Abstract
ETS gene fusions involving ERG, ETV1, ETV4, ETV5, and FLI1 define a distinct class of prostate cancer (PCa), and this might have a bearing on diagnosis, prognosis, and rational therapeutic targeting. In the current study, we focused on the clinicopathological significance of ETV4 in Chinese PCa patients and the mechanisms whereby ETV4 overexpression mediates tumor invasion in the prostate. Overall, ETV4 overexpression was identified in 30.4 % (45/148) of PCa cases by immunohistochemistry. Accordingly, ETV4 was rearranged in only 1.6 % (2/128) of PCa patients. Clinically, ETV4 overexpression was significantly correlated with Gleason score (P = 0.045) and pathological tumor stage (P = 0.041). Multivariate Cox regression analysis indicated that ETV4 is an unfavorable independent prognostic factor (P = 0.040). Functional studies further showed that small interfering RNA (siRNA) knockdown of ETV4 significantly decreases proliferation and invasion of PC-3 cell and partially reverses epithelial-mesenchymal transition in vitro. Notably, ETV4 knockdown significantly downregulated expression of urokinase plasminogen activator (uPA) and its receptor (uPAR) at messenger RNA (mRNA) and protein levels. Chromatin immunoprecipitation assay demonstrated that ETV4 regulates uPA expression through direct binding to its promoter region. Additionally, ETV4 knockdown was also observed to significantly inhibit expression of matrix metalloproteinase (MMP)-2 and MMP-9. In conclusion, for the first time, our study suggested that ETV4 is an independent poor prognostic factor in Chinese PCa patients. Silencing of ETV4 suppresses invasion of PCa cells by inhibiting the expression of uPA/uPAR as well as MMPs. Further studies will be needed to determine whether ETV4 could be regarded as a potential target for the management and prevention of PCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, recurrent gene fusions involving ETS transcription factors ERG, ETV1, ETV4, ETV5, or FLI1, fused to either androgen-regulated gene TMPRSS2 or other upstream partners, have been identified in 40–70 % of prostate cancers (PCas) [1, 2], Among these aberrations, ERG rearrangement is the most prevalent. Other ETS family members, primarily ETV1, but also including ETV4 and ETV5, together constitute less than 10 % of PCa cases [2]. ETS gene fusions probably define a distinct class of PCa, and this might have a bearing on diagnosis, prognosis and rational therapeutic targeting [2, 3].
ETV4 (PEA3/E1AF) has been reported to be overexpressed and promotes metastatic progression in various types of solid cancers, including the esophagus [4], colon [5], and breast [6]. In PCa, we and others have reported that ETV4 overexpression in a subset of PCa cases is associated with translocation of ETV4 to the promoter of a gene highly expressed in prostate (TMPRSS2, KLK2, DDX5, and CANT1) [3, 7, 8]. Most recently, Hollenhorst et al. [9] showed that ETV4 is required for anchorage-independent growth in PC-3 cells. Similarly, Pellecchia et al. [10] suggested that overexpression of ETV4 is oncogenic in prostate cells through promotion of both cell proliferation and epithelial-mesenchymal transition (EMT). In consistent with these findings, Maruta et al. [11] demonstrated that ETV4 expression is associated with extra-prostatic growth and matrix metalloproteinase-7 (MMP-7) expression. Although several studies revealed that ETV4 may collaborate with other factors to promote tumor development [4, 5, 12, 13], the mechanism whereby overexpression of the ETV4 gene mediates oncogenesis and progression in the prostate remains unclear.
The urokinase plasminogen activator (uPA) and its receptor (uPAR) have been implicated in tumor invasion and metastasis [14]. uPA production is regulated by growth factor activation of signal-transduction pathways, which in turn activate transcription factors that control urokinase gene expression [15, 16]. In 2005, Oikawa et al. [15] demonstrated the presence of ETS binding sites adjacent to AP1 in the promoter and/or enhancer regions of uPA, suggesting a potential link between ETS factors and uPA expression. Given the potential ability of ETV4 to broadly affect multiple pathways, we hypothesize that ETV4 might crosstalk with uPA/uPAR to promote PCa progression and metastasis.
Matrix metalloproteinases (MMPs) have well-recognized roles in tumor invasion and metastasis [17, 18]. In PCa, the invasive phenotypes of cells with high ETV4 expression are thought to be due in part to their ability to regulate the expression of MMPs [15]. Clinically, Maruta et al. [11] demonstrated that increased expression of ETV4 is involved in tumor aggression of PCa, and this finding may be influenced by regulation of matrix metalloproteinase-7 (MMP-7) expression. Several previous reports have shown that ETV4 stimulates several MMP genes and thus alters invasion and metastasis of cancer cells [15–17].
In the current study, we therefore aimed to characterize the regulation and interaction of ETV4 and uPA/uPAR as well as MMPs, two important metastatic mediators in PCa. Additionally, the expression and clinicopathological association of ETV4 in a large cohort of Chinese PCa patients were also investigated.
Materials and methods
Patients
Our study consisted of 168 PCa patients who underwent surgery from 2003 to 2010 at the Qilu Hospital of Shandong University (Jinan, China) and Linyi People’s Hospital (Linyi, China). None of the patients received preoperative radiation or androgen deprivation therapy, and follow-up data were available for 148 patients, ranging from 5 to 125 months (mean 51 months). Informed written consents were obtained from the PCa patients. This study and the consent procedures were approved by the Institutional Review Board at the Medical School of Shandong University.
Tissue microarray construction
Two tissue microarrays (TMAs) were constructed for fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) analysis using a manual tissue arrayer. Representative tumor areas were marked on hematoxylin and eosin-stained sections, and duplicate tissue cores per patient were arrayed on a recipient paraffin bock, resulting in 14 × 12 arrays for each of the 84 cases.
Immunohistochemistry
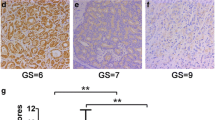
IHC was performed as previously described [19]. Briefly, antigen retrieval was performed by microwave pretreatment in 0.01 M citrate buffer (pH 6.0) for 10 min. Slides were incubated with ETV4 polyclonal antibody (1:50; Epitomics, #T1571) at 4 °C overnight. The slides were blindly evaluated by two independent observers (M.Q. and B.H.) based on the previously described scoring system [20]. Briefly, the scores of two parameters were multiplied by the staining intensity (range 0 to 3) and the percentage of positive cells (range 0 to 4 [0, (0–10 %), 1 (11–25 %), 2 (26–50 %), 3 (51–75 %), and 4 (76–100 %)]). Slides with scores of 8 or higher were classified as overexpression and slides with scores lower than 8 as non-overexpression.
Fluorescence in situ hybridization
ETV4 rearrangement was validated by a previously described dual-color interphase break-apart Fluorescence in situ hybridization (FISH) assay [3]. Bacterial artificial chromosomes were obtained from the BACPAC Resource Center (Oakland, CA, USA), and probes RP11-436 J4 (5′ to ETV4) and RP11-100E5 (3′ to ETV4) were prepared as described [3]. Slides were examined using an ImagingZ1 microscope (Carl Zeiss, Oberkochen, Germany). FISH signals were scored manually (100x oil immersion) in morphologically intact and non-overlapping nuclei by a pathologist (B.H.), and a minimum of 50 cancer cells from each site were recorded.
Chemicals and reagents
The human uPA recombinant protein (rUPA) was purchased from Abnova corporation (Taipei, Taiwan).
Cell lines and cell culture
Human PCa cell lines including PC-3, DU-145, and Lncap were obtained from ATCC (Manassas, VA, USA) and maintained in RPMI-1640 medium supplemented with 10 % fetal bovine serum (Hyclone Laboratories Inc., Logan, UT, USA) at 37 °C in a humidified 5 % CO2 incubator.
siRNA-mediated knockdown
Small interfering RNA (siRNA) transfection on PC-3 cells was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Specific siRNA sequences targeting the human ETV4, uPA, and uPAR were all designed and synthesized respectively (GenePharma Pharmaceutical Co., Shanghai, China), and the sequences were provided in Supplementary Table 1. The NC group was defined as negative control, and the mock group was the ones supplemented with the transfection reagent only.
Cell proliferation and invasion assays
Cell proliferation was measured using the MTS assay with CellTiter 96 AQueous One Solution Reagent (Promega, Mannheim, Germany) following the manufacturer’s protocol. Invasion assay was performed as previously described [21]. For quantification of invading cells, cells were counted in five randomly selected microscopic fields (×200). Three independent experiments were performed.
RNA extraction and quantitative real-time PCR
RNA extraction and qRT-PCR was performed as previously described [21]. All primer sequences were listed in Supplementary Table 2. Relative mRNA levels are calculated by 2−ΔCt method. Three independent experiments were completed and each reaction was performed in triplicate.
Western blot
Western blot was performed as previously described [21]. The membranes were incubated overnight with antibodies against Vimentin (1:1000; Cell Signaling Technology), E-cadherin (1:1000; Cell Signaling Technology), and ETV4 (1:1000; Abnova, #H00002118-M01). The secondary antibody was horseradish peroxidase (HRP)-coupled anti-mouse IgG (Dako, Glostrup, Denmark) (1:1500), and the signals were detected with an enhanced chemiluminescence (ECL) kit (Amersham, Buckinghamshire, UK). Three independent experiments were conducted.
Enzyme-linked immunosorbent assay for uPA
Enzyme-linked immunosorbent assay (ELISA) was performed as previously described [21]. The protein levels of uPA in medium were measured using ELISA kits (R&D System, USA) following the manufacturer’s protocol. The data were expressed as the mean of determinations from two independent experiments.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) was performed as previously described [22]. Briefly, 1 × 107 cells were cross-linked with 1 % formaldehyde and immunoprecipitated using the ChIP assay kit as recommended by the manufacturer (Beyotime Biotechnology, China). Cells were incubated with 5 μg of anti-ETV4 antibody (Abnova, #H00002118-M01) or control IgG (Santa Cruz) per reaction. Immunoprecipitates were used as templates for PCR amplification of uPA promoter region with the following primers: uPA, 5′-ACTTGCCTAAGACTGC-3′ (sense) and 5′ -GGACTGCCACTGAAC-3′ (antisense).
Statistical analysis
All the statistical analyses were performed using the Statistical Package for Social Sciences, version 19.0 (SPSS 19.0), with a significance level of 0.05 (two-tailed probability). The distributions of continuous and categorical variables between cases and controls were compared using Student’s t-test. Correlations between the clinicopathological parameters and ETV4 overexpression were determined by χ 2 test. Survival analyses were conducted using Kaplan-Meier method, and differences were determined by the log-rank test. The prognostic significance of several factors was assessed by the Cox proportional hazards regression model.
Results
ETV4 gene aberrations and expression in PCa
A total of 148 PCa cases were successfully analyzed by IHC and 128 cases by FISH. Overall, ETV4 was rearranged in 1.6 % (2/128) of PCa patients, one of which (1/2) demonstrated deletion of the 5′ end of ETV4. For ETV4 IHC analysis, we combined both negative and weakly ETV4 positive tumors into one group and compared it with moderately and strongly ETV4-positive PCa patients. Overall, ETV4 overexpression was identified in 30.4 % (45/148) of interpretable cases. As expected, the two tumors with ETV4 rearrangement had substantially increased gene expression. However, a large fraction of PCa cases with ETV4 overexpression were absent for gene rearrangement, suggesting that there are other mechanisms of ETV4 overexpression. Representative FISH and IHC images of ETV4 were shown in Fig. 1.
Expressions and genetic aberrations of ETV4 in PCa cases by IHC and FISH. Representative IHC images of ETV4 are shown in a–d. (original magnification, ×200). a Negative staining; b weak staining; c moderate staining; d strong staining. FISH images of ETV4 rearrangement in PCa are shown in e and f. e ETV4 rearrangement negative case, as indicated by two pairs of co-localized green and red signals. f ETV4 rearrangement positive (translocation) case showed one pair of split 5′ and 3′ signals
Overexpression of ETV4 is associated with poor prognosis
The relationship between ETV4 expression and clinicopathological parameters was shown in Table 1. Notably, ETV4 overexpression was significantly correlated with Gleason score (P = 0.045) and pathological tumor (pT) stage (P = 0.041), but not with age, pre-PSA levels, or distant metastasis.
To determine whether overexpression of ETV4 was a prognostic factor for PCa, we compared PCa overall survival rate between patients with or without ETV4 overexpression. On the basis of the Kaplan-Meier analysis, the group of patients with ETV4 overexpression had a much greater rate of mortality than patients who had non-overexpression of ETV4 (P = 0.004, Fig. 2). ETV4 overexpression was shown to be a significant prognostic predictor of PCa overall survival (HR [95 % confidence interval (CI)] 2.260 [1.278–3.966], P = 0.005) in univariate analysis (Table 2). PSA values at diagnosis (P = 0.009), Gleason score (P = 0.001), and pT stage (P = 0.005) were also significantly related to cancer-related survival in univariate analysis (Table 2). Notably, in multivariate analysis with forward stepwise selection, ETV4 overexpression remained a significant predictor (P = 0.040) with a hazard ratio of 2.113 (95 % CI 1.611–3.268) (Table 2).
Kaplan-Meier curves illustrating cancer-related survival among PCa patients stratified by ETV4 expression. The analysis with Kaplan-Meier method clearly showed that PCa patients having tumors with ETV4 overexpression had the worse cancer-related survival compared with the group with absence of ETV4 overexpression
Downregulation of ETV4 inhibits proliferation, invasion and EMT in PCa cells
In consistent with the previous findings, the mRNA and protein expression levels of ETV4 were highest in PCa cell line PC-3 but lowest in Lncap cells (PC-3 > DU-145 > Lncap, Fig. 3a). Using MTS assay, we found that after 48 and 72 h of ETV4 siRNA treatment, the number of PC-3 cells was reduced to 47.6 ± 10.5 and 20.7 ± 5.6 %, respectively (Fig. 3b). Similarly, siRNA knockdown of ETV4 significantly decreased invasive capacity of PC-3 cells compared to the control. Compared with the control group, the invasive capacity of PC-3 cells was reduced by 4.3 ± 0.2-fold downregulation of ETV4 treatment for 24 h (Fig. 3c).
ETV4 overexpression promotes proliferation, invasion, and EMT of PC-3 cells. a mRNA and protein level of ETV4 in prostate cancer cells. b Cell proliferation as assessed by MTS assay at 24 h (upper) and 48 h (lower) post si-ETV4 transfection in PC-3 cells. c Cellular invasive capacity of PC-3 cells as assessed by invasion chambers in PC-3 cells with or without 10 ng/ml rUPA after ETV4 silencing. d The expression of E-cadherin and Vimentin was determined by qPCR (upper) and Western blotting (lower) of PC-3 cells. The data shown and each bar represent the mean ± SD of three independent samples. (*P < 0.05; **P < 0.01). si-ETV4, siRNA of ETV4; NC negative control
EMT is a dynamic process that promotes cell motility and invasion which plays a pivotal role during malignant tumor progression and metastasis [23]. In the present study, as shown in Fig. 3d, ETV4 silencing increased the expression of epithelial marker E-cadherin and decreased the expression of mesenchymal marker vimentin at mRNA and protein level, respectively.
The cross-talk of ETV4 and uPA/uPAR
Since ETV4 and uPA/uPAR have been suggested to be the most common factors involved in tumor progression including PCa [11, 24, 25], we next investigated the interaction between these two important factors, both of which have endogenous high levels in PC-3 cells. As shown in Fig. 4a, the expression of uPA/uPAR upon ETV4 siRNA knockdown showed a coordinated decrease 13.36-fold and 8.33-fold lower, respectively, than the NC group. Similar results were obtained at protein level. Interestingly, siRNA knockdown of uPA significantly suppressed ETV4 expression at both the transcript and protein levels. In parallel, mRNA expression level of ETV4 was ~4-fold decreased but was not obviously altered at protein level by uPAR silencing (Fig. 4b, c). As shown in Fig. 3c, rUPA (10 ng/ml) can significantly rescued the suppressive effect of ETV4 silencing on the invasive capacity of PC-3 cells. Interestingly, the mRNA expression level of uPAR was significantly upregulated after exposure of 10 ng/ml rUPA in PC-3 cells after ETV4 silencing (Supplementary Fig. 1). Taken together, these data suggest a positive reciprocal regulation between ETV4 and uPA. ETV4 could promote PCa invasive progression through uPA/uPAR signaling.
The interaction of ETV4 with uPA/uPAR as well as MMPs in PCa. a qRT-PCR (upper) and ELISA (lower) analysis showed that depletion of ETV4 resulted in the downregulation of uPA/uPAR in PC-3 cells at mRNA and protein levels. b–c qRT-PCR (upper) and Western blot (lower) analysis of ETV4 expression in PC-3 cells after transfection with control siRNA or siRNAs targeting uPA or uPAR. d ChIP analysis of the recruitment of ETV4 on uPA promoter in PC-3 cells. Schema of uPA promoter region was tagged. e Expression of MMP-2 and MMP-9 were examined by qRT-PCR (left) and Western blot (right), respectively. (*P < 0.05;**P < 0.01)
uPA is a direct transcriptional target of ETV4
As shown in Fig. 4a, ETV4 knockdown significantly suppressed uPA expression at both transcript and protein levels in PC-3 cells. To better understand the mechanisms by which ETV4 regulates uPA, ChIP assay was performed on PC-3 cells using the antibody against ETV4 or control IgG. As shown in Fig. 4d, ETV4 could bind to the promoter (encompassing −1494 to −1652 bp) of uPA. By contrast, no binding was detected in the control group with IgG antibody.
siRNAs of ETV4 and/or uPA decreases MMP-2 and MMP-9 expression
In an attempt of identifying potential target genes of ETV4 and uPA, siRNA treatment targeting either ETV4 or uPA was applied. Of note, the expressions of MMP-2 and MMP-9 were significantly decreased in PC-3 cells, accordingly. Of interest, ETV4/uPA siRNAs co-transfection showed a duplicate effect on MMP-2 and MMP-9 compared with the single transfection (P < 0.001) (Fig. 4e). Collectively, our data suggested that ETV4 and uPA might be involved in the regulation of MMP-2 and MMP-9 in PC-3 cells.
Discussion
Overexpression of ETV4 has been identified in various types of human malignancies [4–6]. Although majority of studies have shown the involvement of ETV4 in cancer progression, others suggested that ETV4 may function as a tumor suppressor [26]. In the current study, we systematically characterized clinicopathological significance of ETV4 expression in a large cohort of Chinese PCa patients. ETV4 overexpression was detected in 30.3 % (45/148) of Chinese PCa cases, and its overexpression was significantly correlated with high Gleason score and higher pathological tumor stage. More importantly, we identified ETV4 as an independent prognostic factor indicating poor prognosis in PCa patients. So far, it remains a great challenge how to precisely stratify PCa patients according to their clinical prognosis in alignment with therapeutic options. Recently, a series of reports have identified several novel biomarkers which appear to carry prognostic significance. Of these, we reported that ERG rearrangement and SOX4 overexpression appears to hold potential as predictors of outcome [19, 22]. Further studies will be needed to determine whether ETV4 could be utilized to differentiate aggressive PCas and indolent ones.
ETV4, as an ETS family transcription factor, plays important roles in cell proliferation, cell cycle, anchorage-independent growth, cell mobility, and metastasis [9, 10]. Pellecchia et al. [10] reported that overexpression of ETV4 is oncogenic in prostate cells through promotion of both cell proliferation and EMT. ETV4 inhibition reduced not only cell mobility and anchorage-independent growth, but also cell proliferation, cell cycle progression, and tumor growth in a xenograft model. Similarly, Aytes et al. [13] found that mice engrafted with cells expressing the ETV4 shRNA had relatively fewer lung and liver metastases. In consistent with these findings, we confirmed that ETV4 knockdown significantly decreased PC-3 cell proliferation and invasion, the latter of which was partially through reversing EMT in vitro. To date, the mechanisms of ETV4 overexpression in PCa remains unclear. Several studies suggested that ETV4 is rearranged in a subset of PCa cases, resulting in overexpression of ETV4 [3, 7, 8]. However, ETV4 rearrangement is a rare event in PCa. Indeed, ETV4 rearrangement was identified in only 2 of 128 PCa cases in our cohort. Therefore, the mechanisms of ETV4 overexpression in PCa merits further investigation.
Although ETV4 has been implicated in tumor invasion and metastasis, the mechanism by which ETV4 mediate PCa development and progression remains unclear. Previous studies suggested two possible mechanisms underlying the role of ETV4 on PCa metastasis in vitro and in vivo: (1) activation of signaling pathways, such as aberrant ERK-MAP kinase pathway [4], enhanced Rho/Rho-associated kinase pathway [12] and active PI3-kinase and Ras signaling [13]; (2) a direct regulation of cancer-related genes including Notch-1/Notch-4 [27], MYC [9], MMP-1 [4], and MMP-7 [11].
In the current study, ETV4 knockdown led to a coordinate decrease of uPA and uPAR in PC-3 cells, and following studies demonstrated that ETV4 could regulate uPA through direct binding to its promoter. Interestingly, siRNA knockdown of uPA in PC-3 cells could also suppress the expression of ETV4, indicating a positive loop might exist between ETV4 and uPA. In addition, we also observed a significant decrease of MMP-2 and MMP-9, which are the most frequent alterations observed in invasive type of PCa [17]. It has been reported that uPA modulates its own synthesis via the uPAR pathway [28], and uPA binding to uPAR triggers the subsequent activation of metalloproteinase [29]. Therefore, based on these findings, we suggested that ETV4 is involved in invasion and metastasis of PCa by regulating the expression of MMPs in addition to the uPA/uPAR system, which might work in synergy to enhance tumor cell invasion.
In conclusion, our study suggested that ETV4 is an independent poor prognostic factor in Chinese PCa patients. Importantly, we demonstrated that ETV4-uPA/uPAR-MMPs axis is operative in promoting PCa invasion. Further studies will be needed to determine whether ETV4 could be regarded as a potential target for the management and prevention of PCa.
References
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–8.
Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8(7):497–511.
Han B, Mehra R, Dhanasekaran SM, Yu J, Menon A, Lonigro RJ, et al. A fluorescence in situ hybridization screen for E26 transformation-specific aberrations: identification of DDX5-ETV4 fusion protein in prostate cancer. Cancer Res. 2008;68(18):7629–37.
Keld R, Guo B, Downey P, Gulmann C, Ang YS, Sharrocks AD. The ERK MAP kinase-PEA3/ETV4-MMP-1 axis is operative in oesophageal adenocarcinoma. Mol Cancer. 2010;9:313.
Nosho K, Yoshida M, Yamamoto H, Taniguchi H, Adachi Y, Mikami M, et al. Association of Ets-related transcriptional factor E1AF expression with overexpression of matrix metalloproteinases, COX-2 and iNOS in the early stage of colorectal carcinogenesis. Carcinogenesis. 2005;26(5):892–9.
Yuen HF, Chan YK, Grills C, McCrudden CM, Gunasekharan V, Shi Z, et al. Polyomavirus enhancer activator 3 protein promotes breast cancer metastatic progression through Snail-induced epithelial-mesenchymal transition. J Pathol. 2011;224(1):78–89.
Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, Helgeson BE, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66(7):3396–400.
Hermans KG, Bressers AA, van der Korput HA, Dits NF, Jenster G, Trapman J. Two unique novel prostate-specific and androgen-regulated fusion partners of ETV4 in prostate cancer. Cancer Res. 2008;68(9):3094–8.
Hollenhorst PC, Paul L, Ferris MW, Graves BJ. The ETS gene ETV4 is required for anchorage-independent growth and a cell proliferation gene expression program in PC3 prostate cells. Genes Cancer. 2011;1(10):1044–52.
Pellecchia A, Pescucci C, De Lorenzo E, Luceri C, Passaro N, Sica M, et al. Overexpression of ETV4 is oncogenic in prostate cells through promotion of both cell proliferation and epithelial to mesenchymal transition. Oncogenesis. 2012;1:e20.
Maruta S, Sakai H, Kanda S, Hayashi T, Kanetake H, Miyata Y. E1AF expression is associated with extra-prostatic growth and matrix metalloproteinase-7 expression in prostate cancer. APMIS. 2009;117(11):791–6.
Hakuma N, Kinoshita I, Shimizu Y, Yamazaki K, Yoshida K, Nishimura M, et al. E1AF/PEA3 activates the Rho/Rho-associated kinase pathway to increase the malignancy potential of non-small-cell lung cancer cells. Cancer Res. 2005;65(23):10776–82.
Aytes A, Mitrofanova A, Kinkade CW, Lefebvre C, Lei M, Phelan V, et al. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc Natl Acad Sci U S A. 2013;110(37):E3506–15.
Kumano M, Miyake H, Muramaki M, Furukawa J, Takenaka A, Fujisawa M. Expression of urokinase-type plasminogen activator system in prostate cancer: correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. Urol Oncol. 2009;27(2):180–6.
Oikawa T. ETS transcription factors: possible targets for cancer therapy. Cancer Sci. 2004;95(8):626–33.
Evans CP, Stapp EC, Dall'Era MA, Juarez J, Yang JC. Regulation of u-PA gene expression in human prostate cancer. Int J Cancer. 2001;94(3):390–5.
Hua H, Li M, Luo T, Yin Y, Jiang Y. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68(23):3853–68.
Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel). 2014;6(3):1298–327.
Qi M, Yang X, Zhang F, Lin T, Sun X, Li Y, et al. ERG rearrangement is associated with prostate cancer-related death in Chinese prostate cancer patients. PLoS One. 2014;9(2):e84959.
Wang L, Zhang J, Yang X, Chang YW, Qi M, Zhou Z, et al. SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis. 2013;16(4):301–7.
Wang C, Wang L, Su B, Lu N, Song J, Yang X, et al. Serine protease inhibitor Kazal type 1 promotes epithelial-mesenchymal transition through EGFR signaling pathway in prostate cancer. Prostate. 2014;74(7):689–701.
Wang L, Li Y, Yang X, Yuan H, Li X, Qi M, et al. ERG-SOX4 interaction promotes epithelial-mesenchymal transition in prostate cancer cells. Prostate. 2014;74(6):647–58.
Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42.
Shariat SF, Roehrborn CG, McConnell JD, Park S, Alam N, Wheeler TM, et al. Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J Clin Oncol. 2007;25(4):349–55.
Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34(2):122–36.
Xing X, Wang SC, Xia W, Zou Y, Shao R, Kwong KY, et al. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat Med. 2000;6(2):189–95.
Clementz AG, Rogowski A, Pandya K, Miele L, Osipo C. NOTCH-1 and NOTCH-4 are novel gene targets of PEA3 in breast cancer: novel therapeutic implications. Breast Cancer Res. 2011;13(3):R63.
Li C, Zhang J, Jiang Y, Gurewich V, Chen Y, Liu JN. Urokinase-type plasminogen activator up-regulates its own expression by endothelial cells and monocytes via the u-PAR pathway. Thromb Res. 2001;103(3):221–32.
Noh H, Hong S, Huang S. Role of urokinase receptor in tumor progression and development. Theranostics. 2013;3(7):487–95.
Acknowledgments
Supported by the National Natural Science Foundation of China (Grant No. 81171951); China Postdoctoral Science Foundation Funded Project (Project No. 2012 M521344); Natural Science Foundation of Shandong Province (Grant No. ZR2011HQ040),
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Qi M. and Liu Z. contributed to this work equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLS 26 kb)
ESM 2
(XLS 27 kb)
Supplementary Fig. 1
The alteration of uPA and uPAR mRNA level in PC-3 cells. The uPA and uPAR mRNA levels after exposure of 10ng/ml rUPA was quantified by qRT-PCR in PC-3 cells after ETV4 silencing (*p<0.05, compared to the control). Con, control group; rUPA, recombinant uPA protein (GIF 9 kb)
Rights and permissions
About this article
Cite this article
Qi, M., Liu, Z., Shen, C. et al. Overexpression of ETV4 is associated with poor prognosis in prostate cancer: involvement of uPA/uPAR and MMPs. Tumor Biol. 36, 3565–3572 (2015). https://doi.org/10.1007/s13277-014-2993-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2993-7