Abstract
Aflibercept (Ziv-aflibercept, VEGF Trap, AVE005) is an engineered protein that functions as a decoy receptor to bind vascular endothelial growth factor A (VEGF-A). Hemorrhagic events, including epistaxis, gastrointestinal bleeding, and pulmonary bleeding, is one of its major adverse effects, but the incidence rate and overall risk has not been systematically studied. Therefore, we conducted a meta-analysis of published clinical trials to investigate the incidence and relative risk of hemorrhagic events in cancer patients treated with aflibercept. Electronic databases including PubMed, Embase, Cochrane databases, and American Society of Clinical Oncology abstracts were searched. Eligible studies were phase II and III prospective clinical trials of cancer patients treated with aflibercept with toxicity profile on hemorrhagic events. Overall incidence rates, relative risk (RR), and 95 % confidence intervals (CI) were calculated using fixed or random effects models depending on the heterogeneity of the included studies. A total of 4,538 patients with a variety of solid tumors from 13 prospective clinical trials were included for the meta-analysis. The overall incidences of all-grade and high-grade hemorrhagic events in cancer patients were 22.1 % (95 % CI, 16.5–29.7 %) and 4.2 % (95 % CI, 3.9–4.6 %), respectively. The relative risks of hemorrhagic events of aflibercept compared to control were increased for all-grade (RR = 2.63; 95 % CI, 2.07–3.34) and high-grade (RR = 2.45, 95 % CI, 1.62–3.72) hemorrhagic events. The risk of developing high-grade hemorrhagic events with aflibercept was comparable to that of bevacizumab (RR = 1.26; 95 % CI, 0.89–1.79). Aflibercept is associated with an increased risk of developing hemorrhagic events in patients with solid tumors. Close monitoring and management of hemorrhagic events are recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis is an important process in tumor growth, progression, and metastasis. Vascular endothelial growth factor (VEGF) is the main pro-angiogenic factor [1]. VEGF binds to both VEGFR-1 and VEGFR-2. The VEGF/VEGFR-2 pathway is thought to be the dominant promoter of angiogenesis [2]. VEGF signaling provides potential targets for antiangiogenic therapy in malignant tumors. Targeting VEGF by angiogenesis inhibitors have demonstrated clinical benefit in treating various solid tumors, including anti-VEGF antibody (bevacizumab), VEGF Trap (aflibercept), and VEGF tyrosine kinase inhibitors (sorafenib, sunitinib, vandetanib, pazopanib, axitinib, etc).
Aflibercept (Ziv-aflibercept, VEGF Trap, AVE005) is a recombinant fusion protein comprised of the extracellular domain from VEGFR-1 and VEGFR-2 fused with Fc region of human IgG1. It binds to VEGF-A, VEGF-B, and placental growth factor (PIGF), subsequently preventing ligand binding to VEGFR-1 and VEGFR-2. In vitro assays of aflibercept in human umbilical vein endothelial cells demonstrated the ability to completely block the phosphorylation of VEGFR-2 by VEGF-A, thereby blocking VEGF-A-induced cell proliferation. The affinity of aflibercept for VEGF is superior to that of bevacizumab, and it is a more potent VEGF blocker than bevacizumab [3]. As a single agent, aflibercept led to decreases in tumor vessels and angiogenesis, tumor growth, and metastasis. Aflibercept also demonstrated synergy with other systemic treatments in a number of studies, leading to greater inhibition of tumor growth and change in tumor vasculature. Aflibercept is currently approved as second-line treatment for patients with metastatic colorectal cancer by US Food and Drug Administration.
VEGF inhibitors improve the clinical outcome of patients with solid tumors, but these agents have shown some adverse effects. The safety profile of aflibercept was similar to that of other angiogenesis inhibitors. Most events are of low grade, but some could be life-threatening. Hypertension, proteinuria, and thromboembolism are recognized as the hallmark class-related adverse effects associated with antiangiogenic therapy. Hemorrhagic events is also one of the most common adverse events of anti-VEGF agents [4]. Hemorrhage or bleeding events, including epistaxis, gastrointestinal bleeding and pulmonary hemorrhage, are found in some patients after aflibercept therapy. Epistaxis is the most common form of hemorrhage found with aflibercept. Due to the multiplicity of actions of VEGF on vascular walls, inhibition of VEGF signaling by anti-VEGF agents, such as aflibercept, predisposes vascular walls to hemorrhage. Vascular dysfunction is another area of concern with angiogenesis inhibitors such as aflibercept, as VEGF regulates vascular proliferation and permeability. The recognition and management of hemorrhagic events in cancer patients treated with aflibercept is an important issue since hemorrhagic events may cause severe outcomes. The incidence and relative risk of hemorrhagic events with aflibercept is unclear. Thus, we performed a meta-analysis of prospective clinical trials to determine the incidence and relative risk of hemorrhagic events among cancer patients treated with aflibercept.
Materials and methods
Search strategy
Several databases including PubMed, Embase, and Cochrane databases were searched for studies to include in the meta-analysis. Abstracts presented at the annual meetings of the American Society of Clinical Oncology (ASCO) was also searched manually. The upper date limit of March 2014 was applied, with no lower date limit. Searches include the following terms: (“aflibercept” or “VEGF-trap” or “AVE0005”) and (“cancer” or “carcinoma” or “sarcoma”), and (“clinical trial” or “randomized controlled trial”). The references cited by the included studies were also used to complete the search.
Aflibercept is approved for the treatment of patients with previously treated colorectal cancer at a dose of 4 mg/kg every 2 weeks (Q2W). Therefore, clinical trials using aflibercept at the approved dosage were included. Clinical trials using aflibercept at doses of 6 mg/kg every 3 weeks (Q3W) were also included to assess the possible increased incidence of hemorrhagic events with these treatments.
Eligible criteria for inclusion in this meta-analysis are as follows: (1) prospective phase II and III clinical trials in cancer patients; (2) participants assigned to treatment with aflibercept at 4 mg/kg Q2W or 6 mg/kg Q3W; (3) the language was restricted in English; (4) data available regarding incidence or events of hemorrhagic events, including ecchymosis or petechiae, epistaxis, eye hemorrhage, gastrointestinal hemorrhage, gum hemorrhage, injection-site hemorrhage, hematemesis, hematuria, hemoptysis, nonspecific hemorrhage, hemothorax, melena, menorrhagia, metrorrhagia, purpura, rectal hemorrhage, retroperitoneal hemorrhage, CNS hemorrhage, and vaginal hemorrhage; and (5) if multiple publications of the same trial were retrieved, only the most recent publication was included. Phase I studies were excluded because of the different drug dosage and the relatively small number of patients on these trials. Abstracts of all candidate articles were read by two independent readers (LP and YZ). Articles that could not be categorized based on title and abstract alone were retrieved for full-text review. Disagreements were resolved by consensus between the two readers.
Study selection
Two investigators (LP and YZ) independently assessed the eligibility of the articles and abstracts identified by the search, and any discrepancy was resolved by consensus. Hemorrhagic events was extracted from the safety and toxicity profile in the primary studies. These clinical end points were all recorded according to versions 3.0 of the Common Terminology Criteria for Adverse Events (CTCAE) of National Cancer Institute (http://ctep.cancer.gov/reporting/ctc_archive.html). The CTC version 3.0 describes the grading of hemorrhagic events as follows: grade 1 indicating mild bleeding (intervention not indicated); grade 2 indicating symptomatic bleeding (medical intervention indicated); grade 3 indicating the need for transfusion, interventional radiology, or endoscopic or operative intervention (i.e., hemostasis of bleeding site); grade 4 indicating life-threatening consequences (major urgent intervention indicated); and grade 5 indicating death. We included all incidences of hemorrhagic events of grade 1 or above in our analysis.
Data analysis
Information was retrieved from the primary studies, using a standardized data collection form, including the following items: year of publication, first author, underlying malignancies, number of patients, treatment arm. If data from any of the above categories were not reported in the study, items were treated as “NR (not reported).” The data of the number of patients with all-grade and high-grade (≥grade 3) of hemorrhagic events and the number of patients receiving single-agent aflibercept were extracted from the toxicity profile. For each study, we derived the proportion and 95 % confidence interval (CI) of patients with hemorrhagic events. For studies with a control arm in the same trial, we also calculated and compared the relative risk (RR) of hemorrhagic events. For one study that reported zero events in the control arm, we applied the classic half-integer correction to calculate the RR and variance [5]. authors of the primary studies were not contacted for additional or unreported information. Between-study heterogeneity was estimated using the χ 2-based Q statistic [6]. Heterogeneity was considered statistically significant when P < 0.05 or I 2 > 50 %. If heterogeneity existed, data were analyzed using a random effects model. In the absence of heterogeneity, a fixed effects model was used. To calculate the pooled incidence, an inverse variance statistical method was used. We also explored the differences in incidence of hemorrhagic events between aflibercept and bevacizumab. We used bevacizumab as the control (with RR = 1.0) to calculate the RR of hypertension for aflibercept [7]. A statistical test with a P value less than 0.05 was considered significant. The presence of publication bias was evaluated by using the Begg’s and Egger’s tests [8, 9]. All of the calculations were performed by STATA version 11.0 (Stata Corporation, College Station, TX).
Results
Study selection and characteristics
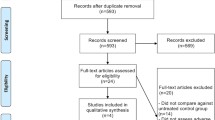
Our search yielded a total of 256 articles on aflibercept from the literature. After reviewing each publication, 13 original studies of full publication met our inclusion criteria, comprising 4,538 patients for final analysis. The selection process is summarized in Fig. 1. The major baseline characteristics of the 13 eligible studies were reported in Table 1, encompassing five randomized controlled trials (RCTs) and eight phase II clinical trials. Underlying malignancies include ovarian cancer (two trials) [10, 11], mCRC (colorectal cancer) (two trials) [12, 13], non-small cell lung cancer (two trials) [14, 15], prostate cancer (one trial) [16], pancreatic cancer (one trial) [17], sarcoma (one trial) [18], endometrial cancer (one trial) [19], melanoma (one trial) [20], glioma (one trial) [21], and urothelial cancer (one trial) [22]. The sample size of the included studies ranged from 22 to 611 patients (median sample size, 106 patients). The studies were published between 2010 and 2014. For calculation of the RRs, five RCTs were pooled. We performed this meta-analysis in accordance with the guidelines of the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) statement [23].
Incidence of all-grade hemorrhagic events
The results of the meta-analysis were shown in Fig. 2. The incidence of all-grade hemorrhagic events ranged from 9.0 to 48.2 %; the lowest incidence was noted in a phase II single-arm trial among patients with urothelial cancer [22], and the highest incidence was observed in patients with NSCLC [14]. Our meta-analysis revealed a significant heterogeneity among included studies (I 2 = 98.8 %, P < 0.001), and the calculated summary incidence of all-grade hemorrhagic events among patients receiving aflibercept was 22.1 % (95 % CI, 16.5–29.7 %) using a random effects model (Fig. 2a).
Forest plot for meta-analysis of incidence relative risk of all-grade and high-grade hemorrhagic events in cancer patients treated with aflibercept. Each study was shown by the name of the lead author and year of publication. The summary incidence was also shown in the figure. Plots are arranged as follows: a incidence of all-grade hemorrhagic events; b incidence of high-grade hemorrhagic events
Incidence of high-grade hemorrhagic events
The incidence of high-grade hemorrhagic events data ranges from 0 to 6.8 %. The highest incidence was observed in a phase II trial conducted by Coleman et al. in patients with endometrial cancer [19], and the lowest incidence was observed in patients with ovarian cancer [10]. The calculated summary incidence of high-grade hemorrhagic events among patients receiving aflibercept was 4.2 % (95 % CI, 3.9–4.6 %) using a fixed effects model (I 2 = 0.0 %, P = 0.565) (Fig. 2b).
Relative risk of hemorrhagic events
We then determined the RR of aflibercept-induced hemorrhagic events compared with control arm. For the calculation of relative risk, the included studies must involve the comparison of aflibercept against placebo, or the comparison of aflibercept with chemotherapy agent against placebo with the same chemotherapy agent. Altogether, five RCTs were pooled [16, 17, 12, 14, 11], comprising four phase 3 studies and one phase 2 study. The pooled RR showed that aflibercept treatment increased the risk of developing all-grade hemorrhagic events in cancer patients with a RR of 2.63 (95 % CI, 2.07–3.34; P < 0.001, Fig. 3a) (I 2 = 70.6 %, P = 0.009). The incidence for high-grade hemorrhagic events was significantly increased in cancer patients receiving aflibercept compared with control (RR = 2.45; 95 % CI, 1.62–3.72, P < 0.001, Fig. 3b) (I 2 = 0.00 %, P = 0.556).
Forest plot for meta-analysis of relative risk of all-grade and high-grade hemorrhagic events in cancer patients treated with aflibercept compared with control. Each study was shown by the name of the lead author and year of publication. Plots are arranged as follows: a relative risk of aflibercept-associated all-grade hemorrhagic events versus control; b relative risk of aflibercept-associated high-grade hemorrhagic events versus control
Risk of specified and unspecified hemorrhagic events
In comparison with controls, aflibercept was associated with a significantly increased risk of specified all-grade (RR, 3.34; 95 % CI, 2.85–3.91; P < 0.001) and specified high-grade hemorrhagic events (RR = 3.32; 95 % CI, 1.70–6.48; P < 0.001). Additionally, a non-significantly increased risk of unspecified all-grade (RR = 1.71; 95 % CI, 0.89–3.30; P = 0.106) and a significantly increased risk of unspecified high-grade hemorrhagic events (RR = 1.87; 95 % CI, 1.10–3.17; P = 0.021) was observed (Fig. 4).
Forest plot for meta-analysis of relative risk of specified and unspecified hemorrhagic events in cancer patients treated with aflibercept compared with control. Each study was shown by the name of the lead author and year of publication. Plots are arranged as follows: a relative risk of aflibercept-associated specified all-grade hemorrhagic events versus control; b relative risk of aflibercept-associated specified high-grade hemorrhagic events versus control; c relative risk of aflibercept-associated unspecified all-grade hemorrhagic events versus control; d relative risk of aflibercept-associated unspecified high-grade hemorrhagic events versus control
Difference in hemorrhagic events incidence between bevacizumab and aflibercept
In addition to aflibercept, other anti-angiogenesis drugs, such as bevacizumab, sorafenib, sunitinib, vandetanib, and axitinib, have been associated with the development of hemorrhagic events (Table 2). We explored the difference of high-grade incidence in hemorrhagic events induced by aflibercept in comparison of bevacizumab. The results showed that the risk of developing high-grade hemorrhagic events with aflibercept was comparable to that of bevacizumab (RR = 1.26; 95 % CI, 0.89–1.79; P = 0.19).
Publication bias
Begg’s funnel plot and Egger’s test were performed to evaluate the publication bias of the eligible studies. Ten and 13 studies investigating all-grade and high-grade hemorrhagic events induced by aflibercept yielded an Egger’s test score of P = 0.001 and P = 0.025 , respectively, indicating the presence of publication bias in the studies (Fig. 5).
Discussion
Angiogenesis is a crucial process in tissue development and growth. VEGF is the most potent and extensively studied. VEGF binding to VEGF receptors (VEGFR1, VEGFR2) initiates angiogenesis signaling process, including increased vascular permeability and endothelial cell proliferation [2]. Antiangiogenic drugs is postulated to block new blood vessel formation and lead to capillary regression [24]. VEGF inhibition is a validated anticancer strategy, and several agents have been designed to target VEGF and angiogenesis pathways.
Aflibercept (VEGF Trap, Ziv-aflibercept, or AVE005) is a recombinant protein consisting of domain 2 from VEGFR-1 fused to domain 3 from VEGFR-2, attached to the hinge region of the Fc domain of IgG1. In contrast to bevacizumab, aflibercept not only targets VEGF-A but also VEGF-B and PIGF, forming a pharmacologic blockade of the VEGF pathway. Aflibercept has a higher VEGF A binding affinity than bevacizumab [3]. It is approved by the Food and Drug Administration for use in combination with FOLFIRI regimen for second-line treatment of patients with metastatic colorectal cancer. Its application in other types of cancer is also undergoing extensive clinical assessment.
VEGF not only stimulates endothelial cell proliferation but also promotes endothelial cell survival and helps maintain vascular integrity. If VEGF is blocked, the repair capacity of endothelial cells are impaired and cause defects that expose pro-coagulant phospholipids on the luminal plasma membrane or underlying matrix, thus increasing the risk of hemorrhage [25]. Moreover, weakening of the wall of major vessels by tumor erosion, necrosis, cavitation, or other concurrent pathological conditions are likely to play a role in the occurrence of life-threatening hemorrhage [26]. Hemorrhagic events is one of the major side effects of aflibercept, and reported incidences vary substantially among clinical trials. The aim of this study is to gain a better understanding of the overall incidence and relative risk of hemorrhagic events in cancer patients treated with aflibercept. The present meta-analysis has combined 13 publications including five randomized controlled trials and eight phase II trials. Our meta-analysis results demonstrate that aflibercept is associated with an increased risk of developing hemorrhagic events. Our meta-analysis demonstrates that hemorrhagic events associated with aflibercept is mostly grades 1 and 2. Epistaxis was reported as the most frequent hemorrhagic event, and other events (GI hemorrhage, GU hemorrhage, hemoptysis, pulmonary hemorrhage, cerebral hemorrhage) were just cited as less frequent. In the incidence analysis, 1,027 patients were included for all-grade hemorrhagic events, and 1,147 were included for high-grade hemorrhagic events. The numerical difference was due to the fact that some trials reported only high-grade but not all-grade hemorrhagic events [18, 21]. The overall incidence of all-grade and high-grade hemorrhagic events was 22.1 % (95 % CI, 16.5–29.7 %) and 4.2 % (95 % CI, 3.9–4.6 %), respectively.
Our analysis data from randomized controlled trials showed a significant two-times risk of hemorrhagic events with aflibercept. The relative risks of hemorrhagic events of aflibercept compared to control were increased for all-grade (RR = 2.63; 95 % CI, 2.07–3.34) and for high-grade (RR = 2.45; 95 % CI, 1.62–3.72) hemorrhagic events. Data were insufficient to analyze the differences of various underlying malignancies. As an exploratory analysis, we analyzed the risk of specified and unspecified hemorrhagic events with aflibercept. Results showed that the risk specified all-grade and high-grade hemorrhagic events were significantly increased with the use of aflibercept.
We also explore the difference in the incidence of hemorrhagic events associated with aflibercept compared with bevacizumab. The results show that the risk of developing high-grade hemorrhagic events with aflibercept is comparable to that of bevacizumab. We did not compare the incidence of all-grade hemorrhagic events between aflibercept and bevacizumab because the meta-analysis regarding hemorrhagic events in cancer patients treated with bevacizumab did not analyze all-grade hemorrhagic events [27]. As the development of aflibercept continues, this agent will come to head-to-head comparison with bevacizumab and VEGFR TKIs (sunitinib, sorafenib, pazopanib, cediranib, axitinib, and so on).
Hemorrhagic event is associated with aflibercept, which is highlighted by a black-box warning issued by the US Food and Drug Administration, recommending monitoring patients for signs and symptoms of GI bleeding and other severe bleeding For patients with high-grade hemorrhagic events, aflibercept should not be administered.
Our meta-analysis has several limitations. First, these studies are conducted at various institutions by different investigators and may have potential bias in reporting the types of adverse events. Secondly, our meta-analysis was based on data from trials that have published results in the literature, but not individual patient data. Thirdly, there was heterogeneity among the primary studies. It is possibly due to different design of the clinical trial and modes of treatment used in each study. In addition, our meta-analysis precludes a more comprehensive analysis such as adjusting for baseline factors and other differences that existed between the trials from which the data were pooled.
In summary, our meta-analysis is the first study to systematically estimate the incidence and relative risk of hemorrhagic events associated with aflibercept in cancer patients. The current analysis suggested that the use of aflibercept increased the risk of all-grade and high-grade hemorrhagic events. The relative risks of hemorrhagic events of aflibercept compared to control were increased for all-grade and high-grade hemorrhagic events. These results would provide important information for clinicians who use aflibercept to treat patients with solid tumors.
References
Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611.
Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49.
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–8.
Elice F, Rodeghiero F. Side effects of anti-angiogenic drugs. Thromb Res. 2012;129 Suppl 1:S50–3.
Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28(13):2280–5.
Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–37.
Fischer A, Wu S, Ho AL, Lacouture ME. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Investig New Drugs. 2013;31(3):787–97.
Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335–71.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Tew WP, Colombo N, Ray-Coquard I, Del Campo JM, Oza A, Pereira D, et al. Intravenous aflibercept in patients with platinum-resistant, advanced ovarian cancer: results of a randomized, double-blind, phase 2, parallel-arm study. Cancer. 2014;120(3):335–43.
Gotlieb WH, Amant F, Advani S, Goswami C, Hirte H, Provencher D, et al. Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol. 2012;13(2):154–62.
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–506.
Tang PA, Cohen SJ, Kollmannsberger C, Bjarnason G, Virik K, MacKenzie MJ, et al. Phase II clinical and pharmacokinetic study of aflibercept in patients with previously treated metastatic colorectal cancer. Clin Cancer Res. 2012;18(21):6023–31.
Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol. 2012;30(29):3640–7.
Leighl NB, Raez LE, Besse B, Rosen PJ, Barlesi F, Massarelli E, et al. A multicenter, phase 2 study of vascular endothelial growth factor trap (aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol. 2010;5(7):1054–9.
Tannock IF, Fizazi K, Ivanov S, Karlsson CT, Flechon A, Skoneczna I, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol. 2013;14(8):760–8.
Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49(12):2633–42.
Mackay HJ, Buckanovich RJ, Hirte H, Correa R, Hoskins P, Biagi J, et al. A phase II study single agent of aflibercept (VEGF Trap) in patients with recurrent or metastatic gynecologic carcinosarcomas and uterine leiomyosarcoma. A trial of the Princess Margaret Hospital, Chicago and California Cancer Phase II Consortia. Gynecol Oncol. 2012;125(1):136–40.
Coleman RL, Sill MW, Lankes HA, Fader AN, Finkler NJ, Hoffman JS, et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;127(3):538–43.
Tarhini AA, Frankel P, Margolin KA, Christensen S, Ruel C, Shipe-Spotloe J, et al. Aflibercept (VEGF Trap) in inoperable stage III or stage iv melanoma of cutaneous or uveal origin. Clin Cancer Res. 2011;17(20):6574–81.
de Groot JF, Lamborn KR, Chang SM, Gilbert MR, Cloughesy TF, Aldape K, et al. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2011;29(19):2689–95.
Twardowski P, Stadler WM, Frankel P, Lara PN, Ruel C, Chatta G, et al. Phase II study of Aflibercept (VEGF-Trap) in patients with recurrent or metastatic urothelial cancer, a California Cancer Consortium Trial. Urology. 2010;76(4):923–6.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–91.
Kilickap S, Abali H, Bevacizumab CI. bleeding, thrombosis, and warfarin. J Clin Oncol. 2003;21(18):3542. author reply 3.
Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–95.
Hang XF, Xu WS, Wang JX, Wang L, Xin HG, Zhang RQ, et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2011;67(6):613–23.
Qi WX, Tang LN, Sun YJ, He AN, Lin F, Shen Z, et al. Incidence and risk of hemorrhagic events with vascular endothelial growth factor receptor tyrosine-kinase inhibitors: an up-to-date meta-analysis of 27 randomized controlled trials. Ann Oncol. 2013;24(12):2943–52.
Acknowledgments
We are indebted to the authors of the primary studies. This study was supported by a grant from the Natural Science Foundation of Zhejiang Province, China (Grant number: LQ13H160010) and a grant from Doctoral Fund of Ministry of Education of China (Grant Number: 20130101120091).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, L., Bu, Z., Zhou, Y. et al. Hemorrhagic events in cancer patients treated with aflibercept: a meta-analysis. Tumor Biol. 35, 9419–9427 (2014). https://doi.org/10.1007/s13277-014-2189-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2189-1