Abstract
Purpose
High-grade bleeding is a serious adverse event associated with bevacizumab, a humanized monoclonal antibody targeting vascular endothelial growth factor and widely used in the current cancer treatments. The aim of this study was to gain a better understanding of the overall incidence and risk of high-grade bleeding in cancer patients who receive bevacizumab therapy.
Methods
We performed a meta-analysis of relevant randomized controlled trials (RCTs) identified in PubMed, Cochrane library, Embase, and American Society of Clinical Oncology conferences. Overall relative risks (RRs), incidence rates, and 95% confidence intervals (CIs) were calculated using a random-effects model. The primary clinical endpoint was high-grade bleeding (grade 3 or above).
Results
A total of 14,277 patients with a variety of solid tumors from 22 RCTs were included in the present analysis. The addition of bevacizumab to cancer chemotherapy significantly increased the risk of high-grade bleeding (RR 1.60, 95% CI 1.19–2.15), with RRs of high-grade bleeding among patients receiving bevacizumab at 2.5 and 5 mg/kg per week of 1.27 (95% CI 0.95–1.71) and 3.02 (95% CI 1.85-4.95), respectively. The overall incidence of high-grade bleeding among patients receiving bevacizumab was 2.8% (95% CI 2.1–3.8). Higher risks were observed in patients with non-small-cell lung cancer (RR 3.41, 95% CI 1.68–6.91), renal cell carcinoma (RR 6.37, 95% CI 1.43–28.33), and colorectal cancer (RR 9.11, 95% CI 1.70–48.79) who were receiving bevacizumab at 5 mg/kg per week.
Conclusions
Among the patients included in the trials analyzed in this meta-analysis, the addition of bevacizumab to cancer chemotherapy significantly increased the risk of high-grade bleeding. The risk may be dose-dependent and may vary with tumor type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Angiogenesis plays a pivotal role in tumor growth, progression, and metastasis [1, 2], and the inhibition of angiogenesis has been a major focus of new cancer therapeutics. In the past several years, angiogenesis inhibitors targeting the vascular endothelial growth factor (VEGF) pathway have come under extensive investigation. Bevacizumab (Avastin; Genentech, South San Francisco, CA), a recombinant humanized monoclonal antibody against VEGF, is widely used in current cancer treatments. It is the first angiogenesis inhibitor approved as a first-line treatment for metastatic colorectal cancer in combination with fluorouracil-based and oxaliplatin-based chemotherapy [3–9]. Bevacizumab has also been approved for the treatment of advanced non-small-cell lung cancer in combination with carboplatin and paclitaxel [10]. Additionally, bevacizumab has been shown to improve progression-free survival in patients with metastatic renal cell carcinoma and metastatic breast cancer [11–15]. The efficacy of bevacizumab in many other malignancies, such as ovarian cancer and gastric cancer, is currently undergoing extensive assessment.
As with other angiogenesis inhibitor drugs, bevacizumab is associated with substantial side-effects, such as hypertension, proteinuria, bleeding, arterial and venous thromboembolic events, and wound-healing complications [16]. High-grade bleeding associated with bevacizumab is a common side-effect observed in clinical trials and may have life-threatening consequences [4, 5, 7–15, 17–27]. However, the risk of high-grade bleeding in cancer patients receiving bevacizumab that has been reported in clinical trials has not been completely consistent, and none of these trials was large enough to define the overall risk. In addition, an individual trial may be limited to the study of one tumor type. Therefore, we propose that pooling analyses of the current studies may provide a better understanding of the overall risk of high-grade bleeding among cancer patients who receive bevacizumab. To address this issue, we carried out a systematic review of the published studies and combined the results from relevant randomized controlled trials (RCTs) for a meta-analysis.
Methods
Data sources
The study was performed using a prespecified search strategy and study eligibility criteria. We did an extensive search of PubMed (up to September 2010), Cochrane Central Register of Controlled Trials (up to Cochrane Library Issue 3, 2010), and Embase (1980 to September 2010) with the aim of identifying relevant RCTs for our meta-analysis. Abstracts and meeting presentations from the American Society of Clinical Oncology conferences held between January 2000 and September 2010 were also searched for relevant RCTs. We restricted the search to RCTs. Search term combinations were “bevacizumab”, “avastin”, and “cancer”. The language of the research papers was not restricted. All reference lists from the relevant articles and reviews were hand searched for additional eligible studies. Experts in the field were also consulted, who subsequently confirmed the results of the search for RCTs and were unable to identify any additional eligible study. The articles that were not freely available to us were requested from the authors.
Study selection
Two reviewers (XFH and WSX) independently carried out a literature search and examined relevant RCTs for further assessment. Only those RCTs that directly compared cancer patients treated with and without bevacizumab, respectively, were selected for analysis. Phase I and single-arm phase II trials were excluded due to their lack of control groups. Specifically, clinical trials that met the following criteria were included in the meta-analysis: (1) prospective phase II and phase III RCTs in patients with cancer; (2) random assignment of participants to bevacizumab treatment or control groups in addition to concurrent chemotherapy and/or treatment with a biological agent; (3) available data, including the event or incidence of bleeding and sample size for analysis.
Qualitative assessment
Evaluation of the methodological quality of the RCTs included in the meta-analysis was performed independently by two reviewers (XFH and WSX) using the Jadad scoring system as follows [28]. One point was awarded for the presence of randomization, blinding, and data on study withdrawals, respectively. If the randomization or blinding procedures was appropriate, one point was awarded for each procedure; no points were awarded if no data were provided on the methodology of the above-mentioned procedures. Finally, if any of these procedures were not deemed appropriate, one point was deducted for each of the “inappropriate” procedures. The maximum score that could be attributed to an RCT was 5. An RCT with a score >2 was considered to be an RCT of adequately good quality [29, 30].
Data extraction
Two reviewers (XFH and WSX) independently extracted data from the trials included in the meta-analysis using a predesigned review form. In the case of any disagreement between the two reviewers, a third reviewer extracted the data, and the results were attained by consensus. We contacted the authors of trials for the missing data when necessary. Data on study characteristics (methodology, underlying malignancy of included patients, concurrent treatment, follow-up duration, number of patients, bevacizumab dose, and publication details) and clinical endpoints were extracted.
Clinical endpoint
Bleeding events in the safety profile of each trial were selected as the main clinical endpoint of our meta-analysis and recorded in the included trial according to versions 1, 2, or 3 of the Common Terminology Criteria for Adverse Events (CTCAE; http://ctep.cancer.gov). These three versions are similar in terms of their grading of bleeding, with grade 1 indicating mild bleeding (intervention not indicated), grade 2 indicating symptomatic bleeding (medical intervention indicated), grade 3 indicating the need for transfusion, interventional radiology, or endoscopic or operative intervention (i.e., hemostasis of bleeding site), grade 4 indicating life-threatening consequences (major urgent intervention indicated), and grade 5 indicating death. We included the incidence of allgrade and high-grade bleeding (grade 3 or above) in our analysis.
Data analysis and statistical methods
We used Stata ver. 10.0 (StataCorp, College Station, TX) for all statistical analyses. The number of patients with bleeding was summarized from the data extracted on all patients assigned bevacizumab treatment in the individual trial. For each trial, the proportion of patients with bleeding was calculated and the 95% confidence interval (CI) was derived. We explored a dose–effect relationship by further dividing bvacizumab therapy into low-dose (5 or 7.5 mg/kg per dose per schedule, which is equivalent to 2.5 mg/kg per week) and high-dose (10 or 15 mg/kg per dose per schedule, which is equivalent to 5 mg/kg per week) therapy. The relative risk (RR) of bleeding in patients assigned to bevacizumab treatment was also calculated and compared only with those assigned to a control treatment in the same trial.
For the meta-analysis, we used a random-effects model applying the method of DerSimonian and Laird, which accounts for both within-study and between-study variation [31]. We assessed statistical heterogeneity among studies included in our meta-analysis with the Q statistic, and we quantified inconsistency with the I 2 statistic. We judged as invalid the assumption of heterogeneity if p < 0.1. To investigate possible reasons for heterogeneity, we did subgroup analyses by underlying malignant disease. The publication bias was assessed by examining the funnel plot.
Results
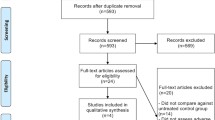
The flow diagram (Fig. 1) shows the detailed screening and selection process that we applied before including trials in our meta-analysis. The search was performed in PubMed, the Cochrane Central Register of Controlled Trials, Embase, and the American Society of Clinical Oncology conferences. We obtained 61 full papers from 357 studies for detailed evaluation and ultimately identified 22 RCTs, including eight phase II and 14 phase III studies, which fulfilled all of the criteria for inclusion in the meta-analysis.
The main characteristics of the 22 included RCTs are presented in Table 1. Malignant diseases included were non-small-cell lung cancer (four studies), colorectal cancer (eight studies), breast cancer (three studies), renal-cell carcinoma (three studies), pancreatic carcinoma (two studies), gastric cancer (one study), and mesothelioma (one study). The total population of the included trials comprised 14,277 patients. Patients were enrolled according to prespecified eligibility criteria for each trial. The baseline Eastern Cooperative Oncology Group (ECOG) performance status for most patients was between 0 and 1. Randomized treatment allocation sequences were generated in all trials. Eight trials were double-blinded and placebo controlled [12, 15, 17, 21–25]; three other trials had placebo as controls [5, 8, 27]; the rest of the trials had active controls [4, 7, 9–11, 13, 14, 18–20, 26]. Bleeding was assessed and recorded according to the CTCAE version 1, 2, or 3 criteria. Version 1 was used in two trials [7, 18], version 2 was used in eight trials [4, 5, 9–11, 13, 19, 24], and version 3 was used in two trials [8, 15]; the remainder of the trials did not specify the CTCAE version. Follow-up time was not specified in eight trials [7, 14, 17, 20, 23, 25–27]. The quality of all the trials included in the meta-analysis was acceptable. We examined the funnel plot [standard error (SE) of log RRs plotted against RRs) to estimate publication bias and obtained a symmetric inverse funnel distribution.
In the present meta-analysis, we calculated the overall RR of high-grade bleeding associated with bevacizumab treatment compared with the control treatment. Data were available for 7136 patients who received bevacizumab and 6,310 patients who received control treatments in the 22 trials included in our meta-analysis. The risk of high-grade bleeding in controls was very low in most of the studies. The overall RR of high-grade bleeding associated with bevacizumab versus the control was 1.60 (95% CI 1.19–-2.15) (Fig. 2). We found the overall RR of all bleeding was 2.65 (95% CI 2.08–3.38).
The incidence of high-grade bleeding ranged between 0.4 and 7.7%, with the highest and lowest incidence reported in the trial performed by Herbst et al. [19] and and Miller et al. [13], respectively. Using a random-effects model, we determined that the overall incidence of high-grade bleeding in patients receiving bevacizumab was 2.8% (95% CI, 2.1–3.8%) (Fig. 3) and that the incidence of all-grade bleeding was 25% (95% CI 18–34%).
To understand further the role of bevacizumab in the pathogenesis of high-grade bleeding, we assessed whether the dose of bevacizumab is related to the risk of developing high-grade bleeding. The RR of high-grade bleeding associated with low-dose bevacizumab (2.5 mg/kg per week) was 1.27 (95% CI 0.95–1.71) as calculated from 11 RCTs (Fig. 4), while the RR for high-dose bevacizumab (5 mg/kg per week) was 3.02 (95% CI 1.85–4.95) as calculated from 11 RCTs (Fig. 5). Based on these results, it would appear that the risk of high-grade bleeding with bevacizumab treatment was dose dependent among the patients in these trials.
Patients with different tumors might be at different risks of bleeding due to differences in tumor biology and the associated treatment. We determined whether having a specific type of cancer was associated with a higher risk for high-grade bleeding relative to other cancers. As shown in Fig. 5, the risk of high-grade bleeding varied according to tumor type. Relatively high RRs for high-grade bleeding associated with high-dose bevacizumab were found among patients with non-small-cell lung cancer (RR 3.41, 95% CI 1.68– 6.91), renal cell carcinoma (RR 6.37, 95% CI 1.43–28.33), and colorectal cancer (RR 9.11, 95% CI 1.70–48.79); relatively low RRs were seen in patients with metastatic breast cancer (RR 1.33, 95% CI 0.32–5.46) and pancreatic cancer (RR 1.54, 95% CI 0.51–4.64). Similarly, the overall incidence of high-grade bleeding in patients receiving high-dose bevacizumab was 3.2% (95% CI, 2.1–4.7%), with a relatively high incidence in non-small-cell lung cancer patients (5%, 95% CI 3.6–6.9%) and colorectal cancer patients (5.1%, 95% CI 2.2–12.2%) and a relatively low incidence in metastatic breast cancer patients (0.6%, 95% CI 0.2–1.7%)(Fig. 6). The addition of low-dose bevacizumab to cancer therapy did not significantly increase the risk of high-grade bleeding (Fig. 4), and the different type of cancer patients receiving low-dose bevacizumab therapy had a similar incidence of high-grade bleeding (data not shown).
Discussion
The study presented here is a systematic review with meta-analysis aimed at investigating the overall incidence and risk of high-grade bleeding among cancer patients who receive bevacizumab therapy. The results showed that the addition of bevacizumab to cancer therapy significantly increased the risk of high-grade bleeding, with an overall RR of 1.60 (95% CI 1.19–2.15) and incidence of 2.8% (95% CI 2.1–3.8%) among patients with a variety of solid tumors from 22 RCTs (Figs. 2, 3). In addition to bevacizumab, other angiogenesis inhibitors, such as sorafenib and sunitinib, which block the activity of VEGF receptors, are also associated with a significant increase in the risk of bleeding [32]. Based on the results of previous studies, the incidences of high-grade bleeding for sorafenib and sunitinib are 2.2 (95% CI 1.3–3.6) and 3.0% (95% CI 1.3–6.8) [32], respectively; in comparison, we found an incidence of 2.8% (95% CI 2.1–3.8%) for bevacizumab in this study. Thus, it would appear that the absolute risk of high-grade bleeding induced by these different angiogenesis inhibitors is similar.
The association of bevacizumab with bleeding may be directly related to its inhibitory effect on VEGF signaling. VEGF is important for endothelial cells to maintain the architecture and integrity of the microvasculature. When VEGF signaling is blocked by bevacizumab, the repair and renewal capacity of endothelial cells in response to trauma could be impaired, which would induce the increased risk of bleeding [33, 34]. In clinical trials, the onset of high-grade bleeding in bevacizumab-treated patients can occur at any time during therapy. However, the risk factors for the development of high-grade bleeding, an important issue in reducing the risk of occurrence, have not been fully elucidated.
The bleeding associated with bevacizumab treatment may be attributable to known risk factors, such as age, race, sex, bevacizumab dose, and underlying cancer, or to the concurrent use of anticoagulants. We first assessed whether the dose of bevacizumab was related to the risk of high-grade bleeding. We were unable to identify an association between a dose–response for bevacizumab and high-grade bleeding in any one trial included in the meta-analysis. For example, Miller et al. [13] reported the lowest rate of high-grade bleeding (0.4%) with bevacizumab 5 mg/kg per week, while Cutsem et al. [21] reported the highest rate of high-grade bleeding (7%) with bevacizumab 2.5 mg/kg per week. The findings of our meta-analysis suggest that the increased risk of high-grade bleeding is dose dependent, as the overall RR of high-grade bleeding was found to be greater in patients receiving a dose of 5 mg/kg per week than in those receiving a dose of 2.5 mg/kg per week (Figs. 4, 5). The addition of bevacizumab 5 mg/kg per week to the cancer chemotherapy regimen significantly increased the risk of high-grade bleeding, while the addition of bevacizumab 2.5 mg/kg per week did not.
Our study also showed that the risk of high-grade bleeding with bevacizumab was relatively higher in patients with certain type of tumors who had received bevacizumab (Figs. 5, 6), suggesting that the risk may vary with tumor type. For the dose of 5 mg/kg per week, the overall RR and incidence were relatively higher in patients with non-small-cell lung cancer, renal cell carcinoma, and colorectal cancer (Fig. 5). The potentially higher RR of bevacizumab-related high-grade bleeding in patients with non-small-cell lung cancer is associated with squamous histology, tumor location close to major blood vessels, and tumor necrosis or cavitation [35]. Sandler et al. suggested that baseline cavitation was the main risk factor of high-grade bleeding for patients with non-small-cell lung cancer [36]. Bevacizumab is believed to cause central necrosis and to enlarge the tumor cavity in these patients. This, in combination with the immature blood vessels within the cavity, increases the risk of bleeding. The potentially higher RR of bevacizumab-related high-grade bleeding in patients with renal cell cancer may be due to the unique biology of renal cell cancer itself. Renal cell cancer patients usually have an inactivation of the von-Hippel Lindau (VHL) gene, which could upregulate VEGF [37]. Therefore, blockade of the VEGF pathway in patients with renal cell tumors who have pre-existing upregulated VEGF in the endothelial cell microenvironment may substantially impair endothelial function, thereby increasing the risk of bleeding. In addition, many patients with renal cell cancer have renal insufficiency, resulting in a decreased clearance of bevacizumab. Patients with colorectal cancer also have a higher risk of high-grade bleeding. Flynn et al. reported that the most common site of bevacizumab-related high-grade bleeding in patients with colorectal cancer is the gastrointestinal/rectal tract [38]. These investigators found that patients with primary tumor of the rectum had a higher rate of high-grade bleeding than did those with primary tumor of the colon, suggesting that the location of the primary cancer may be associated with the risk of high-grade bleeding. However, as yet there is a lack of data on the possible mechanism. Studies focusing on this issue are required.
Although the study recently published by Hapani et al. comprised a meta-analysis of the overall risk of bevacizumab-associated bleeding, the risk factors of bleeding were not clearly elucidated [39]. We obtained similar results in terms of the overall incidence of bevacizumab-associated bleeding as Hapani et al. In contrast to this earlier meta-analysis, we examined 22 RCTs with more patients and paid more attention to the risk factors for the development of bevacizumab-associated high-grade bleeding, an important issue in reducing the risk of occurrence. However, any meta-analysis is not without limitations. First, our findings were affected by the limitations of the individual clinical trials included in the analysis. We attempted to analyze the effect of treatment duration on the incidence of bleeding events, but data on the occurrence of the bleeding event during the course of the respective trial were frequently not reported. Furthermore, the risk of bleeding can be increased by the concurrent use of anti-coagulants (e.g., warfarin), antiplatelet treatment (e.g., aspirin, clopidogrel, ticlopidine), or both. However, such patients are sometimes excluded from clinical trials, even though the use of these drugs is very common in clinical practice, specifically in individuals with malignant disease. Second, heterogeneity is associated with some relevant aspects (such as patient clinical profiles, concurrent chemotherapy, and lengths of follow-up). However, differences among trials are inevitable since each individual trial looks at a different population(s) with different treatment protocols, and there is always some heterogeneity, even within individual trials. But heterogeneity does not preclude pooling of their results because individual patient is directly compared only with other patients within the same trial, and not across the trials [40, 41]. Given this uncertainty resulting from clinical heterogeneity, we performed subgroup analysis in our meta-analysis. Third, although our findings showed that the patients with non-small-cell lung cancer, renal cell carcinoma, and colorectal cancer have a higher risk of high-grade bleeding, this finding might be limited by the small sample size of patients with each of the tumor type. Finally, except for tumor type and bevacizumab dose, other potential risk factors, such as age, race, sex, and history of hematologic diseases, could not be evaluated in our meta-analysis.
In conclusion, despite the limitations of our meta-analysis, we conclude that the addition of bevacizumab to a cancer chemotherapy regimen is associated with a significantly increased risk of developing high-grade bleeding. The risk may be dose-dependent, and it may vary with tumor type. Clinicians should be aware of the possibility that any patient treated with bevacizumab may develop high-grade bleeding, especially in patients at high risk. Future studies are recommended to investigate risk reduction and the possible use of bevacizumab in selected patients.
Reference
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29:15–18
Gerber HP, Ferrara N (2005) Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 65:671–680
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 23:3502–3508
Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60–65
Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF (2005) Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 23:3697–3705
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Miles D, Chan A, Romieu G, Dirix LY, Cortes J, Pivot X, Tomczak P, Taran T, Harbeck N, Steger GG (2008) Randomized, double-blind,placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO. Proc Am Soc Clin Oncol 26:LBA1011
Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 26:5422–5428
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a odifyzed, double-blind phase III trial. Lancet 370:2103–2111
Genentech. Bevacizumab prescribing information. Available at; http://www.Gene.com/gene/products/information/oncology/avastin/. Accessed 27 Oct 2008
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27:1227–1234
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22:2184–2191
Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A (2007) Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 25:474–4750
Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Wolmark N (2008) Initial safety report of NSABP C-08, a randomized phase III study of odify ed 5-fluorouracil (5-FU)/leucovorin (LCV) and oxaliplatin (OX) (mFOLFOX6) with or without bevacizumab (bev) in the adjuvant treatment of patients with stage II/III colon cancer. Proc Am Soc Clin Oncol 26 [15 Suppl]: Abstr 4006
Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27:2231–2237
Kindler HL, Niedzwiecki D, Hollis D, Oraefo E, Schrag D, Hurwitz H, McLeod HL, Mulcahy MF, Schilsky RL, Goldberg RM (2007) A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): a preliminary analysis of Cancer and Leukemia Group B (CALGB). Proc Am Soc Clin Oncol 25:4508
Karrison T, Kindler HL, Gandara DR, Lu C, Guterz TL, Nichols K, Chen H, Stadler WM, Vokes E (2007) Final analysis of a multi-center, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin (GC) plus bevacizumab (B) or placebo (P) in patients (pts) with malignant mesothelioma (MM). J Clin Oncol 25:7526
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 27:2231-2237
Kang Y, Ohtsu A, Van Cutsem E, Rha SY, Sawaki A, Park S, Lim H, Wu J, Langer B, Shah MA (2010) AVAGAST: A randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC). J Clin Oncol 28:LBA4007
Price TJ, Gebski V, van Hazel GA, Robinson BA, Broad A, Ganju V, Cunningham D, Wilson K, Tunney V, Tebbutt NC (2008) International multi-centre randomised Phase II/III study of Capecitabine (Cap), bevacizumab (Bev) and mitomycin C (MMC) as first-line treatment for metastatic colorectal cancer (mCRC): Final safety analysis of the AGITG MAX trial. J Clin Oncol 26:4029
Hambleton J, Novotny WF, Hurwitz H, Fehrenbacher L, Cartwright T, Hainsworth J, Heim W, Berlin J, Kabbinavar F, Holmgren E (2004) Bevacizumab does not pressure bleeding in patients with metastatic colorectal cancer receiving concurrent anticoagulation. J Clin Oncol 22:3528
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Khan KS, Daya S, Jadad A (1996) The importance of quality of primary studies in producing unbiased systematic reviews. Arch Intern Med 156:661–666
Moher D, Jadad AR, Tugwell P (1996) Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care 12:195–208
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Je Y, Schutz FA, Choueiri TK (2009) Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol 10:967–974
Kamba T, McDonald DM (2007) Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96:1788–1795
Kilickap S, Abali H, Celik I (2003) Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol 21:3542–3543
SandlerAB JDH, Brahmer J et al (2006) A study of clinical and radiographic risk factors associated with early onset severe pulmonary hemorrhage in bevacizumab (Avastin) treated patients with advanced non-small cell lung cancer. Proc Am Soc Clin Oncol 24:7068
Sandler AB, Schiller JH, Gray R, Dimery I, Brahmer J, Samant M, Wang LI, Johnson DH (2009) Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with Carboplatin and Paclitaxel plus bevacizumab. J Clin Oncol 27:1405–1412
Choueiri TK, Vaziri SA, Jaeger E, Elson P, Wood L, Bhalla IP, Small EJ, Weinberg V, Sein N, Simko J, Golshayan AR, Sercia L, Zhou M, Waldman FM, Rini BI, Bukowski RM, Ganapathi R (2008) von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol 180:860–866
Flynn PJ, Sugrue MM, Feng S, Purdie DM, Grothey A, Sargent DJ, Berlin JD, Kabbinavar FF, Dong W, Kozloff MF (2008) Incidence of serious bleeding events (sBE) in patients (pts) with metastatic colorectal cancer (mCRC) receiving bevacizumab (BV) as part of a first-line regimen: Results from the BRITE observational cohort study (OCS). J Clin Oncol 26:4104
Hapani S, Sher A, Chu D, Wu S (2010) Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 79:27–38
Lau J, Ioannidis JPA, Schmid CH (1997) summing up the evidence: one answer is not always enough. Lancet 351:123–127
Thompson SG (1994) Why sources of heterogeneity in metaanalysis should be investigated. Br Med J 309:1351–1355
Acknowledgment
We are indebted to the authors of the primary studies, for without their contributions, this work would have been impossible.
Funding of the study
None
Conflict of interest
The authors state that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao Feng Hang and Wen Sheng Xu contributed equally to this article and can be considered to be co-first authors
Rights and permissions
About this article
Cite this article
Hang, X.F., Xu, W.S., Wang, J.X. et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 67, 613–623 (2011). https://doi.org/10.1007/s00228-010-0988-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0988-x