Abstract
Overwhelming evidence has demonstrated that Bit1 and AIF as mitochondrial proteins are implicated in the development and progression of a variety of tumors. However, their expressions and biological functions in esophageal squamous cell carcinoma (ESCC) remain to be delineated. In the present study, we found that Bit1, AIF, and Bcl-2 levels in ESCC tissues were significantly higher than those in normal esophageal epithelial tissues and dysplasia tissues (P < 0.05). Stepwise investigation demonstrated that Bit1 and Bcl-2 levels were both tightly associated with lymphatic metastasis and TNM staging (P < 0.05), and the levels of Bit1 mRNA as well as AIF and Bcl-2 proteins were both closely related to tumor differentiation (P < 0.05), but not related to the patients' age and gender (P > 0.05). Importantly, Bit1 mRNA and protein levels in ESCC with lymphatic metastasis and TNM staging in III and IV were markedly higher than that without lymphatic metastasis and TMN staging in I and II. Further analysis showed that expression of Bit1 protein was both positively correlated with expressions of AIF and Bcl-2 proteins (r = 0.408 and 0.405, respectively; P < 0.05). Correctively, our data cited earlier suggest that Bit1 plays pivotal roles in the development and progression of ESCC, and its biological functions in ESCC may be closely associated with AIF and Bcl-2 levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer (EC) is one of the most common aggressive human tumors with high morbidity and mortality in the world [1, 2], and high incidence rates have been estimated for Southern and Eastern Africa, Eastern Asia, and north central China [3–6]. At present, most EC falls into two classes: squamous cell carcinoma and adenocarcinoma, in which squamous cell carcinoma represents approximately 90 % of all EC worldwide [7]. Furthermore, the prognosis of esophageal squamous cell carcinoma (ESCC) is quite poor, the overall 5-year survival rate is about 15 %, and most patients harbor distant metastasis at the time of diagnosis [8–10]. Therefore, it is particularly necessary to seek new molecular markers for the diagnosis and therapy of patients with ESCC.
Bcl-2 inhibitor of transcription 1 (Bit1) protein encodes 179 amino acids with a known structure for its putative active site [11]. Upon loss of cellular adherence (anoikis) of both tumor and normal cells, Bit1, described as a mitochondrial protein, elicits apoptosis when released from the mitochondria into the cytosol but turns out to be the precursor of mitochondrial peptidyl-tRNA hydrolase 2 [11], indicating that Bit1 is involved in cell apoptosis. Recently, Bit1 was found to be tightly associated with the occurrence and development of cervical carcinoma and was required for the survival of cervical cancer Hela cells [12]; an opposite report about Hela cells revealed that the stable Bit1 knockdown contributed to the proliferation of Hela cells [13]. Alternatively, Bit1 level was significantly inhibited in an advanced stage of breast cancer and inhibition of Bit1 level improved cell adhesion and migration, suggesting that Bit1 functions as a tumor suppressor gene in breast carcinoma [13]. Recent investigation revealed that Bit1 level in ovarian cancer was significantly higher than that in normal epithelial tissues [14], suggesting that Bit1 functions as an oncogene in ovarian cancer. These conflicting data delineating the opposite functions of Bit1 in different tumors as a dominant oncogene or tumor suppressor gene may be mainly dependent on the type of tumors, which will impel us to investigate the function and expression of Bit1 in ESCC.
Likewise, apoptosis-inducing factor (AIF) is implicated in cell apoptosis in a caspase-independent manner, which mainly occurs when it translocates to cell nucleus [15]. Recently, multiple studies focus on the relationship between AIF and tumors, including small lymphocytic lymphoma [16], diffuse large B-cell lymphoma [16], colorectal cancer [17], lung adenocarcinoma [18], ovarian cancer [19], etc. More notably, our previous study demonstrated that TAp63γ expression evoked the release of AIF and Bit1 from the mitochondria to trigger cell apoptosis in ESCC [20]. Moreover, Bcl-2 family proteins, whose members have both anti-apoptotic and pro-apoptotic activity, have a central role in the control of mitochondria-induced apoptosis [21]. These data suggest that Bit1, AIF, and Bcl-2 may have common intrinsic connections in the development and progression of a variety of tumors.
However, to date, the functions and intrinsic correlations of Bit1, AIF, and Bcl-2 in ESCC remain elusive; therefore, in the present study, we investigated the Bit1, AIF, and Bcl-2 levels in ESCC tissues, analyzed the correlations between Bit1, AIF, and Bcl-2 levels and clinicopathological features, and further investigated the correlations of Bit1 and AIF as well as Bcl-2 in ESCC tissues. Our preliminary results presented herein will provide the valuable basis for stepwise function analysis of Bit1 in ESCC.
Materials and methods
Tissue samples
Frozen esophageal tissues for quantitative real-time PCR obtained in the period October to November of 2010 were cut off during the operations in the department of cardiothoracic surgery in the Cancer Hospital of Anyang, Henan Province. Forty-eight cases of ESCC specimens (33 highly and moderately differentiated and 15 lowly differentiated), 50 cases of tumor-adjacent dysplasia specimens, and 50 cases of adjacent tumor-free esophageal specimens matched with the ESCC specimens were enrolled in this study.
Paraffin-embedded sections of esophageal tissues for immunohistochemistry analysis obtained in the period January to August of 2010 were retrieved from the files of the Department of Pathology, the First Affiliated Hospital of Zhengzhou University. Among these cases, there were three groups which included 60 cases of ESCC specimens (48 highly and moderately differentiated and 12 lowly differentiated), 59 cases of tumor-adjacent dysplasia specimens, and 45 cases of normal epithelial samples.
Written informed consent was taken by the cited participants. None of the patients had radiotherapy or chemotherapy before surgery and were confirmed as ESCC by an experienced pathologist. The clinicopathological characteristics of all the patients are summarized in Tables 2 and 4.
Quantitative real-time RT-PCR
Total RNA was extracted from 100 mg of esophageal frozen specimens by using TRIzol reagent (Invitrogen, USA). The cDNA was prepared using a reverse transcription reagent kit (TaKaRa Company, Dalian, China) according to the manufacturer's instructions. Gene expression was quantified using ABI 7500 Real-Time PCR System (USA) for the target gene Bit1 and the housekeeping gene β-actin with the following primers: Bit1, 5′-AGAGGTAGCTCACGCGATAGAA-3′ (forward) and 5′-GCATCCCAAAGCATACTCGAA-3′ (reverse) (product length, 196 bp) as well as β-actin, 5′-TGGCACCCAGCACAATGAA-3′ (forward) and 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (reverse) (product length, 186 bp); β-actin was utilized as normalization. Quantitative RT-PCR was performed in a total reaction volume of 20 μl containing 10 μl SYBR Premix Ex Taq, 0.4 μl forward primers, 0.4 μl reverse primers, 0.4 μl ROX Reference Dye II, 6.8 μl water, and 2 μl cDNA. Thermal cycling conditions were as follows: 30 s at 95 °C for initial denaturation, followed by 40 cycles of 5 s at 95 °C and 34 s at 60 °C. Each sample was tested in triplicate, and Bit1 relative level was normalized using the 2−ΔΔCt method relative to β-actin according to a previous description [22]. Evaluation of the result of quantitative real-time RT-PCR was performed according to a previous report [23].
Immunohistochemistry
Bit1 protein expression was detected by using the Strept Avidin–Biotin Complex (SABC) Reagent Kit (Boster Biological Engineering Co., Ltd., Wuhan, China) in the three groups of the paraffin-embedded blocks of esophageal tissues with Bit1, Bcl-2, and AIF polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer's procedures. PBS, instead of Bit1, Bcl-2, and AIF primary antibodies, was used as a negative control.
Evaluation of staining
All of the normal and neoplastic cells that both exhibited cytoplasmic immunoreactivity with clearly brown-yellow were regarded as Bit1, Bcl-2, and AIF positive staining. A scoring system for Bit1, Bcl-2, and AIF positive staining was carried out according to a previous publication with minor modification [24]. The percentage of positive staining cells was counted by randomly selecting ten fields to count the positive cells under the high power microscope. The percentage of positive immunostaining cells was scored as follows: 0 (none), 1 (<10 %), 2 (10–50 %), 3 (51–80 %), or 4 (>80 %). Staining intensity was scored in the following: 0 (negative), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). Multiplication of the scores for the percentage of positive staining cells and staining intensity resulted in four classifications from 0 to +++: negative, 0 (score 0–1); weak staining, + (score 2–3); moderate staining, ++ (score 4–5); strong staining, +++ (score 6–7).
Statistical analysis
SPSS 17.0 statistical software package (SPSS, Inc., Chicago, IL, USA) was used for statistical treatment. For the measurement data, data were presented as mean ± SD. Differences among the groups were determined using one-way ANOVA, and then multiple comparisons between groups were performed using LSD t-test. For the count data, rank sum test was used in the comparisons between groups. The relationships among protein expressions in ESCC were performed using Spearman rank correlation test. A value of P less than 0.05 was considered as statistically significant.
Results
Elevated Bit1 mRNA level in ESCC tissues
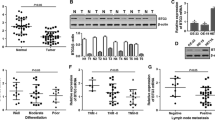
Quantitative real-time RT-PCR was utilized to detect Bit1 mRNA level in 48 cases of ESCC tissues, 50 cases of dysplasia tissues, and 50 cases of normal esophageal epithelial tissues. The results demonstrated that Bit1 mRNA level in ESCC tissues (1.581 ± 1.235) was significantly higher than those in dysplasia tissues (1.141 ± 0.623) and normal esophageal epithelial tissues (1.086 ± 0.588), and there were significant differences among the three groups (F = 4.024, P = 0.020) (Fig. 1; Table 1); conversely, there was no difference in Bit1 mRNA level between dysplasia tissues and normal esophageal epithelial tissues (P > 0.05) (Fig. 1; Table 1). These data presented herein suggest that Bit1 at high levels may be tightly associated with the development and progression of ESCC.
Overexpression of Bit1 mRNA in ESCC. Total RNA was extracted from 48 cases of ESCC tissues, 50 cases of dysplasia tissues, and 50 cases of normal esophageal epithelial tissues subjected to the first-strand cDNA synthesis kit, and quantitative real-time PCR analysis was performed according to “Materials and methods”. a Bit1 mRNA levels in ESCC tissues were significantly higher than that in normal esophageal epithelial tissues, and significant difference was displayed between ESCC tissues and normal esophageal epithelial tissues. b Quantitative real-time PCR analysis revealed higher levels of Bit1 mRNA in ESCC tissues than in dysplasia tissues, and there were evident differences between ESCC tissues and dysplasia tissues
Relationship between Bit1 mRNA level and clinicopathological features in ESCC tissues
To elucidate the possible role of Bit1 mRNA level in the development and progression of ESCC, we performed the investigation regarding the association of Bit1 mRNA level in ESCC patients with various clinicopathological features, such as gender, age, tumor differentiation, lymphatic metastasis, and TNM staging. As indicated in Table 2, a positive correlation between Bit1 mRNA level and lymphatic metastasis, TNM staging, and tumor differentiation was found (both P < 0.05), but not related to the patients' gender and age (all P > 0.05). More notably, Bit1 mRNA level in ESCC tissues with lymphatic metastasis was markedly higher than that in ESCC tissues without lymphatic metastasis (P = 0.001) (Fig. 2a), and similar results were displayed in TNM staging (P = 0.003) (Fig. 2b), implying that Bit1 at a higher level predicts stronger aggressiveness in ESCC.
Bit1 mRNA at high level predicts higher aggressiveness in ESCC. Bit1 mRNA relative levels in ESCC tissues with various clinicopathological features including lymphatic metastasis and TNM staging, and SPSS 17.0 software was utilized to investigate the statistical results. a Bit1 mRNA level in ESCC tissues with lymphatic metastasis was higher than that in ESCC tissues without lymphatic metastasis, and there was significant difference between the two groups. b Bit1 mRNA levels in ESCC tissues with TNM staging in III–IV was higher than that in ESCC tissues with TNM staging in I–II, and there was significant difference between the two groups
Overexpression of Bit1 protein in ESCC tissues
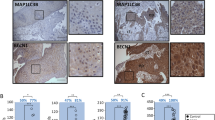
Immunohistochemistry was used to detect the Bit1 protein level in 60 cases of ESCC tissues, 59 cases of dysplasia tissues, and 45 cases of normal esophageal epithelial tissues. The results revealed that Bit1 protein was mainly localized in the cytoplasm of ESCC tissues with brown staining (Fig. 3a–c). Among all the 60 cases of ESCC, 59 cases of dysplasia tissues, and 45 cases of normal esophageal epithelial tissues, 50 (83.33 %), 21 (35.60 %), and three (6.67 %) exhibited the overexpression of Bit1 protein, respectively (Table 3). Compared to that of normal esophageal epithelial tissues and dysplasia tissues, there was an obvious increase in Bit1 protein level in ESCC tissues (P < 0.05) (Table 3). These protein expression profiles suggest that Bit1 may function in the progression of ESCC.
Immunohistochemistry analysis for the expressions of Bit1, Bcl-2, and AIF proteins in various esophageal tissues (SABC, ×400). a Strong positive expression of Bit1 protein in ESCC tissues; b positive expression of Bit1 protein in dysplasia tissues; c negative expression of Bit1 protein in normal esophageal epithelial tissues; d positive expression of Bcl-2 protein in ESCC tissues; e weak positive expression of Bcl-2 protein in dysplasia tissues; f negative expression of Bcl-2 protein in normal esophageal epithelial tissues; g positive expression of AIF protein in ESCC tissues; h weak positive expression of AIF protein in dysplasia tissues; i negative expression of AIF protein in normal esophageal epithelial tissues
Elevated expressions of Bcl-2 and AIF proteins in ESCC tissues
To further delineate the expressions of Bcl-2 and AIF proteins closely related to Bit1 in ESCC tissues, immunohistochemistry was utilized to investigate the expressions of Bcl-2 and AIF proteins in different esophageal tissues. The results revealed that positive expression rate of Bcl-2 protein in ESCC tissues (100 %) was significantly higher than those in dysplasia tissues (91.50 %) and normal esophageal epithelial tissues (77.78 %) (P < 0.05) (Fig. 3d–f; Table 3). In addition, AIF protein level in ESCC tissues (50.00 %) was markedly higher than those in dysplasia tissues (28.81 %) and normal esophageal epithelial tissues (22.22 %) (P < 0.05); however, there was no difference in AIF level between dysplasia tissues and normal esophageal epithelial tissues (P > 0.05) (Fig. 3g–i; Table 3).
Correlations between Bit1 protein expression and clinicopathological features in ESCC tissues
To clarify the potential role of Bit1 protein in the development and progression of ESCC, we performed an investigation about the association of Bit1 protein expression in ESCC patients with various clinicopathological features, such as gender, age, tumor differentiation, lymphatic metastasis, and TNM staging. As shown in Table 4, a positive correlation between Bit1 protein expression and lymphatic metastasis and TNM stage was found (both P < 0.05), but not related to the patients' gender, age, and differentiation (all P > 0.05). More notably, Bit1 protein expression in ESCC tissues with lymphatic metastasis was markedly higher than that in ESCC tissues without lymphatic metastasis (P = 0.000) (Table 4), and similar results were found in TNM staging (P = 0.000) (Table 4), predicting that Bit1 protein at a higher level is tightly associated with stronger aggressiveness in ESCC.
Correlations between Bcl-2 and AIF protein expressions and clinicopathological features in ESCC tissues
To further investigate the possible role of Bit1 in ESCC, we analyzed the relationships between the two proteins, Bcl-2 and AIF, which were closely associated with Bit1 in a variety of tumors [12, 20], and clinicopathological features in ESCC tissues. We found that Bcl-2 expression was closely associated with tumor differentiation, lymphatic metastasis, and TNM stage (P < 0.05), but not related to the patients' age and gender (P > 0.05) (Table 4). Most remarkably, AIF was only associated with tumor differentiation in all studied clinicopathological features (P < 0.05) (Table 4). These data suggest that Bcl-2 and AIF may play an important role in the development and progression of ESCC.
Expression of Bit1 protein is positively correlated to the levels of Bcl-2 and AIF proteins
It is documented that Bit1 exerting biological functions is closely associated with Bcl-2 and AIF expressions in a variety of tumors [12, 20]. To preliminarily elucidate the possible molecular mechanisms of Bit1-mediated apoptosis, we explored the relationship between Bit1 protein expression and Bcl-2 as well as AIF proteins expressions. We found that expressions of Bcl-2 and AIF proteins were positively correlated with expression of Bit1 protein (r = 0.405 and 0.408, respectively; both P < 0.05) (Table 5).
Discussion
Increasing evidence has demonstrated that Bit1 is implicated in various biological processes during the development and progression of tumors, including proliferation, apoptosis, survival, invasion, and metastasis [12–14, 20, 25–27]; most importantly, Bit1 plays an essential role in a large number of signaling pathways closely related to the development and progression of tumors, such as NF-kappa B signaling pathway [12], ERK signaling pathway [25], integrin-specific signaling pathway [28], etc., implying the important function of Bit1 in the development and progression of multiple different tumors. Recently, Hua et al. [14] performed the investigation regarding the expression of Bit1 in 78 cases of serious papillary adenocarcinoma and 78 cases of corresponding normal epithelial ovarian tissues by immunohistochemistry; the result revealed that positive ratios of Bit1 expression were 100 % in 78 cases of serious papillary adenocarcinoma tissues but only 33.3 % of positive ratios in 78 cases of corresponding normal epithelial ovarian tissues, suggesting that Bit1 was tightly associated with ovarian serious papillary adenocarcinomas. Further investigation demonstrated that Bit1 overexpression in ovarian cancer was markedly correlated with histologic grade and overall survival rate [14]. These studies gave prominence to the important role of Bit1 in the development and progression of tumors. Therefore, in the present study, we investigated Bit1 mRNA and protein level by quantitative real-time RT-PCR and immunohistochemistry; we found that Bit1 expression in ESCC tissues was significantly higher than those in dysplasia tissues and normal esophageal epithelial tissues (P < 0.05), suggesting that Bit1 may function as an oncogene in the progression of ESCC.
It is well documented that Bcl-2 and AIF expressions are tightly associated with the development and progression of tumors [29–33], especially implicated in apoptosis of tumor cells [15, 19, 34, 35], which may be an underlying molecular therapeutic target [35, 36]. Bcl-2 as an extensively investigated apoptosis-inhibitive gene has been demonstrated to be overexpressed in multiple different tumors including breast carcinoma [37] and gastric carcinoma [38], but only 18 % overall expression for ESCC [39]. Jeong et al. [40] found that AIF protein expression was detected in both cancer cells and normal mucosal epithelial cells in all of the 103 colorectal carcinoma tissues with higher intensities of AIF immunostaining than the normal cells in 83 cases (80.5 %). Additionally, an increased expression of AIF in malignant gastric epithelial cells compared to the normal mucosal cells was also found [41]. These investigations indicate that Bcl-2 and AIF proteins play pivotal roles in the development and progression of the tumors. To further clarify the role of Bcl-2 and AIF proteins in ESCC, expressions of Bcl-2 and AIF proteins were analyzed by immunohistochemistry. We found that the positive expression rate of Bcl-2 in ESCC was 100 %, which may be somewhat different from the results of Hsia et al. [39]. The result of AIF expression in ESCC displayed a similar tendency with that of Bcl-2; however, there was no difference in AIF level between dysplasia tissues and normal esophageal epithelial tissues. Our results suggest that Bcl-2 and AIF at a high level may play a central role in the occurrence and development of ESCC; however, the precise molecular mechanism for this event remains to be elucidated.
Increasing evidence has demonstrated that clinicopathological features, such as tumor differentiation, TNM stage, and lymph node metastasis, are the most critical prognostic factors for determining the clinical outcomes in esophageal cancer [42]. To date, few studies that examine the relationships between Bit1 and AIF expressions and clinicopathological features of ESCC have been available. Here, to investigate the potential role of the Bit1 and AIF expressions in the development and progression of ESCC, we analyzed the correlations of Bit1 mRNA and protein as well as AIF protein with clinicopathlogical parameters of ESCC. We found a positive correlation of Bit1 protein expression with lymphatic metastasis and TNM stage, but not related to the patients' gender, age, and differentiation. Stepwise analysis revealed that Bit1 mRNA and Bcl-2 overexpressions were closely associated with tumor differentiation, lymphatic metastasis, and TNM stage, but AIF overexpression was only related to tumor differentiation. Notably, discrepancy of Bit1 mRNA and protein in tumor differentiation may be involved in the changes of transcriptional and translational levels of Bit1; nevertheless, precise mechanisms for this event remain to be identified. Collectively, these data suggest that a combination of Bit1 and Bcl-2 may be a novel predictor for ESCC metastasis; further investigations regarding the relationship of the three proteins remain to be explored.
Bit1 was previously reported to be a mitochondrial protein that regulated cell death through modulating Bcl-2 expression [12]. Ectopic expression of Bcl-2 had no effect on apoptosis mediated by cytoplasmically expressed Bit1; however, these proteins partially prevented AES-induced apoptosis, possibly by stabilizing the mitochondria and thereby suppressing the release of endogenous mitochondrial Bit1 [28]. Our previous investigation demonstrated that mitochondria-released proapoptotic proteins, AIF and Bit1, were important factors in a TAp63gamma-induced EC9706 cell apoptosis pathway [20], suggesting that AIF and Bit1 may be both associated with cell apoptosis. To further elucidate the relationships between Bit1 and Bcl-2 as well as AIF, correlation analysis results revealed that Bit1 expression was both positively related to the expressions of Bcl-2 and AIF, which will provide a new strategy for us to investigate the function of Bit1 in ESCC in the future.
In summary, our results presented herein suggest that overexpression of Bit1 may play an essential role in the development and progression of ESCC, and positive correlations of Bit1 expression with Bcl-2 and AIF levels will provide new evidence for Bit1 molecular target therapy.
References
Ilson DH, Kelsen DP. Combined modality therapy in the treatment of esophageal cancer. Semin Oncol. 1994;21(4):493–507.
Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83(1):18–29.
van Rensburg SJ. Epidemiologic and dietary evidence for a specific nutritional predisposition to esophageal cancer. J Natl Cancer Inst. 1981;67(2):243–51.
Sewram V, De Stefani E, Brennan P, Boffetta P. Mate consumption and the risk of squamous cell esophageal cancer in Uruguay. Cancer Epidemiol Biomarkers Prev. 2003;12(6):508–13.
Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40(8 Pt 1):2633–44.
Xing D, Tan W, Lin D. Genetic polymorphisms and susceptibility to esophageal cancer among Chinese population (review). Oncol Rep. 2003;10(5):1615–23.
Ekman S, Dreilich M, Lennartsson J, Wallner B, Brattstrom D, Sundbom M, et al. Esophageal cancer: current and emerging therapy modalities. Expert Rev Anticancer Ther. 2008;8(9):1433–48.
Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–27.
Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol. 1999;26(5 Suppl 15):12–20.
Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105(1):98–100.
De Pereda JM, Waas WF, Jan Y, Ruoslahti E, Schimmel P, Pascual J. Crystal structure of a human peptidyl-tRNA hydrolase reveals a new fold and suggests basis for a bifunctional activity. J Biol Chem. 2004;279(9):8111–5.
Griffiths GS, Grundl M, Leychenko A, Reiter S, Young-Robbins SS, Sulzmaier FJ, et al. Bit-1 mediates integrin-dependent cell survival through activation of the NFkappaB pathway. J Biol Chem. 2011;286(16):14713–23.
Karmali PP, Brunquell C, Tram H, Ireland SK, Ruoslahti E, Biliran H. Metastasis of tumor cells is enhanced by downregulation of Bit1. PLoS One. 2011;6(8):e23840.
Hua W, Miao S, Zou W, Yang H, Chen B: Pathological implication and function of Bcl2-inhibitor of transcription in ovarian serous papillary adenocarcinomas. Neoplasma. 2012.
Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23(16):2785–96.
Li S, Wan M, Cao X, Ren Y. Expression of AIF and HtrA2/Omi in small lymphocytic lymphoma and diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2011;135(7):903–8.
Perraud A, Akil H, Nouaille M, Petit D, Labrousse F, Jauberteau MO, et al. Implications of cleaved caspase 3 and AIF expression in colorectal cancer based on patient age. Oncol Rep. 2012;27(6):1787–93.
Zhang W, Wang X, Chen T. Resveratrol induces mitochondria-mediated AIF and to a lesser extent caspase-9-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. Mol Cell Biochem. 2011;354(1–2):29–37.
Miyake K, Bekisz J, Zhao T, Clark CR, Zoon KC. Apoptosis-inducing factor (AIF) is targeted in IFN-alpha2a-induced Bid-mediated apoptosis through Bak activation in ovarian cancer cells. Biochim Biophys Acta. 2012;1823(8):1378–88.
Fan T, Jiang G, Suo Z, Liu H, Xu P, Ji Z, et al. Down-regulation of the apoptosis-inducing factor or Bcl-2 inhibitor of transcription by RNA interference can alleviate TAp63gamma-induced apoptosis in esophageal squamous carcinoma EC9706 cells. Int J Oncol. 2009;35(2):359–67.
Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22(53):8590–607.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Bossard C, Bieche I, Le Doussal V, Lidereau R, Sabourin JC. Real-time RT-PCR: a complementary method to detect HER-2 status in breast carcinoma. Anticancer Res. 2005;25(6C):4679–83.
Slotta-Huspenina J, Berg D, Bauer K, Wolff C, Malinowsky K, Bauer L, et al. Evidence of prognostic relevant expression profiles of heat-shock proteins and glucose-regulated proteins in oesophageal adenocarcinomas. PLoS One. 2012;7(7):e41420.
Kairouz-Wahbe R, Biliran H, Luo X, Khor I, Wankell M, Besch-Williford C, et al. Anoikis effector Bit1 negatively regulates Erk activity. Proc Natl Acad Sci U S A. 2008;105(5):1528–32.
Bouchentouf M, Benabdallah BF, Rousseau J, Schwartz LM, Tremblay JP. Induction of anoikis following myoblast transplantation into SCID mouse muscles requires the Bit1 and FADD pathways. Am J Transplant. 2007;7(6):1491–505.
Hua W, Chen B, Zhang W, Miao S, Zhang H, Xin X. Monoclonal antibodies against human bit1, an apoptosis-associated mitochondrial protein. Hybridoma (Larchmt). 2009;28(3):167–71.
Jan Y, Matter M, Pai JT, Chen YL, Pilch J, Komatsu M, et al. A mitochondrial protein, Bit1, mediates apoptosis regulated by integrins and Groucho/TLE corepressors. Cell. 2004;116(5):751–62.
Chu SH, Ma YB, Feng DF, Li ZQ, Jiang PC: Correlation between SATB1 and Bcl-2 expression in human glioblastoma multiforme. Mol Med Report. 2012.
Gao Q, Yang S, Kang MQ. Influence of survivin and Bcl-2 expression on the biological behavior of non-small cell lung cancer. Mol Med Rep. 2012;5(6):1409–14.
Ratajczak P, Leboeuf C, Wang L, Briere J, Loisel-Ferreira I, Thieblemont C, et al. BCL2 expression in CD105 positive neoangiogenic cells and tumor progression in angioimmunoblastic T-cell lymphoma. Mod Pathol. 2012;25(6):805–14.
Prasad ML, Patel SG, Shah JP, Hoshaw-Woodard S, Busam KJ. Prognostic significance of regulators of cell cycle and apoptosis, p16(INK4a), p53, and bcl-2 in primary mucosal melanomas of the head and neck. Head Neck Pathol. 2012;6(2):184–90.
Millan A, Huerta S. Apoptosis-inducing factor and colon cancer. J Surg Res. 2009;151(1):163–70.
Wang Z, Huang C, Zeng J, Deng Q, Zeng H, Liu Z, et al. Effects of the proapoptotic regulator Bcl-2/adenovirus EIB 19-kDa-interacting protein 3 on the chemosensitivity of human colon cancer cell lines. Oncol Lett. 2012;4(6):1195–202.
Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol. 2012;30(25):3127–35.
Decaudin D, Marzo I, Brenner C, Kroemer G. Mitochondria in chemotherapy-induced apoptosis: a prospective novel target of cancer therapy (review). Int J Oncol. 1998;12(1):141–52.
Kobayashi S, Iwase H, Ito Y, Yamashita H, Iwata H, Yamashita T, et al. Clinical significance of bcl-2 gene expression in human breast cancer tissues. Breast Cancer Res Treat. 1997;42(2):173–81.
Krajewska M, Fenoglio-Preiser CM, Krajewski S, Song K, Macdonald JS, Stemmerman G, et al. Immunohistochemical analysis of Bcl-2 family proteins in adenocarcinomas of the stomach. Am J Pathol. 1996;149(5):1449–57.
Hsia JY, Chen CY, Hsu CP, Shai SE, Yang SS, Chuang CY, et al. Expression of apoptosis-regulating proteins p53, Bcl-2, and Bax in primary resected esophageal squamous cell carcinoma. Neoplasma. 2001;48(6):483–8.
Jeong EG, Lee JW, Soung YH, Nam SW, Kim SH, Lee JY, et al. Immunohistochemical and mutational analysis of apoptosis-inducing factor (AIF) in colorectal carcinomas. APMIS. 2006;114(12):867–73.
Lee JW, Jeong EG, Soung YH, Kim SY, Nam SW, Kim SH, et al. Immunohistochemical analysis of apoptosis-inducing factor (AIF) expression in gastric carcinomas. Pathol Res Pract. 2006;202(7):497–501.
Roder JD, Stein HJ, Siewert JR. Oesophageal carcinoma. Berlin: Springer; 1995. p. 37.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81071723).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fan, T., Tian, F., Yi, S. et al. Implications of Bit1 and AIF overexpressions in esophageal squamous cell carcinoma. Tumor Biol. 35, 519–527 (2014). https://doi.org/10.1007/s13277-013-1073-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1073-8