Abstract
High-mobility group box (HMGB) proteins are ubiquitous, abundant nuclear non-histone chromosomal proteins that play a critical role in binding to distorted DNA structures and subsequently regulating DNA transcription, replication, repair, and recombination. Both HMGB1 and HMGB2 exhibit a high expression in several human cancers and are closely associated with tumor progression and a poor prognosis. However, the expression patterns of these molecules in pancreatic ductal adenocarcinoma (PDAC) remain to be elucidated. As most cases of postoperative relapse of PDAC occur within the first 2 years, the clinical significance of accurate biomarkers is needed. Therefore, we investigated the correlation between the immunohistochemical HMGB1 and HMGB2 expression and the clinicopathological characteristics and prognosis using 62 paraffin-embedded tumor samples obtained from patients with surgically resected PDAC. The HMGB1/2 expression was considered to be positive when 10 % or more of the cancer cells showed positive nuclear, not merely cytoplasmic, staining. Consequently, the expression of HMGB1/2 was observed in 54 (87.1 %) and 31 (50.0 %) patients, respectively. Unexpectedly, a positive HMGB1 expression was found to have a significantly close relationship with a negative HMGB2 expression. The univariate and multivariate analyses demonstrated that the patients with a HMGB1+ and HMGB2− status had markedly lower disease-specific survival rates, especially within the first 2 years postoperatively, whereas those with a HMGB1+ status alone did not. Therefore, the combination of a HMGB1+ and HMGB2− expression potentially predicts a poor prognosis in patients with PDAC, and these new biomarkers may be useful parameters for clinical management in the early postoperative phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is one of the most lethal malignancies worldwide, including Japan. Indeed, approximately 30,000 new cases are diagnosed each year and more than 28,000 patients die of the disease in Japan alone (http://ganjoho.jp/professional/index.html, 2013). In addition, pancreatic cancer is responsible for approximately 227,000 deaths each year worldwide, with pancreatic ductal adenocarcinoma (PDAC) being the most commonly occurring histopathological type of pancreatic cancer [1]. Various clinicopathological characteristics, such as the size of operable tumors, the patient’s initial Eastern Cooperative Oncology Group performance status (ECOG PS), and the presence of distant metastasis in cases of inoperable PDAC, have been proposed to be prognostic indicators, although the results remain inconsistent and inconclusive to date [2]. Currently, the 5-year overall survival rate for patients diagnosed with PDAC is significantly lower than 4 %. Additionally, long-term survival is very rare, even after surgical resection, which offers the only chance for a cure, with overall 5-year survival rates ranging from 10 to 25 % [3, 4], as more than 80 % of cases of postoperative relapse (local or distant) occur within the first 2 years [5]. Hence, it is very important to predict which patients are prone to recurrence/metastasis and mortality after surgery using practically accurate biomarkers, even those under evaluation. Indeed, the clinical picture of PDAC is strongly determined by the complex interplay among additional cellular alterations, e.g., epigenetic modulation of the gene expression, at least in part [6, 7].
High-mobility group box (HMGB) proteins are ubiquitous, abundant nuclear non-histone chromosomal proteins that bend DNA and bind preferentially to distorted DNA structures, thus promoting the assembly of site-specific DNA-binding proteins, which subsequently regulates DNA transcription, replication, repair, and recombination [8, 9]. HMGB1 and HMGB2 are the main members of the HMGB protein family and are very highly conserved, with a shared amino acid sequence of more than 85 % and similar molecular structures [10]. Indeed, both of these HMGB proteins play a crucial role in regulating normal mammalian growth and development [8–10]. In addition, HMGB1 and HMGB2 function as sensors of DNA modification and facilitate the DNA damage response, thus functioning as novel promising targets for chemotherapeutic intervention in various cancer cell lines [8–11]. Moreover, it has been reported that HMGB1 and/or HMGB2 are specifically expressed in several human solid tumors, including hepatocellular carcinoma [12], cutaneous squamous cell carcinoma [13], prostatic adenocarcinoma [14], colorectal and gastric adenocarcinoma [15, 16], breast ductal carcinoma [17], bladder urothelial carcinoma [18], renal cell carcinoma [19], and malignant melanoma [20], and that an immunohistochemically high expression of HMGB1/2 is significantly correlated with a poor outcome. However, to our knowledge, there are no previous reports of possible associations between the HMGB1/2 expression and clinicopathological characteristics, such as the histopathological tumor stage, vessel permeation, or prognosis, in the setting of PDAC. Furthermore, the detailed features of the diverse roles of HMGB1 in angiogenesis and cancer progression most likely remain uncovered [21], and the involvement of HMGB2 in carcinogenesis remains to be elucidated. Moreover, HMGB2 has not been reported to leave the nucleus or individual cells, unlike HMGB1, which is not a housekeeping protein, but rather has a dual function, being expressed in both the nucleus and cytoplasm [22, 23]. In the present study, we show, for the first time, that the combination of a nuclear (not merely cytoplasmic) HMGB1-positive and HMGB2-negative expression is significantly correlated with a poor outcome and that these molecules may be promising biomarkers in patients with PDAC, especially in the early postoperative phase.
Materials and methods
Patients
All the intended procedures of the present study, including use of specimens from human subjects in UOEH in Kitakyushu, Japan, were approved especially by written consent of next of kin for research use of the materials obtained, according to the guidelines of the Japanese Society of Pathology. Pathological reports were reviewed to identify patients who had undergone pancreaticoduodenectomy or distal pancreatectomy for PDAC between 1994 and 2010 at the hospital of UOEH. Two patients with perioperative death, defined as death during the initial hospitalization or within 30 days of surgery, were excluded. A total of 62 patients with available follow-up data comprised the cohort of this retrospective study, after further excluding those with the following characteristics: (a) other prior or concomitant malignant tumors, (b) coexisting medical problems of sufficient disease severity to shorten life expectancy, and (c) the use of neoadjuvant chemotherapy or radiotherapy prior to surgery. Clinical information was gathered from the patients’ records, and no subjects had previously undergone biopsies of the PDAC lesions. The duration of survival was defined as the interval from the date of surgery to death or the most recent clinic visit. Fifty-nine (95.2 %) patients received adjuvant chemotherapy after surgery, as follows: fluorouracil (3 cases), fluorouracil plus cisplatin (4 cases), gemcitabine (41 cases), and gemcitabine plus S-1 (TS-1; Taiho Pharmaceutical, Tokyo, Japan; 11 cases). All patients were followed up every month within the first postoperative year and at approximately 2- to 4-month intervals thereafter using chest X-ray and thoracic and abdominal CT scans or measurements of tumor markers. CT was performed every 6 months for 3 years after surgery. Additional examinations, including brain CT, MRI, and bone scintigraphy, were performed in cases involving signs or symptoms of recurrence. The serum levels of carbohydrate antigen (CA) 19-9 were measured at the time of the final diagnosis (i.e., surgery) in 53 of the 62 patients. We selected and validated a cut-off value for a high-serum CA19-9 level (90 U/mL) based on the findings of previous analyses of PDAC [2].
Tissue specimens
Three pathologists examined all resected specimens to confirm the histopathological features. The tumor node metastasis (TNM) system of the Union for International Cancer Control (UICC) 7th Edition was used for staging [24], and all PDACs were graded based on the three-tiered histological grading system of the World Health Organization (WHO) classification for tumors of the pancreas [25], with a grade equal to or higher than G2 considered to indicate a high-grade tumor. Three (9.8 %) of the 62 patients had tumors of stage IV with distant metastasis, exhibiting macroscopically resectable, small, incidental metastatic foci in the liver. Therefore, these three patients were not managed palliatively. Formalin-fixed, paraffin-embedded tissue blocks were provided by our department of pathology. Each patient was assigned an ECOG PS score at the time of diagnosis. Normal human tissue was obtained from non-tumor portions of the surgically resected specimens and stained with hematoxylin and eosin (H&E), Elastica van Gieson (EVG), or immunohistochemistry preparations in sequential sections. EVG, immunohistochemical podoplanin (D2-40; Nichirei Bioscience Co., Tokyo, Japan; diluted 1:1), and S-100 protein (Dako, Glostrup, Denmark; diluted 1:900) staining very clearly revealed vascular invasion (VI), lymphatic vessel invasion (LI), and perineural involvement (PNI), respectively.
Preparation of antibodies against HMGB1 and HMGB2
Polyclonal antibodies were raised against HMGB1 and HMGB2 via multiple immunizations of New Zealand white rabbits with synthetic peptides, respectively, based on previously published work (synthetic peptide sequence of HMGB1: KGETKKKFKDPNAP (K plus amino acids 83–95)) (synthetic peptide sequence of HMGB2: KSEAGKKGPGRPTG (K plus amino acids 168–180)) [26]. The specificity of our original antibodies was confirmed on Western blotting (Supplementary Figure 1A,B), which demonstrated that the HMGB1- and HMGB2-specific antibodies identified endogenous 28- and 27-kDa nuclear proteins, respectively [26]. Furthermore, we performed immunohistochemistry with peptide (HMGB1 and HMGB2) competition (data not shown). For immunohistochemistry of HMGB1 and HMGB2, we used human cancer cells of well- to moderately differentiated adenocarcinoma of the colon as positive controls.
Cell culture
Human pancreatic invasive PDAC cell lines, PANC-1 and MIA PaCa-2, and human cervical cancer cells, HeLa, were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in DMEM medium containing 10 % fetal calf serum at 37 °C in an atmosphere of 95 % air and 5 % CO2 [2].
Immunofluorescence of the pancreatic cancer cell lines PANC-1 and MIA PaCa-2
The pancreatic cancer cell lines PANC-1 and MIA PaCa-2 were cultured on cover slips, fixed with 95 % acetone for 5 min and allowed to air dry [2]. The cells were then incubated with anti-HMGB1 (diluted 1:100) and anti-HMGB2 (diluted 1:100) antibodies for 1 h at room temperature (RT) then further incubated with Hoechst 33258 (blue-stained) (0.5 mg/mL; Dojindo, Kumamoto, Japan) and visualized with goat anti-rabbit antibodies conjugated with Alexa Fluor Dyes (green- and red-stained, respectively) (Invitrogen, Carlsbad, CA, USA). After being washed with PBS, the specimens were observed under a Nikon ECLIPSE E600 inverted fluorescence microscope (Nikon, Tokyo, Japan).
Plasmid construction, transient transfection, and immunoprecipitation (IP) assay
In order to obtain the pcDNA3–hemagglutinin (HA)-HMGB1 or pcDNA3–HA-HMGB2, full-length HMGB1 or HMGB2 complementary DNA (cDNA) was prepared from each GST expression plasmid [26], and the NH2-terminal HA-tagged HMGB1 or HMGB2 cDNA was ligated into a pcDNA3 vector (Invitrogen, San Diego, CA, USA) [27, 28]. Transient transfection and immunoprecipitation (IP) assays were performed as previously described [27]. Briefly, 1 × 105 HeLa cells were seeded into six-well tissue-culture plates. The following day, each 1-μg HA expression plasmid was transfected using X-tremeGENE 9 (Roche Applied Science, Lewes, UK) according to the manufacturer’s instructions, and the transfectants were cultured at 37 °C for 48 h. The transfectants were then washed with phosphate-buffered saline (PBS) and lysed in buffer X containing 50 mM Tris–HCl (pH 8.0), 1 mM ethylenediaminetetraacetic acid (EDTA), 120 mM NaCl, 0.5 % Nonidet P-40, 10 % glycerol, and 1 mM phenylmethylsulfonyl fluoride. The lysates were centrifuged at 21,000 g for 10 min at 4 °C, after which the supernatant (200 μg) was incubated for 2 h at 4 °C with anti-HA-probe (F-7) AC, and the beads were washed three times with buffer X. The immunoprecipitated samples and pre-immunoprecipitated samples (50 μg) were separated via SDS-PAGE, and a Western blotting analysis was performed with the anti-HMGB1 or anti-HMGB2 antibodies, respectively, as described below.
Cell fractionation and Western blotting
Cell pellets derived from the PANC-1 and MIA PaCa-2 cell lines were resuspended in hypotonic buffer A containing 10 mM Hepes–KOH, pH 7.9, 10 mM KCl, 0.1 mM EDTA–NaOH, pH 8.0, 0.1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM dithiothreitol (DTT), and 0.5 mM phenylmethylsulfonyl fluoride (PMSF) and then incubated for 15 min on ice. Following the addition of Nonidet P-40 to a final concentration of 0.3 %, the cells were gently resuspended and centrifuged at 4,200 g for 5 min. The supernatant was subsequently stored to obtain the cytoplasmic fraction (CF) [2]. The nuclear pellets were resuspended in high-salt buffer C (1/4 volume of buffer A), containing 20 mM Hepes–KOH, pH 7.9, 0.4 M NaCl, 1 mM EDTA–NaOH, 1 mM EGTA, 1 mM DTT, and 0.5 mM PMSF. A half-volume of buffer C containing the nucleus was sonicated for 20 s in order to obtain the nuclear fraction (NF) [2]. Then, 100 μg of each NF and CF were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immun-Blot polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, K.K., Tokyo, Japan) using a semi-dry blotter. The blotted membranes were treated with 5 % (w/v) skimmed milk in 10 mM Tris, 150 mM NaCl, and 0.2 % (v/v) Tween 20 and incubated for 1 h at room temperature with the primary antibody. The following antibodies and dilutions were used: 1:100 dilution for the above anti-HMGB1 and anti-HMGB2 antibodies. The membranes were then incubated for 45 min at room temperature with a peroxidase-conjugated secondary antibody and visualized using an ECL kit (GE Healthcare Bioscience, Buckinghamshire, England, UK).
Immunohistochemistry of the tissue samples
Immunohistochemical staining was performed according to the antibody-linked dextran polymer method for antibody-bridge labeling, with hematoxylin counterstaining (EnVision; DAKO, Glostrup, Denmark). Deparaffinized and rehydrated 4-μm sections were incubated in 10 % H2O2 for 5 min to block the endogenous peroxidase activity. The sections were thereafter rinsed and incubated with rabbit polyclonal anti-HMGB1 (diluted 1:100) and anti-HMGB2 (diluted 1:100) antibodies for 30 min, respectively. The second antibody-peroxidase-linked polymers were then applied, and the sections were incubated with a solution consisting of 20 mg of 3,3′-diaminobenzidine tetrahydrochloride, 65 mg of sodium azide, and 20 mL of 30 % H2O2 in 100 mL of Tris–HCl (50 mM, pH 7.6). After counterstaining with Meyer’s hematoxylin, the sections were observed under a light microscope. Each section was first scanned at low power for all fields (original magnification × 40) using tumor and non-tumor tissues, respectively, in order to determine the heterogeneity of the distribution. The number of positive cells showing nuclear or both nuclear and cytoplasmic (HMGB1/2), but not merely cytoplasmic, staining and the pattern of staining were recorded. Necrotic tissues, stromal cells, and lymphoid cells were not included in the recordings [2, 29–31].
The degree of immunoreactivity for HMGB1 and HMGB2 was assessed in each case semi-quantitatively by evaluating the proportion of positive cells relative to the total number of adenocarcinoma cells. Samples with positive areas comprising equal to or less than 9 % of the neoplasms were considered to be negatively stained. For the nuclear HMGB1/2 expression, samples with positive areas equal to or more than 10 % were considered to be positively stained and graded according to three categories: weak, positive areas of 10–29 %; strong, positive areas of 30–79 %; and very strong, positive areas of more than 80 %. We selected and validated immunohistochemical cut-off scores for HMGB1 and HMGB2 positivity (10 % for both HMGBs), based on the findings of a receiver-operating characteristic (ROC) curve analysis [31, 32]. Finally, all patients were divided into two groups, as follows: those with positive findings, equal to or more than 10 %; and those with negative findings, less than 10 %. We additionally performed immunohistochemistry of the same surgical sections obtained from several representative PDAC patient specimens using the newly developed commercially available anti-HMGB2 mouse monoclonal antibody (ABGENT, San Diego, CA, USA, 1:400 diluted). Since the staining patterns and expression profiles of this monoclonal anti-HMGB2 antibody (data not shown) were very similar to those of our original polyclonal antibody, we are able to confirm that the present anti-HMGB2 antibody has high specificity and was alternatively applied to the HMGB2 immunohistochemical examinations.
All histological and immunohistochemical slides were evaluated by two independent observers (certified surgical pathologists within our department: Shohei Kitada and Sohsuke Yamada) using a blind protocol design (the observers were blinded to the clinicopathological data). The degree of agreement between the observers was excellent (an agreement rate of more than 90 %) for all antibodies investigated, as measured according to the interclass correlation coefficient. For the few (less than 1 %) instances of disagreement, a consensus score was calculated by a third board-certified pathologist (Yasuyuki Sasaguri) in our department [2, 29–31].
Statistical analysis
The significance of correlations was determined based on the χ 2 test or Fisher’s exact test, where appropriate, in order to assess the relationships between the immunohistochemical expression levels and the clinicopathological variables [2, 29]. Survival curves were plotted according to the Kaplan–Meier method and compared using the log-rank test. Hazard ratios and 95 % confidence intervals (95 % CIs) were estimated using univariate or multivariate Cox proportional hazard models. All statistical tests were two-tailed, with a P value of <0.05 considered to be statistically significant. All statistical analyses were performed with the EZR software program (Saitama Medical Center, Jichi Medical University, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0) [30, 31, 33]. More precisely, this program is a modified version of R commander (version 1.6-3) designed to add statistical functions frequently used in biostatistics.
Results
Confirmation of the specificity of HMGB1/2 antibodies and the nuclear and cytoplasmic localization of HMGB1/2 in the PDAC cell lines
First, we examined the specificity of the antibodies against HMGB1 and HMGB2 used in this study. As shown in Fig. 1a, the anti-HMGB1 antibody recognized HA-tagged HMGB1 as well as 28 kDa of endogenous HMGB1. In the same way, the anti-HMGB2 antibody recognized HA-tagged HMGB2 as well as 27 kDa of endogenous HMGB2. No cross-reaction was observed between these two antibodies. In order to further confirm the specificity, we employed an immunoprecipitation (IP) assay. As shown in Fig. 1b, the HA-HMGB1 and HA-HMGB2 immunoprecipitated by the anti-HA antibody were recognized by the anti-HMGB1 and anti-HMGB2 antibodies, respectively. These results confirm the specificity of each antibody (Fig. 1).
Transient transfection of hemagglutinin (HA) and an immunoprecipitation (IP) assay confirmed and strengthened the specificity of our original anti-HMGB1 and anti-HMGB2 antibodies. a Our self-made polyclonal anti-HMGB1/2-specific antibodies recognized both endogenous 28-kDa/27-kDa HMGB1/2 proteins and exogenous HA-tagged HMGB1/2 proteins. b Furthermore, we employed transient transfection using HA-HMGB1- or HA-HMGB2-expressing plasmids in whole-cell lysates (200 μg) prepared from HeLa cells, followed by an IP assay and Western blotting using the anti-HA antibody. Consequently, the HA-HMGB1 and HA-HMGB2 immunoprecipitated by the anti-HA antibody were recognized by the anti-HMGB1 and anti-HMGB2 antibodies, respectively. These results indicate the specificity of each antibody. HA hemagglutinin, IP immunoprecipitation
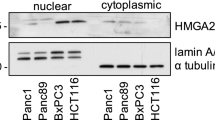
A Western blotting analysis showed that the majority of both the HMGB1 and HMGB2 expression was localized in the NF rather than the CF in the PANC-1 and MIA PaCa-2 cell lines (Supplementary Figure 1A). Next, although the ratio of HMGB1/2 for each cellular fraction mildly varied between the two cell lines, approximately 75 to 90 % of HMGB1/2 was localized in the NF (Supplementary Figure 1B). Immunofluorescence staining of the same PDAC cell lines (PANC-1 and MIA PaCa-2) confirmed the findings of the Western blotting analysis, showing a specific expression of HMGB1 and HMGB2 in both the nuclei and cytoplasm (Supplementary Figure 2). However, a weaker cytoplasmic expression was detectable in both cell lines (Supplementary Figure 2).
Patient characteristics
The cohort included 62 patients (35 males, 27 females) with clinicopathological variables representative of PDAC (Table 1). The average age at surgery was 65 years. All patients (62/62; 100 %) were ECOG 0. The median tumor size was 3.5 cm (range 1.0–7.5 cm). At diagnosis, 40 (64.5 %) patients had lymph node metastasis and 3 (4.8 %) patients had distant metastasis, the latter of whom were managed palliatively. The tumor grade included 19 well-differentiated (G1; 30.6 %), 35 moderately differentiated (G2; 56.5 %), and 8 poorly differentiated (G3; 12.9 %) adenocarcinomas. Based on the UICC criteria, the majority of the patients (39/62; 62.9 %) had stage II disease (stage IIa (12 cases) and stage IIb (27 cases)). Postoperative follow-up was available for all 62 patients (average 21.7 months; range 2–202 months). The median disease-specific postoperative survival (DSS) was 13.4 months, with 1- and 5-year survival rates of 57.9 % and only 12.7 %, respectively. Table 2 displays each patient’s information in detail.
HMGB1/2 expression in normal pancreatic tissues and the PDAC specimens
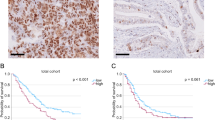
On immunohistochemistry, HMGB1 showed nuclear and weakly cytoplasmic expression patterns, whereas HMGB2 exhibited both a nuclear (in part, equal to or more than 10 %) and cytoplasmic expression in the typically positive cancer cases (Fig. 2). Representative images of the immunohistochemical analyses of the human PDAC samples associated with a worse prognosis are shown in Table 2 (poor outcome cancer: HMGB1+ and HMGB2−; case no. 9 and 27), displaying a nuclear and weakly cytoplasmic (HMGB1) or only cytoplasmic (HMGB2) staining pattern, respectively (Fig. 3). In contrast, a nuclear HMGB1 or HMGB2 expression was not detectable in the adjacent normal ductal epithelium in the paraffin-embedded tissues (Fig. 3).
The nuclear HMGB1 and HMGB2 expression patterns showed specifically positive staining on immunohistochemistry. Representative images of immunohistochemical positivity/negativity for HMGB1 and HMGB2 in the human PDAC samples showing a nuclear and weakly cytoplasmic (HMGB1) or both nuclear (in part, equal to or more than 10 %) and cytoplasmic (HMGB2) staining pattern, respectively, in the HMGB1/2-positive cases (case no. 36), compared to a modestly cytoplasmic staining pattern in the HMGB1/2-negative cases (case no. 25) (original magnification × 100; inset × 400). Each inset provides a representative image of PDAC nuclei and cytoplasm on high-power view. Bar 100 μm (×100) or 20 μm (×400)
The combination of a nuclear HMGB1-positive and HMGB2-negative expression is potentially associated with a poor prognosis in PDAC patients. Representative images of the immunohistochemical analyses of both HMGB1 and HMGB2 in the human PDAC samples (high-grade (G2 to G3) cancer in advanced stage with a worse prognosis: HMGB1+ and HMGB2− expression; case no. 27), displaying a nuclear and weakly cytoplasmic (HMGB1) or only cytoplasmic, not nuclear, (HMGB2) staining pattern, respectively, and the normal ductal specimens (a nuclear negative expression of HMGB1/2; case no. 9) (original magnification × 100; inset × 400). Each inset provides a representative image of a PDAC or bland-looking pancreatic ductal nucleus and cytoplasm on high-power view. Bar 100 μm (×100) or 20 μm (×400). H&E hematoxylin and eosin
HMGB1 was negatively and positively expressed in 8 (12.9 %) and 54 (71.9 %) of the 62 PDAC specimens, respectively (Table 3) (8 negative (12.9 %), 30 weak (48.4 %), 20 strong (32.3 %), and 4 very strong (6.5 %)). In contrast, HMGB2 was negatively and positively expressed in 31 (50.0 %) and 31 (50.0 %) specimens, respectively (Table 3) (31 negative (50.0 %), 23 weak (37.1 %), 7 strong (11.3 %), and 1 very strong (1.6 %)).
Associations between the HMGB1/2 expression and the clinicopathological variables
In order to identify associations between the HMGB1/2 expression (negative versus positive) and the clinicopathological characteristics of the study cohort, the variables were categorized as shown in Table 3. Consequently, there were no significant differences between the patients with negative- and positive-HMGB1 or negative- and positive-HMGB2 tumor expression patterns in terms of age, gender, location, tumor grade, size (>2 cm), surgical margin, tumor stage, including T and N stage, presence of VI and PNI, and a high-serum CA19-9 level (P > 0.05) (Table 3). However, a HMGB2-negative expression was found to be borderline insignificantly associated with the presence of LI (P = 0.11) in all tumors (Table 3 and Supplementary Figure 2), whereas a HMGB1-positive expression was not (P > 0.05). In particular, a HMGB2− and HMGB1+ expression (case no. 9 in Table 2) was evident in LI, VI, and PNI representative adenocarcinoma components, as shown on D2-40, EVG, and S-100 protein staining, respectively (Supplementary Figure 3). Moreover, there was a significantly close relationship between the immunohistochemical HMGB2− and HMGB1+ expression levels (P = 0.002, r = 0.38) (Fig. 3, Supplementary Figure 3, and Table 3). Nevertheless, the combination of a HMGB1+ and HMGB2− expression was only insignificantly associated with various clinicopathological parameters, including a high (G2 and G3) tumor grade or advanced disease stage, with the exception of a high-serum CA19-9 level (P = 0.01) (Table 3).
According to a Kaplan–Meier analysis, there were no significant differences in postoperative DSS between the two groups of PDAC patients with a HMGB1+ (median 12.6 months) and HMGB1− (median 22.2 months) status (P = 0.39, Fig. 4a), except within the first 1 year (P = 0.03). In contrast, the patients with a HMGB2− expression had a significantly shorter postoperative median DSS (12.6 months) than those with a HMGB2+ expression (21.3 months) (P = 0.02, Fig. 4b). Next, when the HMGB1 and HMGB2 expressions were split into groups of either +/−, their immunoprofiles were as follows: 37.1 % + and − (23 cases); 0 % − and + (0 cases); 50.0 % + and + (31 cases); and 12.9 % − and − (8 cases). In addition, the Kaplan–Meier analysis revealed that the HMGB1+ and HMGB2− (+and −) patients had a markedly shorter postoperative median DSS (9.8 months) than the other patients (21.3 months) (P = 0.0004, Fig. 4c).
The combination of a nuclear HMGB1-positive and HMGB2-negative expression was associated with a markedly shorter postoperative DSS in the PDAC patients. a, b Kaplan–Meier curves for DSS in the patients with PDAC within the first 2 years after surgery according to the HMGB1 (a) and HMGB2 (b) expression. c Kaplan–Meier curves for DSS in the patients with PDAC within the first 2 years after surgery according to the combination of a HMGB1+ and HMGB2− expression. DSS disease-specific survival
The combination of the HMGB1+ and HMGB2− expression represents a significant independent prognostic indicator for PDAC
In order to assess whether the combination of a HMGB1+ and HMGB2− expression is an independent predictor of postoperative DSS, a Cox proportional hazards model was created in a forward fashion including only covariates with a statistically significant correlation with DSS using an inclusion threshold of P < 0.05 (Table 4). Consequently, a univariate analysis showed the tumor size (>2 cm), an advanced T stage (T3 and 4), a high-serum CA19-9 level (≥90 IU), and the combination of a HMGB1+ and HMGB2− expression to be borderline insignificant (the former three variables) and significant (the latter variable) predictors of a poor survival (P = 0.05, 0.07, 0.05, and 0.0007, respectively). Furthermore, a multivariate analysis demonstrated that, after correcting for confounding variables, the combination of a HMGB1+ and HMGB2− expression and an advanced T stage remained independent prognostic indicators for DSS (P = 0.04 and 0.04, respectively) (Table 4), whereas the HMGB1+ expression alone did not.
Discussion
The present study demonstrated for the first time that the combination of an immunohistochemically nuclear HMGB1-positive and HMGB2-negative expression is a powerful and potentially independent negative indicator of DSS in patients with postoperative PDAC and, by extension, a novel prognostic marker for PDAC, especially within the first 2 years after surgery. Moreover, the functions of HMGB1 and HMGB2 may be separate but likely complementary. It is well known that the prognosis of pancreatic cancer is very poor and the administration of adjuvant chemotherapy does not improve the prognosis. Approximately 80 % of cases of postoperative recurrence (local or distant) and subsequent death occur within the first 2 years [3–5], which corresponds to the rate observed in the present study (47 of 62 patients; 75.8 %). However, there are currently no reliable predictors of the progressive potential of PDAC. In this sense, the HMGB1/2 expression patterns in both PDAC surgical specimens and preoperative biopsy samples, the latter of which have recently become more frequently collected as a routine procedure, may allow for improved patient selection of candidates for adjuvant/neoadjuvant systemic therapy as well as prediction of the clinical postoperative course, especially in the early phase. Reportedly, adjuvant chemotherapy using a combination of oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX), rather than standard gemcitabine, significantly improves the prognosis of advanced PDAC [34]. The validity of the clinical relevance of these HMGB proteins must therefore be verified in the future in order to prevent unnecessary surgery (i.e., perform only biopsies) and prolong the effects of beneficial surgical treatment.
Collectively, our data are both partly in agreement and disagreement with the findings of previous studies of epithelial cancers. For example, the overexpression of both HMGB1 and HMGB2 in human bladder and skin carcinoma cells is closely associated with histopathological poorly differentiated characteristics and/or invasive/aggressive behavior [13, 18]. HMGB1 has also been shown to tightly regulate the transcription of central factors contributing to cancer growth and metastatic spread, e.g., tumor necrosis factor (TNF) and E-selectin, by interacting with receptors for advanced glycation end-products (RAGE) or Toll-like receptors (TLRs) [8–10, 20, 21]. However, HMGB1 may also play a critical role in the epithelial-to-mesenchymal transition (EMT), subsequently facilitating the spread of metastasis in various carcinomas [30, 31, 35], possibly including PDAC. In contrast to HMGB1, which has been more extensively studied, considerably less is known about HMGB2 in the field of cancer research, despite the high homology of HMGB2 to HMGB1. Surprisingly, in the present study, an inverse HMGB2-negative expression was found to significantly correlate with a HMGB1-positive expression in our PDAC group. Therefore, we hypothesize that HMGB2 has similar effects and roles to those of HMGB1 in cancer development. Interestingly, HMGB2 is reportedly a potential tumor suppressor gene located within the disease-associated region of 4q32-34 in patients with European familial pancreatic cancer [36]. In this context, it is possible that, unlike HMGB1, HMGB2 may have a key function in preventing malignant transformation by inducing cell cycle arrest and/or apoptosis, especially in the setting of PDAC, very similar to p53, which is also a crucial tumor suppressor gene. Consistent with these observations, another in vitro study recently demonstrated that the knockdown of HMGB2 results in significantly reduced chemosensitivity against cytarabine in the A549 lung adenocarcinoma cell line [11]. Taken together, despite our study limitations of assessing a small cohort at a single institution, HMGB1 and HMGB2 may function separately, but in a complementary manner, reminiscent of crosstalk. It is thus noteworthy that the combination of the two biomarkers HMGB1/2, but not HMGB1 alone, potentially predicted a clinicopathologically aggressive potential and/or poor outcome in the patients with postoperative PDAC in the present retrospective study. Furthermore, we found an immunohistochemical nuclear HMGB1-positive and HMGB2-negative expression to be closely associated with a high-serum CA19-9 level (P = 0.01) in our cohort, indicating the uniquely additive effects and/or benefits of the combination of HMGB1/2 and CA19-9. Further follow-up with a larger cohort is therefore needed to confirm the intriguing relationships between HMGB1 and HMGB2 and between HMGB1/2 and more conventional markers, such as CA19-9.
Ubiquitous HMGB family proteins are not always associated with chromatin, but rather are also detected in the cytoplasm, and HMGB1 has been reported to be found in both locations due to its migration between the nucleus and cytoplasm [22, 23], as shown in this study. Of note, cytoplasmic and secretory extracellular HMGB1 proteins play a pivotal role in both innate and specific immune responses as pro-inflammatory cytokines, as well as aid in the metastatic potential of cancer cells [8–10, 20, 21]. In the present examinations, we demonstrated for the first time that HMGB2 may also not be a housekeeping protein, but rather have a dual function, as expressed in both the nucleus and cytoplasm in PDAC cells. On the other hand, it remains unclear whether a cytoplasmic HMGB-positive expression has a substantial impact on the pathophysiological effects on normal ducts versus carcinoma cells. Nevertheless, further molecular and biochemical experiments are needed to clarify the mechanisms of HMGB2 migration from the nucleus to cytoplasm and hence the utility of HMGB2 agonists and/or HMGB2 signaling activators as potential therapeutic agents for PDAC.
In conclusion, the current cohort study showed for the first time that a combination of the HMGB1+ and HMGB2− expression is an independent, novel, and powerful marker of a poor prognosis in PDAC patients, particularly within the first 2 years after curative surgery. Therefore, physicians may assess the values of these critical PDAC-specific biomarkers, HMGB1 and HMGB2, as useful parameters for clinical management, especially in the early postoperative phase.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
Li Z, Yamada S, Inenaga S, et al. Polypeptide N-acetylgalactosaminyltransferase 6 expression in pancreatic cancer is an independent prognostic factor indicating better overall survival. Br J Cancer. 2011;104(12):1882–9.
Cleary SP, Gryfe R, Guindi M, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198(5):722–31.
Katz MH, Hwang R, Fleming JB, Evans DB. Tumor-node-metastasis staging of pancreatic adenocarcinoma. CA Cancer J Clin. 2008;58(2):111–25.
Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7(3):163–72.
Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36.
Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PLoS One. 2011;6(8):e24099.
Thomas JO. HMG1 and 2: architectural DNA-binding proteins. Biochem Soc Trans. 2001;29(Pt4):395–401.
Pallier C, Scaffidi P, Chopineau-Proust S, Agresti A, Nordmann P, Bianchi ME, et al. Association of chromatin proteins high mobility group box (HMGB) 1 and HMGB2 with mitotic chromosomes. Mol Biol Cell. 2003;14(8):3414–26.
Ronfani L, Ferraguti M, Croci L, Ovitt CE, Schöler HR, Consalez GG, et al. Reduced fertility and spermatogenesis defects in mice lacking chromosomal protein Hmgb2. Development. 2001;128(8):1265–73.
Krynetskaia NF, Phadke MS, Jadhav SH, Krynetskiy EY. Chromatin-associated proteins HMGB1/2 and PDIA3 trigger cellular response to chemotherapy-induced DNA damage. Mol Cancer Ther. 2009;8(4):864–72.
Kostova N, Zlateva S, Ugrinova I, Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem. 2010;337(1–2):251–8.
Sharma A, Ray R, Rajeswari MR. Overexpression of high mobility group (HMG) B1 and B2 proteins directly correlates with the progression of squamous cell carcinoma in skin. Cancer Invest. 2008;26(8):843–51.
Gnanasekar M, Thirugnanam S, Ramaswamy K. Short hairpin RNA (shRNA) constructs targeting high mobility group box-1 (HMGB1) expression leads to inhibition of prostate cancer cell survival and apoptosis. Int J Oncol. 2009;34(2):425–31.
Yao X, Zhao G, Yang H, Hong X, Bie L, Liu G. Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol. 2010;136(5):677–84.
Song B, Song WG, Li ZJ, Xu ZF, Wang XW, Wang CX, et al. Effect of HMGB1 silencing on cell proliferation, invasion and apoptosis of MGC-803 gastric cancer cells. Cell Biochem Funct. 2012;30(1):11–7.
Jiao Y, Wang HC, Fan SJ. Growth suppression and radiosensitivity increase by HMGB1 in breast cancer. Acta Pharmacol Sin. 2007;28(12):1957–67.
Wang W, Jiang H, Zhu H, Zhang H, Gong J, Zhang L, et al. Overexpression of high mobility group box 1 and 2 is associated with the progression and angiogenesis of human bladder carcinoma. Oncol Lett. 2013;5(3):884–8.
Takeuchi T, Sakazume K, Tonooka A, Zaitsu M, Takeshima Y, Mikami K, et al. Cytosolic HMGB1 expression in human renal clear cell cancer indicates higher pathological T classifications and tumor grades. Urol J. 2013;10(3):960–5.
Pardo M, García A, Thomas B, Piñeiro A, Akoulitchev A, Dwek RA, et al. The characterization of the invasion phenotype of uveal melanoma tumour cells shows the presence of MUC18 and HMG-1 metastasis markers and leads to the identification of DJ-1 as a potential serum biomarker. Int J Cancer. 2006;119(5):1014–22.
Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14(16):5000–5.
Müller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255(3):332–43.
Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. Bratisl Lek Listy. 2012;113(3):163–71.
Sobin LH, Gospodarowicz MK, Wittekind Ch. TNM classification of malignant tumours 7th edition. Wiley-Blackwell; 2009.
Bosman F, Carneiro F, Hruban RH, Theise N, editors. Ductal adenocarcinoma of the pancreas. In: Hruban RH, Boffetta P, Hiraoka N, lacobuzio-Donahue C, Kato Y, Kern SE, Klimstra DS, Kloppel G, Marita A, Offerhaus GJA, Pitman MB, editors. World Health Organization (WHO) classification of tumours of the digestive system. IARC, Lyon: Springer; 2010, pp. 281–91.
Ise T, Nagatani G, Imamura T, et al. Transcription factor Y-box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res. 1999;59(2):342–6.
Wakasugi T, Izumi H, Uchiumi T, et al. ZNF143 interacts with p73 and is involved in cisplatin resistance through the transcriptional regulation of DNA repair genes. Oncogene. 2007;26(36):5194–203.
Izumi H, Wakasugi T, Shimajiri S, et al. Role of ZNF143 in tumor growth through transcriptional regulation of DNA replication and cell-cycle-associated genes. Cancer Sci. 2010;101(12):2538–45.
Kitada S, Yamada S, Kuma A, et al. Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br J Cancer. 2013;109(2):472–81.
Wu Y, Yamada S, Izumi H, et al. Strong YB-1 expression is associated with liver metastasis progression and predicts shorter disease-free survival in advanced gastric cancer. J Surg Oncol. 2012;105(7):724–30.
Kawatsu Y, Kitada S, Uramoto H, et al. The combination of strong expression of ZNF143 and high MIB-1 labelling index independently predicts shorter disease-specific survival in lung adenocarcinoma. Br J Cancer. 2014, in print.
Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging. 1989;29(3):307–35.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Chang BP, Wang DS, Xing JW, Yang SH, Chu Q, Yu SY. miR-200c inhibits metastasis of breast cancer cells by targeting HMGB1. J Huazhong Univ Sci Technolog Med Sci. 2014;34(2):201–6.
Earl J, Yan L, Vitone LJ, et al. Evaluation of the 4q32-34 locus in European familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1948–55.
Financial support
This work was supported in part by Grants-in-Aid for Scientific Research (24790394, 20590416, and 19590413) from the Ministry of Education, Culture, Sports, Science, and Technology, Tokyo, Japan (to S. Y., K. K., and Y. S).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
T.T. and S.Y. contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
The specificity of our original polyclonal antibodies for HMGB1 and HMGB2 was confirmed, with a larger amount of HMGB1/2 found to be localized in the nuclei of the PDAC cell lines. (A) Western blotting analyses of HMGB1/2 in the PDAC cell lines PANC-1 and MIA PaCa-2 divided into CF and NF. The majority of the HMGB1 and HMGB2 expression was localized in the NF rather than the CF in both of the cell lines. (B) Next, each cell lysate was diluted 1/2 sequentially. The same volume was subjected to SDS–PAGE, and Western blotting was performed with the indicated antibodies. The maximum amount of proteins was 100 μg. Sp-1 is a control for NF, whereas β-tubulin is a control for CF. Based on these data, approximately 75 % to 90 % of the HMGB1/2 expression was localized in the NF in each cell line. CF: cytoplasmic fraction. NF: nuclear fraction. (PDF 126 kb)
Supplementary Figure 2
Immunofluorescence confirmed the Western blotting data for the PDAC cell lines, showing a larger amount of HMGB1/2 localized in the nuclei of the PDAC cells. Immunofluorescence staining of the PANC-1 and MIA PaCa-2 cells also showed a specific expression of HMGB1 (green-stained) and HMGB2 (red-stained) in both the nuclei (blue-stained by Hoechst) and cytoplasm in the PDAC cell lines. Correspondingly, a weaker cytoplasmic expression of HMGB1/2 was detectable in both cell lines. (PDF 46 kb)
Supplementary Figure 3
The HMGB1+ and HMGB2− expression in the PDAC cells exhibited a potentially close relationship with a pathological LI, VI and/or PNI potential, manifesting as poorly differentiated characteristics associated with invasive/aggressive behavior. Representative pictures for H&E, EVG and the immunohistochemical analyses of HMGB1, HMGB2, D2-40 and S-100 protein in the areas of vascular (VI; Case No.27), lymphatic (LI; Case No.9) and perineural (PNI; Case No.9) invasion among the advanced PDAC components (Original magnification: ×100; inset: ×400). EVG, D2-40 and S-100 protein staining very clearly revealed elastic fibers in the vascular medial wall (VI(+)), with a lymphatic endothelium (LI(+)) and neuronal fibers (PNI(+)). Each inset provides a representative image of LI or PDAC nuclei and cytoplasm with a nuclear and cytoplasmic (HMGB1) or only cytoplasmic, not nuclear, (HMGB2) staining pattern, respectively, on high-power view. Bar = 100 μm (×100) or 20 μm (×400). H&E: hematoxylin and eosin. EVG: Elastica van Gieson. LI: lymphatic vessel invasion. VI: vascular invasion. PNI: perineural involvement. (PDF 2018 kb)
Rights and permissions
About this article
Cite this article
Takeda, T., Izumi, H., Kitada, S. et al. The combination of a nuclear HMGB1-positive and HMGB2-negative expression is potentially associated with a shortened survival in patients with pancreatic ductal adenocarcinoma. Tumor Biol. 35, 10555–10569 (2014). https://doi.org/10.1007/s13277-014-2328-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2328-8