Dano K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol. 1998;10:667–71.
Lee JH, Choi JW, Kim YS. Plasma or serum TIMP-1 is a predictor of survival outcomes in colorectal cancer: a meta-analysis. J Gastrointest Liver Dis. 2011;20:287–91.
Wurtz SO, Schrohl AS, Mouridsen H, Brunner N. TIMP-1 as a tumor marker in breast cancer—an update. Acta Oncol. 2008;47:580–90.
Grunnet M, Mau-Sorensen M, Brunner N. Tissue inhibitor of metalloproteinase 1 (TIMP-1) as a biomarker in gastric cancer: a review. Scand J Gastroenterol. 2013;48:899–905.
Oh WK, Vargas R, Jacobus S, Leitzel K, Regan MM, Hamer P, et al. Elevated plasma tissue inhibitor of metalloproteinase-1 levels predict decreased survival in castration-resistant prostate cancer patients. Cancer. 2011;117:517–25.
Pesta M, Kulda V, Kucera R, Pesek M, Vrzalova J, Liska V, et al. Prognostic significance of TIMP-1 in non-small cell lung cancer. Anticancer Res. 2011;31:4031–8.
Honkavuori M, Talvensaari-Mattila A, Puistola U, Turpeenniemi-Hujanen T, Santala M. High serum TIMP-1 is associated with adverse prognosis in endometrial carcinoma. Anticancer Res. 2008;28:2715–9.
Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–52.
Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6.
Liu XW, Bernardo MM, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem. 2003;278:40364–72.
Liu XW, Taube ME, Jung KK, Dong Z, Lee YJ, Roshy S, et al. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells from extrinsic cell death: a potential oncogenic activity of tissue inhibitor of metalloproteinase-1. Cancer Res. 2005;65:898–906.
Akahane T, Akahane M, Shah A, Connor CM, Thorgeirsson UP. TIMP-1 inhibits microvascular endothelial cell migration by MMP-dependent and MMP-independent mechanisms. Exp Cell Res. 2004;301:158–67.
Yamada E, Tobe T, Yamada H, Okamoto N, Zack DJ, Werb Z, et al. TIMP-1 promotes VEGF-induced neovascularization in the retina. Histol Histopathol. 2001;16:87–97.
John AS, Hu X, Rothman VL, Tuszynski GP. Thrombospondin-1 (TSP-1) up-regulates tissue inhibitor of metalloproteinase-1 (TIMP-1) production in human tumor cells: exploring the functional significance in tumor cell invasion. Exp Mol Pathol. 2009;87:184–8.
Schelter F, Grandl M, Seubert B, Schaten S, Hauser S, Gerg M, et al. Tumor cell-derived TIMP-1 is necessary for maintaining metastasis-promoting met-signaling via inhibition of adam-10. Clin Exp Metastasis. 2011;28:793–802.
Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S, et al. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67:8615–23.
Jung YS, Liu XW, Chirco R, Warner RB, Fridman R, Kim HR. TIMP-1 induces an EMT-like phenotypic conversion in MDCK cells independent of its MMP-inhibitory domain. PLoS One. 2012;7:e38773.
Aljada IS, Ramnath N, Donohue K, Harvey S, Brooks JJ, Wiseman SM, et al. Upregulation of the tissue inhibitor of metalloproteinase-1 protein is associated with progression of human non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:3218–29.
Chang YH, Lee SH, Liao IC, Huang SH, Cheng HC, Liao PC. Secretomic analysis identifies alpha-1 antitrypsin (A1AT) as a required protein in cancer cell migration, invasion, and pericellular fibronectin assembly for facilitating lung colonization of lung adenocarcinoma cells. Mol Cell Proteomics. 2012;11:1320–39.
Chiu KH, Chang YH, Wu YS, Lee SH, Liao PC. Quantitative secretome analysis reveals that COL6A1 is a metastasis-associated protein using stacking gel-aided purification combined with iTRAQ labeling. J Proteome Res. 2011;10:1110–25.
Yang PC, Luh KT, Wu R, Wu CW. Characterization of the mucin differentiation in human lung adenocarcinoma cell lines. Am J Respir Cell Mol Biol. 1992;7:161–71.
Cheng HC, Abdel-Ghany M, Elble RC, Pauli BU. Lung endothelial dipeptidyl peptidase IV promotes adhesion and metastasis of rat breast cancer cells via tumor cell surface-associated fibronectin. J Biol Chem. 1998;273:24207–15.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92.
Taube ME, Liu XW, Fridman R, Kim HR. TIMP-1 regulation of cell cycle in human breast epithelial cells via stabilization of p27(KIP1) protein. Oncogene. 2006;25:3041–8.
Jee BK, Park KM, Surendran S, Lee WK, Han CW, Kim YS, et al. KAI1/CD82 suppresses tumor invasion by MMP9 inactivation via TIMP1 up-regulation in the H1299 human lung carcinoma cell line. Biochem Biophys Res Commun. 2006;342:655–61.
Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128:355–63.
Ikenaka Y, Yoshiji H, Kuriyama S, Yoshii J, Noguchi R, Tsujinoue H, et al. Tissue inhibitor of metalloproteinases-1 (TIMP-1) inhibits tumor growth and angiogenesis in the TIMP-1 transgenic mouse model. Int J Cancer. 2003;105:340–6.
Reed MJ, Koike T, Sadoun E, Sage EH, Puolakkainen P. Inhibition of TIMP1 enhances angiogenesis in vivo and cell migration in vitro. Microvasc Res. 2003;65:9–17.
Shen W, Zhu J, Yu Z, Xue Q. TIMP-1 secreted by fibroblasts inhibits tumor cell invasion and metastasis in mouse melanoma. J Biomed Eng. 2009;26:610–4.
Yamauchi K, Ogata Y, Nagase H, Shirouzu K. Inhibition of liver metastasis from orthotopically implanted colon cancer in nude mice by transfection of the TIMP-1 gene into KM12SM cells. Surg Today. 2001;31:791–8.
Kawamata H, Kawai K, Kameyama S, Johnson MD, Stetler-Stevenson WG, Oyasu R. Over-expression of tissue inhibitor of matrix metalloproteinases (TIMP1 and TIMP2) suppresses extravasation of pulmonary metastasis of a rat bladder carcinoma. Int J Cancer. 1995;63:680–7.
Shi Y, Parhar RS, Zou M, Al-Mohanna FA, Paterson MC. Gene therapy of melanoma pulmonary metastasis by intramuscular injection of plasmid DNA encoding tissue inhibitor of metalloproteinases-1. Cancer Gene Ther. 2002;9:126–32.
Wang N, Zhu M, Tsao SW, Man K, Zhang Z, Feng Y. Up-regulation of TIMP-1 by genipin inhibits MMP-2 activities and suppresses the metastatic potential of human hepatocellular carcinoma. PLoS One. 2012;7:e46318.
Wurtz SO, Schrohl AS, Sorensen NM, Lademann U, Christensen IJ, Mouridsen H, et al. Tissue inhibitor of metalloproteinases-1 in breast cancer. Endoc Relat Cancer. 2005;12:215–27.
Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–93.
Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113.
Wang CS, Wu TL, Tsao KC, Sun CF. Serum TIMP-1 in gastric cancer patients: a potential prognostic biomarker. Ann Clin Lab Sci. 2006;36:23–30.
Klintman M, Ornbjerg Wurtz S, Christensen IJ, Braemer Hertel P, Ferno M, Malmberg M, et al. Association between tumor tissue TIMP-1 levels and objective response to first-line chemotherapy in metastatic breast cancer. Breast Cancer Res Treat. 2010;121:365–71.
Schrohl AS, Christensen IJ, Pedersen AN, Jensen V, Mouridsen H, Murphy G, et al. Tumor tissue concentrations of the proteinase inhibitors tissue inhibitor of metalloproteinases-1 (TIMP-1) and plasminogen activator inhibitor type 1 (PAI-1) are complementary in determining prognosis in primary breast cancer. Mol Cell Proteomics. 2003;2:164–72.
Schrohl AS, Look MP, Meijer-van Gelder ME, Foekens JA, Brunner N. Tumor tissue levels of tissue inhibitor of metalloproteinases-1 (TIMP-1) and outcome following adjuvant chemotherapy in premenopausal lymph node-positive breast cancer patients: a retrospective study. BMC Cancer. 2009;9:322.
Inagaki D, Oshima T, Yoshihara K, Tamura S, Kanazawa A, Yamada T, et al. Overexpression of tissue inhibitor of metalloproteinase-1 gene correlates with poor outcomes in colorectal cancer. Anticancer Res. 2010;30:4127–30.
Durkan GC, Nutt JE, Rajjayabun PH, Neal DE, Lunec J, Mellon JK. Prognostic significance of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in voided urine samples from patients with transitional cell carcinoma of the bladder. Clin Cancer Res Off J Am Assoc Cancer Res. 2001;7:3450–6.
Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2001;7:3113–9.
Mahner S, Woelber L, Eulenburg C, Schwarz J, Carney W, Jaenicke F, et al. TIMP-1 and VEGF-165 serum concentration during first-line therapy of ovarian cancer patients. BMC Cancer. 2010;10:139.
Schelter F, Halbgewachs B, Baumler P, Neu C, Gorlach A, Schrotzlmair F, et al. Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by hypoxia-inducible factor-1alpha. Clin Exp Metastasis. 2011;28:91–9.
Gong Y, Scott E, Lu R, Xu Y, Oh WK, Yu Q. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS One. 2013;8:e77366.
Rho SB, Chung BM, Lee JH. TIMP-1 regulates cell proliferation by interacting with the ninth zinc finger domain of PLZF. J Cell Biochem. 2007;101:57–67.
Cui H, Grosso S, Schelter F, Mari B, Kruger A. On the pro-metastatic stress response to cancer therapies: evidence for a positive co-operation between TIMP-1, HIF-1alpha, and MIR-210. Front Pharmacol. 2012;3:134.
Huang L, Cheng HC, Isom R, Chen CS, Levine RA, Pauli BU. Protein kinase cepsilon mediates polymeric fibronectin assembly on the surface of blood-borne rat breast cancer cells to promote pulmonary metastasis. J Biol Chem. 2008;283:7616–27.
Dubinett SM, Elashoff D, Meyerson M. Assessing prognosis in non-small-cell lung cancer: avenues to a more complete picture? J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:3209–11.
Acknowledgments
This study was supported by Grant MOST103-2113-M-006-003-MY3, MOST102-2325-B-006-003, MOST100-2113-M-006-002-MY3 from the National Science Council. We thank the Tissue Bank, Research Center of Clinical Medicine, National Cheng Kung University Hospital, for preparing the tissue microarray and for providing immunohistochemistry technology. Additionally, we are grateful for the assistance of Dr. Kuen-Jer Tsai and Miss Ya-Chun Hsiao for their services involving TMA image quantification using FACS-like Tissue Cytometry with HistoQuest from Optical Imaging Core Laboratory, Research Center of Clinical Medicine, National Cheng Kung University Hospital.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Ying-Hua Chang and Yi-Jen Chiu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chang, YH., Chiu, YJ., Cheng, HC. et al. Down-regulation of TIMP-1 inhibits cell migration, invasion, and metastatic colonization in lung adenocarcinoma. Tumor Biol. 36, 3957–3967 (2015). https://doi.org/10.1007/s13277-015-3039-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3039-5